Antithrombotic Treatment Patterns of Patients with Symptomatic Peripheral Arterial Occlusive Disease in Germany: Evidence from Health Insurance Claims Data
Abstract
1. Introduction
2. Materials and Methods
2.1. BARMER Cohort
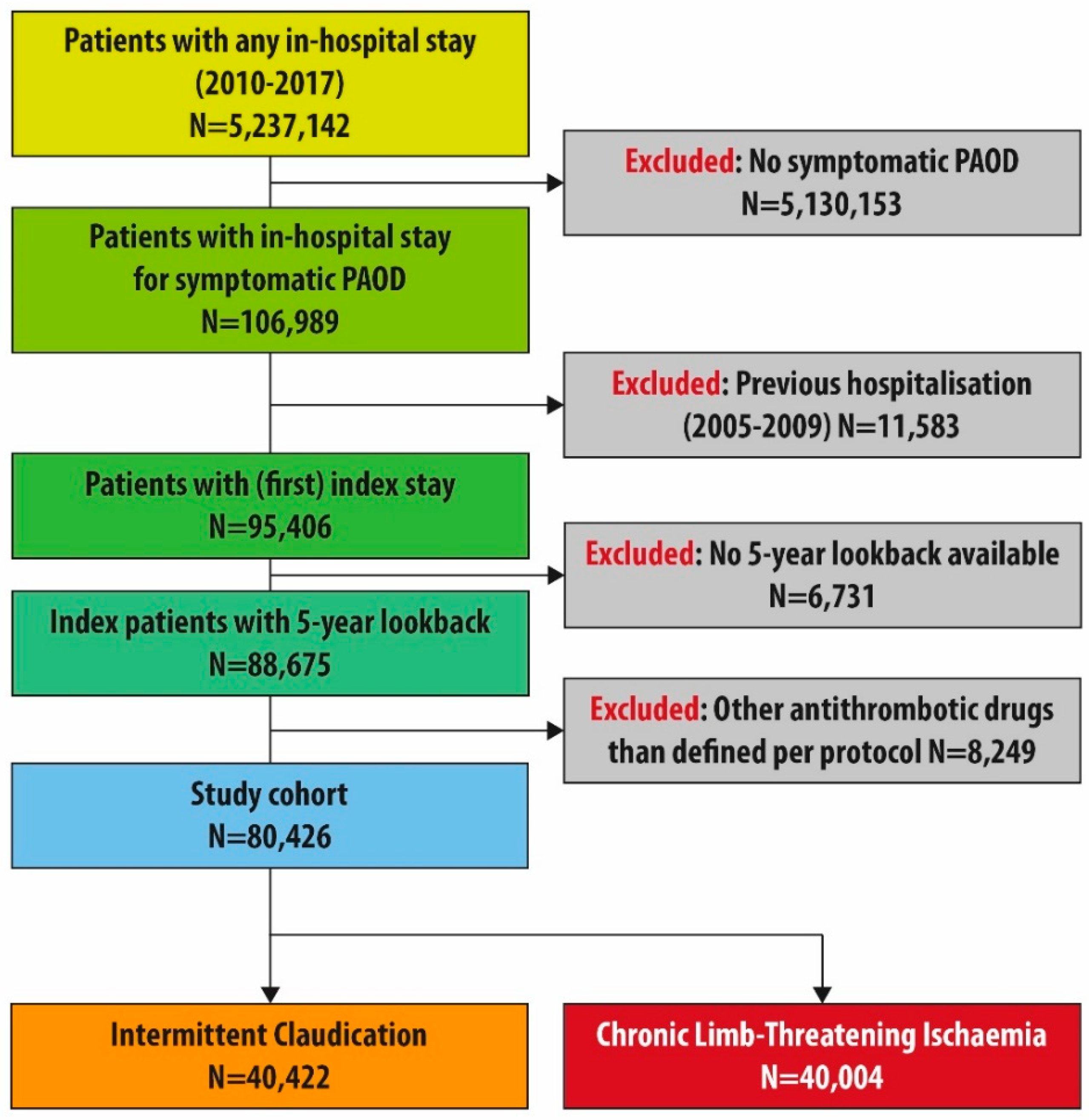
2.2. Study Population
2.3. Study Variables
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Baseline Characteristics of the Subgroup Excluding Patients with Concomitant Indication for Anticoagulation (Sensitivity Analysis)
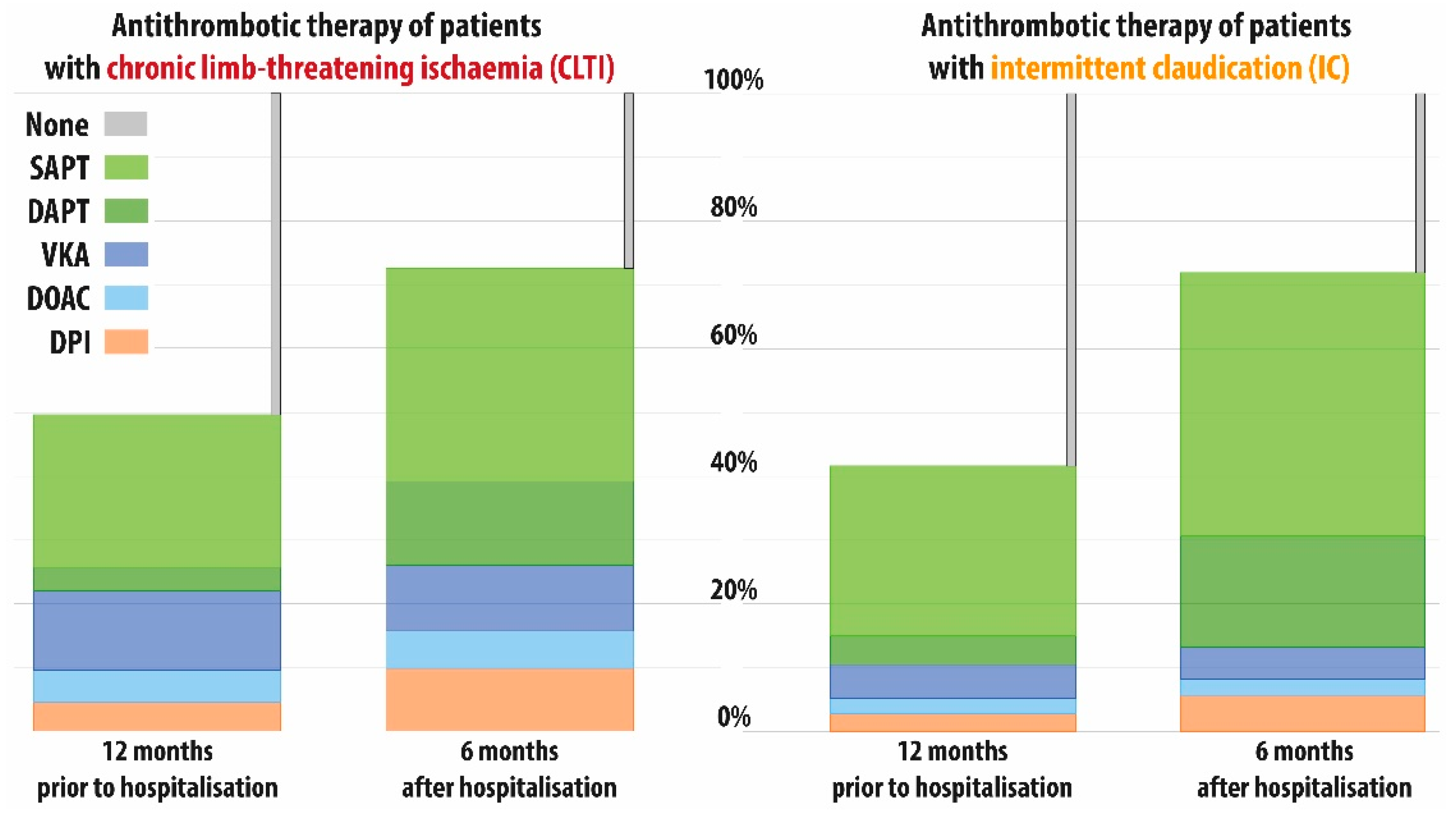
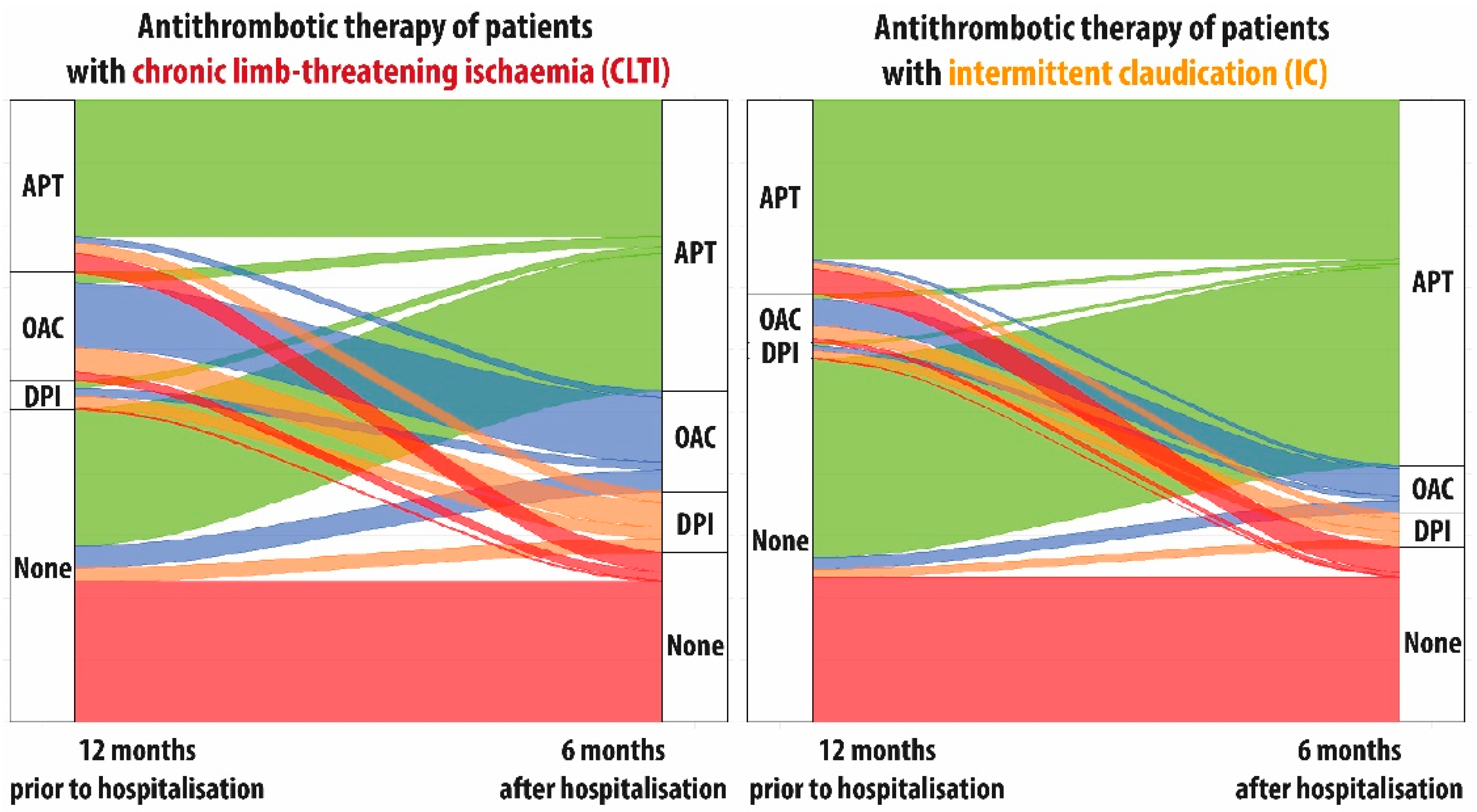
3.3. Treatment Patterns in the Entire Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Criqui, M.H.; Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, J.C. Peripheral arterial disease: Pathophysiology, risk factors, and role of antithrombotic therapy. J. Am. Pharm. Assoc. 2004, 44, S37–S44; quiz S44–S35. [Google Scholar] [CrossRef] [PubMed]
- Peach, G.; Griffin, M.; Jones, K.G.; Thompson, M.M.; Hinchliffe, R.J. Diagnosis and management of peripheral arterial disease. BMJ 2012, 345, e5208. [Google Scholar] [CrossRef]
