Abstract
Swallowing is a complex function that relies on both brainstem and cerebral control. Cerebral neurofunctional evaluations are mostly based on functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), performed with the individual laying down; which is a non-ecological/non-natural position for swallowing. According to the PRISMA guidelines, a review of the non-invasive non-radiating neurofunctional tools, other than fMRI and PET, was conducted to explore the cerebral activity in swallowing during natural food intake, in accordance with the PRISMA guidelines. Using Embase and PubMed, we included human studies focusing on neurofunctional imaging during an ecologic swallowing task. From 5948 unique records, we retained 43 original articles, reporting on three different techniques: electroencephalography (EEG), magnetoencephalography (MEG) and functional near infra-red spectroscopy (fNIRS). During swallowing, all three techniques showed activity of the pericentral cortex. Variations were associated with the modality of the swallowing process (volitional or non-volitional) and the substance used (mostly water and saliva). All techniques have been used in both healthy and pathological conditions to explore the precise time course, localization or network structure of the swallowing cerebral activity, sometimes even more precisely than fMRI. EEG and MEG are the most advanced and mastered techniques but fNIRS is the most ready-to-use and the most therapeutically promising. Ongoing development of these techniques will support and improve our future understanding of the cerebral control of swallowing.
1. Introduction
The swallowing function is critical to ensure survival in all species, allowing both nutrition and airway protection. Its volitional and spontaneous coordination involves about 30 nerves and muscles [1], through three phases: the oral phase, the pharyngeal phase and the esophageal phase. Each phase has its own neuromuscular structures with specific brainstem control centers. The peripheral motor function has been well-described by many authors through multiple techniques such as flexible endoscopy of swallowing, videofluoroscopy, pharyngo-esophageal manometry and cervical electromyography. Its brainstem reflexive regulation is also well-described in anatomical and neurofunctional studies [2,3]. Anatomical studies suggest that a swallowing central pattern generator is located in the nucleus ambiguus and retroambiguus in the medulla [1,4].
However, less is known about the central cortical regulation of swallowing. For many years, swallowing was thought to be a reflex action, it was believed to involve solely the brainstem. It was Hamdy et al.’s work using transcranial magnetic stimulation (TMS) and functional magnetic resonance imaging (fMRI) that suggested otherwise, resulting in TMS and fMRI being the new gold standard for neurofunctional explorations [5,6].
For instance, with fMRI, Martin et al. compared the cortical activation associated with the swallowing function and non-swallowing tongue motility [7]. They showed specific swallowing activation located in the lateral post central gyrus, the supra marginal gyrus, the cuneus and precuneus. Next, Luan et al. explored the connectivity of the complex swallowing network with fMRI, showing it to be bilateral and symmetrical [8].
Dysphagia is the symptomatic expression of swallowing difficulties. Its pathophysiological mechanisms are numerous, in accordance with the numerous structures involved in the swallowing function. Dysphagia can result from many different underlying diseases affecting either the muscles involved in swallowing or the neurological control of swallowing.
At the neurofunctional level, the results from previous neurological studies have limited value in the daily clinical evaluation of dysphagia and may not be easily generalizable from one subject to another, particularly in case of neurologic disease (dystonia) or in children. Indeed, MRI is not widely available, and, above all, the subject must be lying down, which is not a natural position for meals in adults. Moreover, motion artifacts highly impair fMRI results; thus, a prolonged immobility is required for good analysis. Therefore, subjects presenting with dystonia, uncontrolled movement or unable to lay down properly due to neurologic disease and children are not easy populations to perform fMRI, even if they would be of interest. Another neurofunctional imaging method, the positron emitting tomography (PET), shares similar drawbacks, in addition to its radiating nature that increases the risk of neoplastic diseases. Electrocorticography being an invasive technique, requiring a surgical placement over the cortex, it is also not adapted for the aforementioned situations and is reserved for patients undergoing cranial surgery. It appears that non-invasive non-radiating explorations of the cerebral activity would be of particular interest in the study of the swallowing function.
The present study aims to systematically review non-invasive non-radiating neurofunctional tools, other than fMRI and PET, that can be used to explore the cerebral activity during a food swallowing task occurring in a natural setting. Furthermore, we will describe their results in the light of possible applications in routine clinical assessments.
2. Materials and Methods
2.1. Protocol and Registration
We performed this literature review according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [9]. The PRISMA statement guides researchers in their reports of systematic reviews. This protocol was registered on Prospero as ID 319150 (University of York, York, UK).
2.2. Data Sources and Search Strategies
We performed systematic literature searches from Embase (Elsevier, Amsterdam, Netherlands) and PubMed (MEDLINE, Bethesda, MD, USA). All publications prior to 30 September 2021 were included, with no limitations regarding publication dates. The complete search strategies including both subject headings (e.g., MeSH and thesaurus) and free text terms are presented in Table 1.

Table 1.
Search strategies.
Four reviewers participated in the two-step inclusion process. For every article, two reviewers filled each of the eligibility criteria and an excel function permitted to include/exclude each article according to these criteria to prevent errors of selection (e.g., inclusion in spite of the presence of an exclusion criteria). In case of divergent opinion, the reviewers discussed the article until they found a consensus. The selection process flowchart is shown in Figure 1.
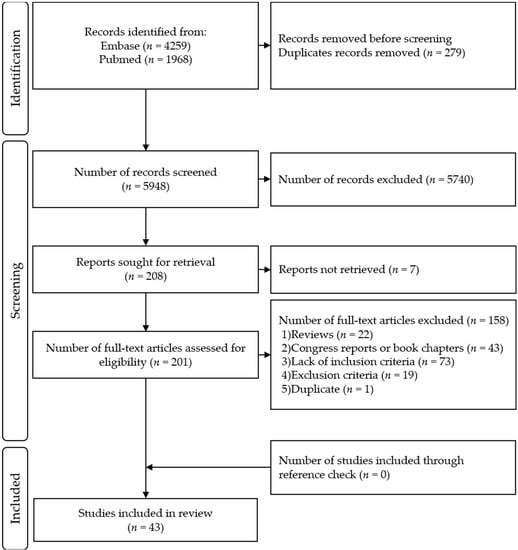
Figure 1.
PRISMA Flow Diagram.
2.3. Eligibility Criteria
The first step of screening was based on titles and abstract reviewing. Inclusion criteria for this step were the following: studies reported on (1) swallowing function and/or dysphagia; (2) brain structures, (3) brain signals (4) with an “ecological” swallowing situation. “Ecological” refers to any food intake situation in a usual/natural position (seated or standing up) with no invasive evaluation method. Invasive (e.g., intracerebral electrodes) and/or radiating techniques (e.g., PET), inappropriate head or body positioning (e.g., laying down for MRI) were considered “non-ecological”. Studies using these methods, without any other eligible task or technique, were thus excluded. Only articles published in English or French were eligible.
Case reports, animal studies, reviews, congress papers, post-mortem or fetal studies and articles involving a technique that was clearly “non-ecological” were excluded.
The second step of inclusion was based on the full article reading. Inclusion criteria were slightly refined with the following additional criteria: (1) during task the subjects were requested to swallow and/or specifically focus on their swallow; (2) awake and alert during task (excludes swallowing during sleep); (3) the meal position had to be stated (whether sitting or standing up); (4) the brain signal had to be clearly recorded during the swallowing task.