- Criqui, M.H.; Ninomiya, J.K.; Wingard, D.L.; Ji, M.; Fronek, A. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J. Am. Coll. Cardiol. 2008, 52, 1736–1742. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. Editor’s Choice-2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur. J. Vasc. Endovasc. Surg. 2019, 58, S1–S109. [Google Scholar] [CrossRef]
- Qureshi, M.I.; Li, H.L.; Ambler, G.K.; Wong, K.H.F.; Dawson, S.; Chaplin, K.; Cheng, H.Y.; Hinchliffe, R.J.; Twine, C.P. Antiplatelet and Anticoagulant Use in Randomised Trials of Patients Undergoing Endovascular Intervention for Peripheral Arterial Disease: Systematic Review and Narrative Synthesis. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 77–87. [Google Scholar] [CrossRef]
- Saito, Y.; Kobayashi, Y.; Tanabe, K.; Ikari, Y. Antithrombotic therapy after percutaneous coronary intervention from the Japanese perspective. Cardiovasc. Interv. Ther. 2020, 35, 19–29. [Google Scholar] [CrossRef]
- Pande, R.L.; Perlstein, T.S.; Beckman, J.A.; Creager, M.A. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation 2011, 124, 17–23. [Google Scholar] [CrossRef]
- Galas, N.; Becker, I.; Ficon, T.; Sakrauski, M.; Reichert, R.; Ahmad, W.; Mylonas, S.; Brunkwall, J.; Majd, P. Prescription rate of anti-atherosclerotic drugs in German nursing homes and its impact on outcome. Vasa 2019, 48, 158–166. [Google Scholar] [CrossRef]