2.4. Methodological Quality, Level of Evidence, and Risk of Bias
Methodological quality assessment was rated by two independent researchers, after which a consensus was reached with the involvement of a third reviewer, when necessary. Two tools were used to assess the quality of the included articles. To assess their level of evidence, we used the National Health and Medical Research Council (NHMRC) Evidence Hierarchy, ranging from I (Systematic Review) to IV (Case Serie) [10]. To assess their methodological quality, we used the QualSyst critical appraisal tool published by Kmet et al. [11]. The QualSyst tool is a systematic and replicable assessment tool evaluating the methodological quality of a broad range of study designs. The Qualsyst has a three-point ordinal scoring system (yes = 2, partial = 1, and no = 0). The total Qualsyst score can be converted into a percentage score: strong quality for scores from 80 to 100%; good quality for 60 to 79%; adequate quality for 50 to 59%; and poor quality below 50% scores.
2.5. Data Extraction and Synthesis of the Results
Once studies were included and their quality was evaluated as sufficient (Qualsyst score above 50%), data across all studies was extracted. To achieve this, comprehensive data extraction forms were used, focusing on: the number of subjects and subjects characteristics (healthy versus dysphagic, their age, gender and hand lateralization); the neurofunctional method used, the number of channels/captors and cerebral coverage; the studied outcome parameters (type of tasks, bolus types and period of recording, e.g., swallowing preparation and/or execution) and statical analysis methods; and lastly, the authors principal conclusions. Geographic bibliometric data were also recorded.
We grouped the studies according to the technology used, as analysis methods vary according to the type of signal recorded. Motor imagery and direct pharyngo-esophageal stimulation without swallowing execution were not considered as part of the focus of this review. Results of the inclusions are presented below.
Based on studied population and performed tasks, we classified the studies as referring to “physiology” (classical swallowing condition in healthy subjects), “adaptive physiology” (modification of a specific characteristic of interest during the task in healthy subjects, such as body and head positioning or sensory stimulation), “pathology” (comparison between dysphagic population and healthy subjects) and “patho-physiologic” (if the study would fall under both “pathology” and “adaptive-physiology”).
Two triggering methods for the swallowing task were differentiated: non-volitional and volitional tasks. The non-volitional tasks, included both “provoked swallowing” (shunt of the oral phase) and “spontaneous swallowing”, whilst the volitional tasks, included both “cued swallowing” (investigator-driven or informatically driven) and “self-paced swallowing” (subject-driven).
2.6. Data Presentation and Analysis
The descriptive data is presented at a global level and per technique. When possible, we described numeric data using means, standard deviations and range (Mean ± SD; Range), unless specified otherwise.
3. Results
3.1. Study Selection
A total of 5948 unique records were retrieved. Each of the four reviewers assessed 50% of all records. After title and abstract selection, 208 full-text articles were screened for eligibility, of those, 43 articles were included in this review (Figure 1). The list of the included articles and their methodological quality as evaluated by QualSyst are presented in Table 2. Their respective results are available in Tables S2–S4.

Table 2.
Methodological quality rating according to the QualSyst critical appraisal tool [11] and the NHMRC level of evidence [10] of the 43 included articles.
3.2. Neurofunctional Imaging Techniques
We identified three techniques used in neurofunctional swallowing assessment that were both non-invasive, non-radiating and usable in standard meal positions: electroencephalography (EEG), magnetoencephalography (MEG) and functional Near Infrared Spectroscopy (fNIRS). A total of 21 (50.0%) articles focused on MEG, 11 (26.2%) on fNIRS and 10 (23.8%) on EEG. Table 3 summarizes the complete Supplementary Table S1 as a presentation of each method’s technical characteristics in comparison to fMRI, the Gold Standard of functional neuroimaging. A detailed report on the contributions provided by each technique, with regards to the cerebral activity during swallowing, will be provided. The results will be presented according to the methods, analyses and limitations of each technique.

Table 3.
Technical characteristics of EEG, fNIRS, MEG: methodology, swallowing sequences and measure of interest as reported in the included studies, compared to fMRI. See Supplementary Table S1 for the complete results.
3.3. Quality Assessment
According to the QualSyst score [11], 36 studies displayed a strong methodology (EEG: 9; MEG: 19; fNIRS: 8) and the remaining 7 had good methodology (EEG: 2; MEG: 2; fNIRS: 3).
Based on NHMRC evidence hierarchy [10], 1 MEG study was classified as level II (“Randomized control trial”), 2 MEG studies as level III-1 (“Pseudo randomized control trial”); 4 studies (EEG: 2; fNIRS: 2) were classified as level III-2 (“Comparative studies with concurrent controls and allocation not randomized [cohort studies], or case control studies”); 9 studies (MEG: 7; fNIRS: 2) were classified as III-3 (“Comparative studies with two or more single-arm studies”); 27 studies (EEG: 9; MEG: 11; fNIRS: 7) were classified as level IV (“Case series”). The full quality evaluation is displayed in Table 2.
Since no studies were found to be of poor quality, all 43 eligible studies were included. On the whole, all the studies selected were assessed as being of good quality; however, the majority of studies displayed a relatively low level of evidence (mostly level III and IV, see Table 2).
3.4. Bibliometric Data
Regardless of the technique used, multiple teams are involved in natural neurofunctional evaluation of swallowing, mainly in Europe, North America and Asia. We found 6 international collaborations. EEG is most used in the USA (N = 6) and Japan (N = 3), and we noted 1 collaboration between USA and Japan, and 1 between USA and Austria. MEG is the most used technique, mostly in Germany (N = 18). Germany had 2 collaborations, 1 with the United Kingdom and 1 with Canada. fNIRS has been the most experimented in Austria (N = 5) and Japan (N = 4). One collaboration between Austria and Germany was noted. The worldwide study distribution is displayed in Figure 2.
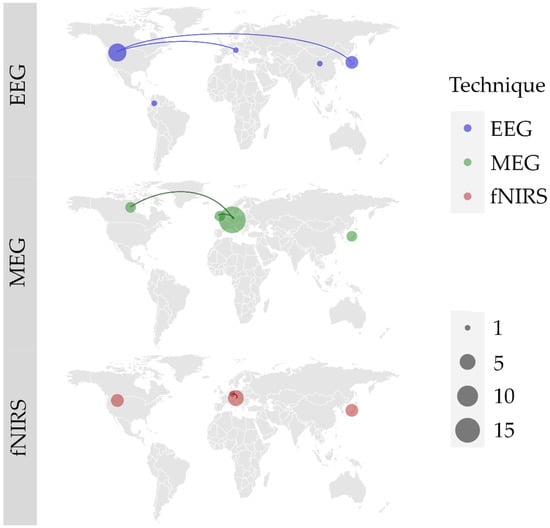
Figure 2.
World locations of author’s affiliations, and collaborations (lines) between research teams for each of the three identified techniques: electroencephalography (EEG); magnetoencephalography (MEG); functional near infrared spectroscopy (fNIRS). Size of the point represents the number of articles published by each and every country.
EEG and MEG studies have been published since the 2000s, while most of fNIRS studies date from the mid 2010s as fNIRS is a younger technology (Figure 3).
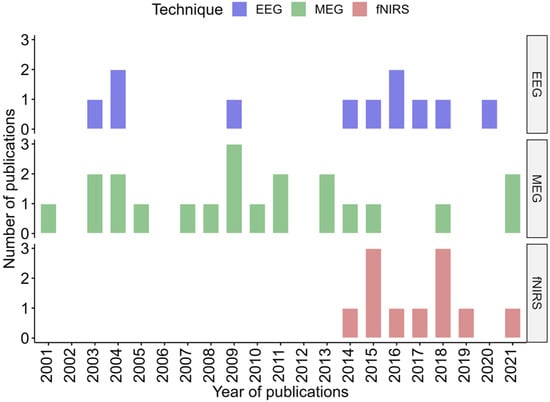
Figure 3.
Distribution timeline, in years, of all included publication for each technique.
3.5. Participants
Amongst the 43 studies, the mean number of participants was 17.5 ± 13.4, ranging from: 3 to 55 (EEG: 24.8 ± 20.1; Range= 7–55; MEG: 14.2 ± 9.2; Range= 3–44; fNIRS: 16.5 ± 9.8; Range = 6–43). Only 10 studies included 20 subjects or more (23.3%) [16,17,18,19,35,36,37,38,53], 31 studies included between 5 and 19 subjects (72.1%) [12,13,14,15,20,21,22,25,26,27,28,29,30,31,32,33,34,39,40,41,42,43,44,45,46,47,48,49,50,51,52], and the 2 studies that had less than 5 subjects (4.7%) [23,24] were the first published on this topic.
A total of 32 (74.4%) studies focused on healthy subjects [12,13,14,15,16,17,18,19,21,23,24,25,26,27,28,29,31,32,34,38,40,42,43,44,46,47,48,49,50,51,53,54], 10 studies (23.3%) included both healthy and dysphagic subjects [20,22,30,33,35,36,37,39,45,52] and 1 study (2.3%) focused on only dysphagic subjects [41]. Table S1 displays these data per technique.
Only one EEG study focused on children aged from 10 to 13 years [22]. All other studies focused on adult subjects, aged between 18 and 80 years. More details can be seen in Table S1.
Regarding gender distribution, 7 (16.3%) studies did not display this data [14,17,18,24,27,33,41] and 1 study (2.3%) seemed to present a mistake describing “14 females and 7 females” [54]. Among the other 35 (81.4%) studies, gender distribution was slightly unbalanced, with a total of 268 females (F) and 305 males (M), with notably more women in fNIRS studies (97F/77M) and more men in MEG studies (101F/153M).
Hand lateralization can affect cortical activity distribution and should be recorded in studies on cortical activity. However, 17 studies (39.5%) did not present this data [16,17,18,21,22,30,33,37,39,41,42,43,46,47,50,52,53]. The 26 (60.5%) other studies focused mainly on right-handed subjects. Throughout these studies, only three ambidextrous subjects from three different studies [19,35,36] and one left-handed subject [35] were included. Unfortunately, their individual results were not specifically described. Therefore, further conclusions might be applicable only to right-handed subjects.
A quarter of studies (11, 25.6%) explored dysphagic subjects, either alone (n = 1; 2.3%) [41] or compared to healthy controls (n = 10; 23.3%) [20,22,30,33,35,36,37,39,45,52]. Looking at dysphagic subjects in more detail, they suffered from different diseases and conditions, including: stroke [20,35,45,52], Parkinson’s disease [37], amyotrophic lateral sclerosis [36], Kennedy disease [33], botulism [30], anterior open bite [22] and functional dysphagia [39].
3.6. Types of Studies
The study types were the following: 17 (40%) “physiology” studies (5 EEG, 7 MEG, 5 fNIRS); 15 (35%) “adaptive physiology” studies (4 EEG, 7 MEG, 4 fNIRS), 2 (5%) “patho-physiology” (1 EEG, 1 fNIRS), and 9 (21%) “pathology” studies (1 EEG, 1 fNIRS, 7 MEG). More specifically, “adaptive physiology” focused on the effects of different conditions: differences in age [34,53], bolus viscosity [16,18], head positioning [17,18], bolus volume [21], distractors [21], taste [48], tactile stimulations [29,31,40,42,43,49], neurofeedback [46] and transcranial direct current stimulation (tDCS) [38,42]. The 2 “patho-physiology” studies focused on the effect of tDCS [20] and the taste perception differences [52] between stroke patients and healthy subjects.
The studies looked at different phases of the swallow A total of 6 (14%) studies focused specifically on swallowing preparation (4 EEG, 2 MEG), 15 (35%) specifically on execution (1 EEG, 3 MEG, 11 fNIRS), and 22 (51%) focused on both (6 EEG, 16 MEG).
When studying the cortical activity of swallowing, the different types of boluses evaluated are of importance. The majority of studies evaluated swallowing with liquids (29 studies = 67%), or saliva (8 studies = 19%), or both (3 studies = 7%). The remaining study (2%) focused on solids through the swallowing of cucumber. The swallowing of liquids was mostly assessed with water in all but one study. The effect of viscosity on the swallow was also reported in 2 studies [16,18], using honey or nectar. Furthermore, one study used flavored jellies [52]. It is of note that taste, more than swallowing itself, was the major point of interest in two studies using water [43] or jelly [52].
The type of swallowing trigger can also influence the observed cortical activity. In terms of triggered swallow, 34 studies (79%) focused on self-paced, 7 studies (16%) on cued, 2 studies (5%) on spontaneous and 2 studies (5%) on provoked swallowing. Three studies compared different triggers: self-paced versus cued swallowing [15], cued versus provoked swallowing [20] and self-paced versus provoked swallowing [25].
See Table 4, Table 5 and Table 6 and their respective extended versions, Supplementary Tables S2–S4 for each study details.

Table 4.
EEG studies data. See complete version in Supplementary Table S2.

Table 5.
fNIRS studies data. See complete version in Supplementary Table S3.

Table 6.
MEG studies data. See complete version in Supplementary Table S4.
4. Discussion
The present review highlighted three different neuro-imaging techniques currently used for swallowing assessment purposes that meet our ecological process of swallowing. As we focused on the study of motor activity in an ecological swallow, other tasks such as motor imagery, direct pharyngo-esophageal stimulation and tongue movement without swallowing studies results were not included.
For each technique, it is important to understand how the cerebral area can be accessed in order to better understand their specific results. They share some similarities in terms of technical procedures, objectives and results but also have their own specificities, which explain their complementary nature.
4.1. Methodological Considerations
All three techniques can efficiently analyze the superficial cortical activity, but for deeper activity, fNIRS is limited and EEG or MEG are necessary. On the one hand, fNIRS is considered as a topographical (it is limited to the surface of the cortex, resembling a surface map) superficial technique since its signal is limited to the cortical area 3 cm below the scalp skin, which matches the most superficial part of the cortex. On the other hand, MEG and EEG are tomographical techniques that can render three-dimensional results. Those two are supposedly able to access both superficial cortices and deep brain regions, such as insula and deep grey nuclei, thanks to specific statistics analyses (e.g., independent component analysis, graph theory and/or wavelet analyses).
With this in mind, we will focus first on the regions of interest for this review; second, the variations according to different task types and bolus substances and lastly, the studies will be differentiated based on their physiologic and/or pathologic objectives and results.
4.1.1. Activity Localization
It is important to delineate the main swallowing regions of interest from this review. On the one hand, all three techniques showed good activity of the caudal pericentral cortex (Supplementary Table S1), gathering both primary sensory and motor cortices (Broadman Areas (BA) 4,1,2,3). These regions likely focus on pharyngeal-laryngeal cortices according to both classical homunculus [59,60] and Ludlow et al.’s review [1]. Other areas can also be studied by both techniques, particularly in the premotor areas (premotor cortex [PMC], supplementary motor area [SMA] in BA6) and the prefrontal cortex (BA9, 10, 45) [20,33,39,47,52]. Over those regions, the three techniques allow for the study of both the precise localization of the activity and its chronology. These regions have also been described in fMRI studies and thus, are good candidates for further exploration in ecologic conditions [4,55].