- Muller-Buhl, U.; Laux, G.; Szecsenyi, J. Secondary Pharmacotherapeutic Prevention among German Primary Care Patients with Peripheral Arterial Disease. Int. J. Vasc. Med. 2011, 2011, 316496. [Google Scholar] [CrossRef][Green Version]
- Hussain, M.A.; Al-Omran, M.; Mamdani, M.; Eisenberg, N.; Premji, A.; Saldanha, L.; Wang, X.; Verma, S.; Lindsay, T.F. Efficacy of a Guideline-Recommended Risk-Reduction Program to Improve Cardiovascular and Limb Outcomes in Patients with Peripheral Arterial Disease. JAMA Surg. 2016, 151, 742–750. [Google Scholar] [CrossRef]
- Cea Soriano, L.; Fowkes, F.G.R.; Johansson, S.; Allum, A.M.; Garcia Rodriguez, L.A. Cardiovascular outcomes for patients with symptomatic peripheral artery disease: A cohort study in The Health Improvement Network (THIN) in the UK. Eur. J. Prev. Cardiol. 2017, 24, 1927–1937. [Google Scholar] [CrossRef]
- Sigvant, B.; Hasvold, P.; Thuresson, M.; Jernberg, T.; Janzon, M.; Nordanstig, J. Myocardial infarction and peripheral arterial disease: Treatment patterns and long-term outcome in men and women results from a Swedish nationwide study. Eur. J. Prev. Cardiol. 2019, 28, 1426–1434. [Google Scholar] [CrossRef]