On the other hand, some differences must be raised, as they are critical for the choice of the technique adapted to one’s experiment. The insula, the anterior cingulum cortex (ACC) and posterior cingulum cortex (PCC) have been shown to play an important role in the swallowing process in fMRI studies [4,55]. However, as a topographical superficial technique, the fNIRS is currently unable to provide good activity data of these deep regions. Whereas MEG results included in our studies, using tomographical source positions analyses, provided results consistent with fMRI results. No EEG studies mentioned these deeper activities, as they mostly focused on preparation potential without gyrus dipole source localization approach, or they focused on the network microstructure. Actually, only one study focused on the dipole source location of the activity and found an activity centered on the motor BA4 and PMC BA6 [19] and one study used the electrode as a topographical source locator [20], without three-dimensional dipole localization approach. We would still expect EEG to also be able to individualize this activity using independent component analysis. Indeed, other studies with classical somatosensory stimulation were able to individualize deeper activities such as the anterior cingulum [61].
Some spatial limitations of these techniques appear to be the putamen and the cerebellum activities shown in fMRI reports [4,55], as neither EEG, nor MEG (and obviously fNIRS) measured these activities in our review. This obstacle might not be crossable, but at least for the cerebellum, EEG and MEG could be of potential use according to Andersen et al. [62]. Similarly, fNIRS, EEG and MEG in their current technological state, appear impractical for exploring the brainstem and specifically the central pattern generator located in the nucleus ambiguus and retroambiguus. As mentioned previously regarding the cerebellum, EEG and MEG might be the future key explorations for this purpose.
4.1.2. Tasks Modalities
Secondly, in order to deepen each regions’ role, we need to separate different types of swallowing tasks, according to two characteristics that should be highlighted: the way to trigger the swallowing task and the bolus type, as each characteristic has its specificities. Through the review, task triggering was either non-volitional or volitional.
Non-volitional swallowing can be divided in two sub-tasks: provoked swallowing (direct pharyngeal instillation by external source) and spontaneous swallowing (the subject being unaware of the swallowing task). Two studies focused on provoked swallowing execution, with transnasal instilled water. In this regard, EEG showed an increase in approximate entropy (compared to resting state) of the central region in healthy subjects, just before the muscular swallowing activity [20]. MEG showed activation of the prefrontal cortex (BA9) during preparation (reflecting anticipation) and of the parietal lobule (BA7) during execution [25]. The transnasal canula sensation could be the cause of the parietal activity during the preparation phase, as suspected by the authors. Interestingly however, it was shown with MRI that tactile stimulation of the mucosa of the anterior nose elicit responses over the caudal part of the primary sensorimotor cortex, without parietal response [63]. To our knowledge, no study has tested the back of the nose/choanae cerebral sensory pattern.
Spontaneous swallowing was the central point of two studies using fNIRS, focused on primary motor and primary sensory cortex. Without any instruction to the subject, they showed activity in the primary sensorimotor cortex [49,50]. It is of interest that the authors of those 2 fNIRS studies claim to be recording the BA4 but, according to the Bioimage suite tool (Yale School of Medicine, New Haven, CT, USA), might be recording the Broca Area (BA44). This detail might illustrate the lack of spatial resolution of current fNIRS system in comparison to EEG, MEG or fMRI, although its development is quickly improving. The fMRI was used in similar fashion (reported as “naïve saliva swallowing”) and showed similar activation of the lateral pericentral and PMC on both sides [64]. They also observed activation of the right insula, the superior temporal gyrus, the middle and inferior frontal gyri (MFG and IFG) and frontal operculum. The two fNIRS study channels were limited to the primary sensory and motor cortex, thus, they could not measure those activities, besides the deepness of certain areas. A broader cortical fNIRS coverage might confirm those fMRI results in the future.
Volitional swallowing can be cued swallowing (no decision from the subject) or self-paced swallowing (with the subject’s decision). For these tasks, studies focused either on the swallowing preparation and/or on the execution. It is also critical to take into account the swallowed substance, either saliva, liquids or solid.
With regards to cued preparation of saliva swallowing, with EEG, Hiraoka et al. were able to measure cued evoked negative preparation potentials called CNV (“contingent negative variations”). CNV appeared earlier and were about 2 to 4 times stronger than the self-paced preparation potential named MRCP (“Movement related cortical potential” which contains the “Bereischaftpotential” [BP]). Those negative potentials were detected in the vertex area (Pz, Fz, Cz, C3, C4) [15]. Regarding water cued swallowing preparation, with EEG, Yuan et al. also showed a broader approximate entropy over the parieto-fronto-temporo-central region in cued condition in comparison to provoked conditions, where it is more limited to the central region [20]. With MEG, Watanabe et al. measured, during cued water swallowing, bilateral preparation responses that seemed to follow the following course, from the PCC, SMA, ACC, SFG, MFG, IFG and lastly, Insula, but only PCC and Insula had statistically significant different onset times (p < 0.003) [27].
During cued swallowing execution, EEG retrieved beta ERD in the motor and premotor regions (BA 4 and 6) with water in comparison to tongue tapping [19]. With fNIRS, an oxyHb increase can be measured in the whole pericentral, temporal and frontal regions associated to water swallowing [47], and also in SMC, PFC and pre-SMA while executing a swallowing task with chopsticks and cucumber slices [54]. This last task also evokes an oxyHb decrease in SMA and PMA after swallowing. This last study is the only to focus on the whole meal process, from the plate to the mouth. This illustrate its use in ecological conditions but could also be a source of confounding factors on its conclusions.
These results are consistent with fMRI results showing anterior cingulum, IFG, MFG, cuneus and precuneus region activation during volitional cued swallowing in comparison to spontaneous swallowing [64,65]. This cingulate activity appears to be linked with task complexity or with an imposed higher rate of swallowing [6]. With 18FDG-PET, Harris et al. showed broader activity of the left sensorimotor cortex, cerebellum, thalamus, precuneus, anterior insula, left and right lateral postcentral gyrus, and left and right occipital cortex [66].
Volitional self-paced swallowing preparation and execution were the most studied in the different articles. During preparation, all EEG studies tended to record a one-phase Bereischaftpotential (BP), a negative preparation potential recorded from the vertex region before self-paced motor tasks, about 1500 ms before swallowing muscular activity [12,13,14,15]. This BP appeared to be stronger with saliva than with water [14]. With MEG and water, a similar activity was shown to be located in the Cingulate gyrus and SMA (thought to be the origin of the BP), but also in MFG, IFG and insula with similar time onset (from −1500 ms to 1000 ms before muscular activity) [24].
Regarding the execution, EEG and MEG studies confirmed that the majority of the activity is localized in the pericentral cortex in the alpha and beta bands of the recorded signal. The evoked potential in an EEG study of saliva self-paced swallowing execution showed greater amplitude than water in Hiraoka et al.’s study [14]. Self-paced swallowing execution MEG studies represent a major part of our review, as it gathers all the work of Dziewas et al., which deserves a focus as it is the most advanced in term of both localization, temporal course of the activity and the implications of pathophysiological alteration on the swallowing network. They showed that in normal conditions, the alpha and beta activities are focused on the pericentral cortex and globally symmetric, but more precisely, they undergo a shift from the left to the right hemisphere from 400 ms before to 600 ms after the muscular activity onset, suggesting a left lateralization of the voluntary phases (oral) and a right lateralization of the reflexive tasks (pharyngeal). These results are consistent with other studies but are the most precise in terms of temporal course in these conditions. This high precision clearly illustrates MEG’s higher temporal resolution than fMRI, which could only measure a global right insular lateralization [64].
4.2. Adaptive Physiology
Besides simple saliva and water studies, some teams studied the adaptive physiology of swallowing through other modifications of the bolus types and volumes, or other factors.
Dziewas’ team is the only one that studied various swallowing condition modifications and their effects on the MEG signal with a consistent self-paced water swallowing protocol over all their studies. They studied the effect of age on cortical activity and showed that elders had a stronger and broader bilateral pericentral activity, predominantly in the β band. In comparison to their usual task, they showed an increased pericentral activity in faster and more challenging swallowing tasks, with specific activation of PMC and parieto-occipital cortex during their hardest task. They illustrated that sensorimotor cortical activity of swallowing is reduced by pharyngeal anesthesia [29] and increased by cold tactile thermal oral stimulation [31]. Following the latter, they tested the sensory effect of capsaicinoids. It appeared that capsaicinoids would have a specific peripheral effect on muscular contraction without specific modification of swallowing cortical activity [43].
Some fNIRS studies also focused on adaptive physiology. In this regard, Kober et al. retrieved a cortical increase in oxyHb concentrations over the IFG in elders [53], similarly to the aforementioned Dziewas study. Mulheren et al. and Lee et al. studied the effect of taste on swallowing cortical activity. In healthy subjects, Mulheren et al. showed no pericentral or premotor (SMA) early effect (2 to 7 s after swallowing) on oxyHb concentrations but found a remanent effect at 17 to 22 s, with significant effect of a sour taste (in comparison to sweetness or water) [48]. Lee et al. focused on the prefrontal activity in healthy and brain-impaired dysphagic subjects. Although they showed globally increased oxyHb concentrations with flavor and decreased oxyHb with sweetness, they found no modifications in brain impaired dysphagic patients [52]. As previously noted, Matsuo’s study with cucumber slices shows the feasibility of fNIRS studies on the ecological meal process, from the plate to the swallowing [54]. It should be noted that they focused only on premotor areas (PMC and SMA) and on cranial sensorimotor cortex, which is likely to exclude the laryngeal cortex and to include superior limb motor cortex. Nevertheless, this is the first neurofunctional study of the whole process of solid swallowing without pause, with other studies being limited to the swallowing part.
Some EEG studies tried to explore the whole process with liquids but introduced pauses between the self-instillation from a glass and the swallowing of the bolus. Cuellar et al.’s cued task comprised a cued self-instillation from cup into the mouth, a 7 s pause then a cued swallowing to reduce arm movement artifacts [19].
The studies of Jestrović et al. with EEG are of note, as they are original and illustrate the microarchitectural characteristics (also called “small-worldness”) of the different global swallowing networks. They used a self-paced multiple successive swallowing from cup without pause between arm and mouth paradigm (but a 2–3 s pause between each swallowing) which made those tasks “self-feeding like”, similarly to Matsuo, in order to study those networks. “Networks” here is plural as they show arguments of different microarchitectural characteristics for different head positions [17,18], bolus types and volumes [16,18,21] and in the presence of distractors [21], suggesting different networks at work. These characteristics could be of use in studying microarchitectural anomalies and better understand pathophysiology of dysphagia and the effect of rehabilitation [17], even though they do not have a “localizationist” value and their future usefulness in clinical conditions needs to be clarified.
To our knowledge, no other neurofunctional imaging studies of swallowing in ecological conditions have been performed with fMRI. The closest to ecological study is the one from Harris et al. with PET [66]. They performed a cued water swallowing task in ecological condition (seated position) during the 18F-FDG uptake and used the remanent fixation to neural network of FDG to perform the PET in lying position. Interestingly, they showed increased glucose metabolism in the left sensorimotor cortex, cerebellum, thalamus, precuneus, anterior insula, left and right lateral postcentral gyrus, and left and right occipital cortex, and decreased glucose metabolism were also seen in the right PMC, right and left sensory and motor association cortices, left posterior insula and left cerebellum. These results are quite consistent with our review, but it must be reiterated that PET being radiative, has been excluded of our review.
4.3. Patho-Physiological Contexts
4.3.1. Pathological Descriptions
As previously emphasized, these techniques have been used in pathological conditions. Dziewas’ team is again the most advanced, as they observed that multiple pathological conditions (Amyotrophic lateral sclerosis [36], strokes [35], Parkinson’s disease (PD) [37], etc…) seem to broaden the activity areas, which tends to be more right-sided in those patients. In these pathologic conditions, MEG found activity in the parietal cortex (BA7, 40, 43) but also in premotor (BA6) and prefrontal cortex (BA44, 45, 47). This broadening seems more associated with the presence of dysphagia. Similar prefrontal activity could also be recorded with fNIRS [45] but might be highly dependent on the type and localization of the lesions and obviously on the position of the limited number of optodes of this technique. This general pattern actually shows specificities with each disease. For example, Teismann et al. studied the cortical modifications during swallowing found in cases of stroke. In the case of hemispheric stroke, regardless of dysphagia, they found a higher activity of the Dorsolateral PFC (DLPFC) and insula compared to healthy controls. The presence of dysphagia modifies pericentral activity as hemispheric stroke patients with dysphagia showed a reduction in ipsilateral pericentral activity with no contralateral activity, whereas non dysphagic subjects showed bilateral activity similar to controls. Lastly, brainstem stroke patients displayed a right lateralization of their pericentral activity. With fNIRS, Kober et al. compared the inferior frontal cortex (DLPFC) cortical activity of 2 hemispheric stroke patients, 2 brainstem stroke patients and 2 healthy subjects [45]. They found different results with the 4 patients. The hemodynamic responses (HDR) appeared to be of lower amplitude in cerebral stroke patients and of higher amplitude in brainstem stroke patients, compared to healthy subjects. The authors interpret these as a sign of cortical plasticity after stroke for the swallowing function. Liu’s meta-analysis on fMRI in stroke dysphagic patients during swallowing found slightly different results. Indeed, in patients, they showed an hyperactivation of the left cingulate and precentral gyri and right posterior cingulate gyrus with hypoactivation of the right cuneus and left MFG. This discrepancy could reflect the difference of tasks position (lying down with fMRI and seated with MEG and fNIRS), difference of populations as strokes are different according to the region and their extension, or difference of temporal resolution between MEG (analysis over 200 ms intervals) and fMRI and fNIRS (analysis over a few seconds period).
The only pathology study with EEG compared children with anterior open bite with children with normal denture and found no difference of swallowing EEG activity between the two conditions [22].
4.3.2. Pathophysiological Experiments
These techniques have already been used for pathophysiological studies and pre-therapeutical objectives. These results were not specifically the objective of our review but are still interesting to point out. Yuan et al. used transcranial Direct Current Stimulation (tDCS) and compared EEG results before and after tDCS and showed an increase in swallowing cortical excitability in healthy and cerebral stroke subject with swallowing apraxia. Dziewas’s team also showed an increase in cortical activity in healthy subjects on MEG with tDCS, associated with an improvement of swallowing skills during challenging tasks (e.g., fast swallowing). Based on that, they successfully used tDCS on dysphagic patients through a clinical prospective double-blind protocol, showing its efficiency on cortical activity [41]. They showed similar results in healthy subjects after thermal tactile oral stimulation (used for rehabilitation) [31] and a reduction in cortical activity after pharyngeal electrical stimulation (PES) [40]. They lastly showed that PES could increase cortical activity after pharyngeal anesthesia, which was not possible with tDCS [46].
EEG and fNIRS might also be powerful swallowing rehabilitation tools, through motor imagery and neurofeedback [46,53,67]. Kober et al. are the more advanced with regards to fNIRS neurofeedback of dysphagia. Their preliminary results suggest an ability of fNIRS to stimulate cortical plasticity of the swallowing network, which could lead to dysphagia improvement [46,53]. This actually needs further thorough evaluations and validations, but it is nevertheless a promising treatment.
4.4. Limitations and Perspectives
The three techniques showed encouraging results for the exploration of the swallowing function in near-ecologic conditions. However, they both have limitations for use during a mealtime that still needs to be taken into account and overcome.
The easiest tool seems to be fNIRS but it has some limitations. The actual limited number of optodes (the fNIRS captors) implies that one must know the region of interest before the experiment. As most of the activity is located in the caudal pericentral cortex and posterior inferior frontal cortex, it leaves a few optodes to explore other areas. Increasing the number of optodes in future systems should improve this drawback. Moreover, the inaccessibility of the insula and the cingulum limits the use of fNIRS. Some studies used high definition fNIRS systems to explore deeper brain layers (resembling a tomographic technique) but their transferability to swallowing exploration is not certain [68]. One last drawback of about half of the studies is the lack of proof of real HDR measurements. Indeed, almost half of studies only used the oxyHb concentration evolution. It is well known that, with fNIRS, HDR is defined as an inverse variation of oxyHb and deoxyHb [69]. On the contrary, if oxyHb and deoxyHb vary in the same way, it is considered to be non-hemodynamic signal and might be due to movement artifacts. Thus, it is hard to tell if those studies recorded HDR signal or artifacts. This problem also questions the validity of fMRI studies as BOLD (Blood oxygen level dependent) signal also only focus on oxyHb. The measures of both oxyHb and deoxyHb with fNIRS might help to validate or correct previous fMRI results on this matter. Moreover, the major interest of this technique is the low number of trials needed (~10) to record good signal, similarly to fMRI. Its use for motor execution seems promising but still needs more robust studies and its potential for swallowing preparation still needs to be assessed.
For deeper brain studies with more precision in both localization and time course of activity, both EEG and MEG are the best mastered techniques for ecological conditions in both preparation and execution of swallowing. However, the classical need for a lot of trials (50 to 100) is less adapted to classical meal modalities. It should be highlighted that decades of optimizations have allowed for their use with fewer trials (down to 5 [21,67]) but their clinical value is more limited. Another often raised drawback is the important effect of muscular contraction artifacts, but as our review suggests, many analyses can suppress those artifacts within these two techniques.
One way to overcome these limitations could be the use of both EEG or MEG (neuronal signal) and fNIRS (hemodynamic signal) to correlate both results. The EEG/fNIRS association has been used in other fields with promising results [70], but still needs to be evaluated for swallowing purposes. This could improve the data quality and reduce the number of needed trials. The association of the hemodynamic data from fNIRS and neuronal activity from EEG is particularly interesting, as they can be both portable (which is not the case for MEG) and some systems already integrate both signals. The fNIRS itself would also benefit from the short channel technology [71,72] to reduce the effects of non-hemodynamic blood flows, however, that has not been used for swallowing up to now.
Another limitation is the comparison with the gold standard which is the MRI. As we already discussed, when we compare these techniques’ results with those of fMRI, there are some discrepancies that might be due to the position itself. Vertical MRI (called “weight-bearing MRI”) have been used for two decades to study the effect of one’s weight on one’s joints [70]. Their use for functional imaging would allow researchers to differentiate the proper effect of the lying down position but the technology has not been used in this way up to now as the scanner’s power is limited to 1.5T [73].
5. Conclusions
Neurofunctional imaging of the swallowing function in ecologic condition is possible through EEG, MEG and fNIRS but still needs to be improved. As each technique has its benefits and drawbacks, the improvement could well arise from multi-signal explorations to allow for meal-time analysis in the future. This will help to improve physiology and pathology comprehension and might lead to the rehabilitation of dysphagia in these subjects.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11185480/s1, Table S1: Technical characteristics of EEG, fNIRS, MEG: methodology, swallowing sequences and measure of interest as reported in the included studies, compared to the fMRI gold standard; Table S2: EEG studies data; Table S3: fNIRS studies data; Table S4: MEG studies data.
Author Contributions
Conceptualization, Y.G., P.G., R.S. and V.W.; methodology, R.S. and V.W.; formal analysis, Y.G.; data curation, Y.G., F.N., M.G. and X.C.; investigation, Y.G., F.N., M.G. and X.C.; writing—original draft preparation, Y.G.; writing—review and editing, Y.G., R.S., E.V. and V.W.; visualization, Y.G.; supervision, P.G. and V.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Anne Lasfargue-Delannoy for her help with english proof reading.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ludlow, C.L. Central nervous system control of the laryngeal muscles in humans. Respir. Physiol. Neurobiol. 2005, 147, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, C.L. Recent advances in laryngeal sensorimotor control for voice, speech and swallowing. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Jean, A. Brain Stem Control of Swallowing: Neuronal Network and Cellular Mechanisms. Physiol. Rev. 2001, 81, 929–969. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, C.L. Central Nervous System Control of Voice and Swallowing. J. Clin. Neurophysiol. 2015, 32, 294–303. [Google Scholar] [CrossRef]
- Hamdy, S.; Aziz, Q.; Rothwell, J.; Singh, K.; Barlow, J.; Hughes, D.G.; Tallis, R.C.; Thompson, D.G. The cortical topography of human swallowing musculature in health and disease. Nat. Med. 1996, 2, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, S.; Mikulis, D.; Crawley, A.; Xue, S.; Lau, H.; Henry, S.; Diamant, N.E. Cortical activation during human volitional swallowing: An event-related fMRI study. Am. J. Physiol. Liver Physiol. 1999, 277, G219–G225. [Google Scholar] [CrossRef]
- Martin, R.E.; MacIntosh, B.J.; Smith, R.C.; Barr, A.M.; Stevens, T.K.; Gati, J.S.; Menon, R.S. Cerebral Areas Processing Swallowing and Tongue Movement Are Overlapping but Distinct: A Functional Magnetic Resonance Imaging Study. J. Neurophysiol. 2004, 92, 2428–2443. [Google Scholar] [CrossRef]
- Luan, B.; Sörös, P.; Sejdić, E. A Study of Brain Networks Associated with Swallowing Using Graph-Theoretical Approaches. PLoS ONE 2013, 8, e73577. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- National Health and Medical Research Council (NHMRC). Guidelines for the Development and Implementation of Clinical Guidelines, 1st ed.; National Health and Medical Research Council (NHMRC): Canberra, Australia, 1995.
- Kmet, L.M.; Cook, L.S.; Lee, R.C. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields. Available online: https://era.library.ualberta.ca/items/48b9b989-c221-4df6-9e35-af782082280e (accessed on 21 December 2021).
- Huckabee, M.-L.; Deecke, L.; Cannito, M.P.; Gould, H.J.; Mayr, W. Cortical control mechanisms in volitional swallowing: The Bereitschaftspotential. Brain Topogr. 2003, 16, 3–17. [Google Scholar] [CrossRef]
- Satow, T.; Ikeda, A.; Yamamoto, J.-I.; Begum, T.; Thuy, D.H.D.; Matsuhashi, M.; Mima, T.; Nagamine, T.; Baba, K.; Mihara, T.; et al. Role of primary sensorimotor cortex and supplementary motor area in volitional swallowing: A movement-related cortical potential study. Am. J. Physiol. Liver Physiol. 2004, 287, G459–G470. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K. Movement-Related Cortical Potentials Associated with Saliva and Water Bolus Swallowing. Dysphagia 2004, 19, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, T.; Yoshida, M.; Yamaguchi, T.; Uchida, A.; Ohba, H.; Oka, S.; Nakajima, I. Contingent negative variations associated with command swallowing in humans. Clin. Neurophysiol. 2009, 120, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Jestrović, I.; Coyle, J.; Sejdić, E. The effects of increased fluid viscosity on stationary characteristics of EEG signal in healthy adults. Brain Res. 2014, 1589, 45–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jestrović, I.; Coyle, J.L.; Sejdić, E. Characterizing functional connectivity patterns during saliva swallows in different head positions. J. Neuroeng. Rehabil. 2015, 12, 61. [Google Scholar] [CrossRef][Green Version]
- Jestrović, I.; Coyle, J.L.; Perera, S.; Sejdić, E. Functional connectivity patterns of normal human swallowing: Difference among various viscosity swallows in normal and chin-tuck head positions. Brain Res. 2016, 1652, 158–169. [Google Scholar] [CrossRef]
- Cuellar, M.; Harkrider, A.; Jenson, D.; Thornton, D.; Bowers, A.; Saltuklaroglu, T. Time–frequency analysis of the EEG mu rhythm as a measure of sensorimotor integration in the later stages of swallowing. Clin. Neurophysiol. 2016, 127, 2625–2635. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, J.; Wu, D.; Huang, X.; Song, W. Effect of transcranial direct current stimulation on swallowing apraxia and cortical excitability in stroke patients. Top. Stroke Rehabil. 2017, 24, 503–509. [Google Scholar] [CrossRef]
- Jestrović, I.; Coyle, J.L.; Perera, S.; Sejdić, E. Influence of attention and bolus volume on brain organization during swallowing. Anat. Embryol. 2018, 223, 955–964. [Google Scholar] [CrossRef]
- Restrepo, C.; Botero, P.; Valderrama, D.; Jimenez, K.; Manrique, R. Brain Cortex Activity in Children with Anterior Open Bite: A Pilot Study. Front. Hum. Neurosci. 2020, 14, 220. [Google Scholar] [CrossRef]
- Loose, R.; Hamdy, S.; Enck, P. Magnetoencephalographic Response Characteristics Associated with Tongue Movement. Dysphagia 2001, 16, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Wantanabe, Y.; Shintani, M.; Tazaki, M.; Takahashi, M.; Yamane, G.-Y.; Ide, Y.; Yamada, Y.; Shimono, M.; Ishikawa, T. Magnetoencephalographic study of the starting point of voluntary swallowing. CRANIO® 2003, 21, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Dziewas, R.; Sörös, P.; Ishii, R.; Chau, W.; Henningsen, H.; Ringelstein, E.; Knecht, S.; Pantev, C. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. NeuroImage 2003, 20, 135–144. [Google Scholar] [CrossRef]
- Furlong, P.; Hobson, A.; Aziz, Q.; Barnes, G.; Singh, K.; Hillebrand, A.; Thompson, D.; Hamdy, S. Dissociating the spatio-temporal characteristics of cortical neuronal activity associated with human volitional swallowing in the healthy adult brain. NeuroImage 2004, 22, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Abe, S.; Ishikawa, T.; Yamada, Y.; Yamane, G.-Y. Cortical regulation during the early stage of initiation of voluntary swallowing in humans. Dysphagia 2004, 19, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Dziewas, R.; Sörös, P.; Ishii, R.; Chau, W.; Henningsen, H.; Ringelstein, E.B.; Knecht, S.; Pantev, C. Cortical processing of esophageal sensation is related to the representation of swallowing. NeuroReport 2005, 16, 439–443. [Google Scholar] [CrossRef]
- Teismann, I.K.; Steinstraeter, O.; Stoeckigt, K.; Suntrup, S.; Wollbrink, A.; Pantev, C.; Dziewas, R. Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci. 2007, 8, 62. [Google Scholar] [CrossRef]
- Teismann, I.K.; Steinstraeter, O.; Warnecke, T.; Zimmermann, J.; Ringelstein, E.B.; Pantev, C.; Dziewas, R. Cortical recovery of swallowing function in wound botulism. BMC Neurol. 2008, 8, 13. [Google Scholar] [CrossRef]
- Teismann, I.K.; Steinsträter, O.; Warnecke, T.; Suntrup, S.; Ringelstein, E.B.; Pantev, C.; Dziewas, R. Tactile thermal oral stimulation increases the cortical representation of swallowing. BMC Neurosci. 2009, 10, 71. [Google Scholar] [CrossRef]
- Teismann, I.K.; Dziewas, R.; Steinstraeter, O.; Pantev, C. Time-dependent hemispheric shift of the cortical control of volitional swallowing. Hum. Brain Mapp. 2009, 30, 92–100. [Google Scholar] [CrossRef]
- Dziewas, R.; Teismann, I.K.; Suntrup, S.; Schiffbauer, H.; Steinstraeter, O.; Warnecke, T.; Ringelstein, E.-B.; Pantev, C. Cortical compensation associated with dysphagia caused by selective degeneration of bulbar motor neurons. Hum. Brain Mapp. 2009, 30, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Teismann, I.K.; Steinstraeter, O.; Schwindt, W.; Ringelstein, E.B.; Pantev, C.; Dziewas, R. Age-related changes in cortical swallowing processing. Neurobiol. Aging 2010, 31, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Teismann, I.K.; Suntrup, S.; Warnecke, T.; Steinsträter, O.; Fischer, M.; Flöel, A.; Ringelstein, E.B.; Pantev, C.; Dziewas, R. Cortical swallowing processing in early subacute stroke. BMC Neurol. 2011, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Teismann, I.K.; Warnecke, T.; Suntrup, S.; Steinsträter, O.; Kronenberg, L.; Ringelstein, E.B.; Dengler, R.; Petri, S.; Pantev, C.; Dziewas, R. Cortical Processing of Swallowing in ALS Patients with Progressive Dysphagia—A Magnetoencephalographic Study. PLoS ONE 2011, 6, e19987. [Google Scholar] [CrossRef] [PubMed]
- Suntrup, S.; Teismann, I.; Bejer, J.; Suttrup, I.; Winkels, M.; Mehler, D.; Pantev, C.; Dziewas, R.; Warnecke, T. Evidence for adaptive cortical changes in swallowing in Parkinson’s disease. Brain 2013, 136, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Suntrup, S.; Teismann, I.; Wollbrink, A.; Winkels, M.; Warnecke, T.; Flöel, A.; Pantev, C.; Dziewas, R. Magnetoencephalographic evidence for the modulation of cortical swallowing processing by transcranial direct current stimulation. NeuroImage 2013, 83, 346–354. [Google Scholar] [CrossRef]
- Suntrup, S.; Teismann, I.; Wollbrink, A.; Warnecke, T.; Winkels, M.; Pantev, C.; Dziewas, R. Altered Cortical Swallowing Processing in Patients with Functional Dysphagia: A Preliminary Study. PLoS ONE 2014, 9, e89665. [Google Scholar] [CrossRef]
- Suntrup, S.; Teismann, I.; Wollbrink, A.; Winkels, M.; Warnecke, T.; Pantev, C.; Dziewas, R. Pharyngeal electrical stimulation can modulate swallowing in cortical processing and behavior—Magnetoencephalographic evidence. NeuroImage 2015, 104, 117–124. [Google Scholar] [CrossRef]
- Suntrup-Krueger, S.; Ringmaier, C.; Muhle, P.; Wollbrink, A.; Kemmling, A.; Hanning, U.; Claus, I.; Warnecke, T.; Teismann, I.; Pantev, C.; et al. Randomized trial of transcranial direct current stimulation for poststroke dysphagia. Ann. Neurol. 2018, 83, 328–340. [Google Scholar] [CrossRef]
- Muhle, P.; Labeit, B.; Wollbrink, A.; Claus, I.; Warnecke, T.; Wolters, C.H.; Gross, J.; Dziewas, R.; Suntrup-Krueger, S. Targeting the sensory feedback within the swallowing network—Reversing artificially induced pharyngolaryngeal hypesthesia by central and peripheral stimulation strategies. Hum. Brain Mapp. 2021, 42, 427–438. [Google Scholar] [CrossRef]
- Suntrup-Krueger, S.; Muhle, P.; Kampe, I.; Egidi, P.; Ruck, T.; Lenze, F.; Jungheim, M.; Gminski, R.; Labeit, B.; Claus, I.; et al. Effect of Capsaicinoids on Neurophysiological, Biochemical, and Mechanical Parameters of Swallowing Function. Neurotherapeutics 2021, 18, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Kober, S.; Wood, G. Changes in hemodynamic signals accompanying motor imagery and motor execution of swallowing: A near-infrared spectroscopy study. NeuroImage 2014, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kober, S.E.; Bauernfeind, G.; Woller, C.; Sampl, M.; Grieshofer, P.; Neuper, C.; Wood, G. Hemodynamic Signal Changes Accompanying Execution and Imagery of Swallowing in Patients with Dysphagia: A Multiple Single-Case Near-Infrared Spectroscopy Study. Front. Neurol. 2015, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Kober, S.E.; Gressenberger, B.; Kurzmann, J.; Neuper, C.; Wood, G. Voluntary Modulation of Hemodynamic Responses in Swallowing Related Motor Areas: A Near-Infrared Spectroscopy-Based Neurofeedback Study. PLoS ONE 2015, 10, e0143314. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, K.; Sakuma, S.; Ariji, Y.; Higuchi, N.; Izumi, M.; Nakata, K. Measurement of cerebral blood volume dynamics during volitional swallowing using functional near-infrared spectroscopy: An exploratory study. Neurosci. Lett. 2015, 588, 67–71. [Google Scholar] [CrossRef]
- Mulheren, R.W.; Kamarunas, E.; Ludlow, C. Sour taste increases swallowing and prolongs hemodynamic responses in the cortical swallowing network. J. Neurophysiol. 2016, 116, 2033–2042. [Google Scholar] [CrossRef]
- Mulheren, R.W.; Ludlow, C.L. Vibration over the larynx increases swallowing and cortical activation for swallowing. J. Neurophysiol. 2017, 118, 1698–1708. [Google Scholar] [CrossRef]
- Kamarunas, E.; Mulheren, R.; Palmore, K.; Ludlow, C. Timing of cortical activation during spontaneous swallowing. Exp. Brain Res. 2018, 236, 475–484. [Google Scholar] [CrossRef]
- Kober, S.E. Hemodynamic signal changes during saliva and water swallowing: A near-infrared spectroscopy study. J. Biomed. Opt. 2018, 23, 015009. [Google Scholar] [CrossRef]
- Lee, J.; Yamate, C.; Taira, M.; Shinoda, M.; Urata, K.; Maruno, M.; Ito, R.; Saito, H.; Gionhaku, N.; Iinuma, T.; et al. Prefrontal cortex activity during swallowing in dysphagia patients. J. Oral Sci. 2018, 60, 329–335. [Google Scholar] [CrossRef]
- Kober, S.E.; Spörk, R.; Bauernfeind, G.; Wood, G. Age-related differences in the within-session trainability of hemodynamic parameters: A near-infrared spectroscopy–based neurofeedback study. Neurobiol. Aging 2019, 81, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Matsuo, M.; Iso, N.; Fujiwara, K.; Moriuchi, T.; Matsuda, D.; Mitsunaga, W.; Nakashima, A. Comparison of cerebral activation between motor execution and motor imagery of self-feeding activity. Neural Regen. Res. 2021, 16, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Malandraki, G.A.; Johnson, S.; Robbins, J. Functional MRI of swallowing: From neurophysiology to neuroplasticity. Head Neck 2011, 33 (Suppl. S1), S14–S20. [Google Scholar] [CrossRef] [PubMed]
- Scrivener, C.L.; Reader, A.T. Variability of EEG electrode positions and their underlying brain regions: Visualizing gel artifacts from a simultaneous EEG-fMRI dataset. Brain Behav. 2022, 12, e2476. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Dan, H.; Sakamoto, K.; Takeo, K.; Shimizu, K.; Kohno, S.; Oda, I.; Isobe, S.; Suzuki, T.; Kohyama, K.; et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. NeuroImage 2004, 21, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Lacadie, C.M.; Fulbright, R.K.; Rajeevan, N.; Constable, R.T.; Papademetris, X. More accurate Talairach coordinates for neuroimaging using non-linear registration. NeuroImage 2008, 42, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Penfield, W.; Rasmussen, T. The Cerebral Cortex of Man; a Clinical Study of Localization of Function; Macmillan: Stuttgart, Germany, 1950. [Google Scholar]
- Penfield, W.; Roberts, L. Speech and Brain Mechanisms; Princeton University Press: Princeton, NJ, USA, 2014; ISBN 1-4008-5467-9. [Google Scholar]
- Waberski, T.; Gobbelé, R.; Darvas, F.; Schmitz, S.; Buchner, H. Spatiotemporal Imaging of Electrical Activity Related to Attention to Somatosensory Stimulation. NeuroImage 2002, 17, 1347–1357. [Google Scholar] [CrossRef]
- Andersen, L.M.; Jerbi, K.; Dalal, S.S. Can EEG and MEG detect signals from the human cerebellum? NeuroImage 2020, 215, 116817. [Google Scholar] [CrossRef]
- Gastl, M.; Brünner, Y.F.; Wiesmann, M.; Freiherr, J. Depicting the inner and outer nose: The representation of the nose and the nasal mucosa on the human primary somatosensory cortex (SI). Hum. Brain Mapp. 2014, 35, 4751–4766. [Google Scholar] [CrossRef]
- Martin, R.E.; Goodyear, B.G.; Gati, J.S.; Menon, R. Cerebral Cortical Representation of Automatic and Volitional Swallowing in Humans. J. Neurophysiol. 2001, 85, 938–950. [Google Scholar] [CrossRef]
- Kern, M.K.; Jaradeh, S.; Arndorfer, R.C.; Shaker, R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am. J. Physiol. Liver Physiol. 2001, 280, G354–G360. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.L.; Julyan, P.; Kulkarni, B.; Gow, D.; Hobson, A.; Hastings, D.; Zweit, J.; Hamdy, S. Mapping Metabolic Brain Activation during Human Volitional Swallowing: A Positron Emission Tomography Study Using [18F]fluorodeoxyglucose. J. Cereb. Blood Flow Metab. 2005, 25, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Jestrović, I.; Coyle, J.L.; Sejdić, E. Decoding human swallowing via electroencephalography: A state-of-the-art review. J. Neural Eng. 2015, 12, 051001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, A.F.; Zhang, F.; Yuan, H.; Ding, L. Brain-wide functional diffuse optical tomography of resting state networks. J. Neural Eng. 2021, 18, 046069. [Google Scholar] [CrossRef]
- Nishiyori, R. fNIRS: An Emergent Method to Document Functional Cortical Activity during Infant Movements. Front. Psychol. 2016, 7, 533. [Google Scholar] [CrossRef] [PubMed]
- Uchitel, J.; Vidal-Rosas, E.E.; Cooper, R.J.; Zhao, H. Wearable, Integrated EEG–fNIRS Technologies: A Review. Sensors 2021, 21, 6106. [Google Scholar] [CrossRef]
- Paranawithana, I.; Mao, D.; Wong, Y.T.; McKay, C.M. Reducing false discoveries in resting-state functional connectivity using short channel correction: An fNIRS study. Neurophotonics 2022, 9, 015001. [Google Scholar] [CrossRef]
- Zhou, X.; Sobczak, G.; McKay, C.M.; Litovsky, R.Y. Comparing fNIRS signal qualities between approaches with and without short channels. PLoS ONE 2020, 15, e0244186. [Google Scholar] [CrossRef]
- Fiani, B.; Griepp, D.W.; Lee, J.; Davati, C.; Moawad, C.M.; Kondilis, A. Weight-Bearing Magnetic Resonance Imaging as a Diagnostic Tool That Generates Biomechanical Changes in Spine Anatomy. Cureus 2020, 12, e12070. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).