- Lee, M.S.; Choi, B.G.; Rha, S.W. Impact of diabetes mellitus on 5-year clinical outcomes following successful endovascular revascularization for peripheral artery disease. Vasc. Med. 2020, 25, 33–40. [Google Scholar] [CrossRef]
- Mustapha, J.; Gray, W.; Martinsen, B.J.; Bolduan, R.W.; Adams, G.L.; Ansel, G.; Jaff, M.R. One-Year Results of the LIBERTY 360 Study: Evaluation of Acute and Midterm Clinical Outcomes of Peripheral Endovascular Device Interventions. J. Endovasc. Ther. 2020, 26, 143–154. [Google Scholar] [CrossRef]
- Soga, Y.; Yokoi, H.; Urakawa, T.; Tosaka, A.; Iwabuchi, M.; Nobuyoshi, M. Long-term clinical outcome after endovascular treatment in patients with intermittent claudication due to iliofemoral artery disease. Circulation 2010, 74, 1689–1695. [Google Scholar] [CrossRef]
- Freisinger, E.; Fuerstenberg, T.; Malyar, N.M.; Wellmann, J.; Keil, U.; Breithardt, G.; Reinecke, H. German nationwide data on current trends and management of acute myocardial infarction: Discrepancies between trials and real-life. Eur. Heart J. 2014, 35, 979–988. [Google Scholar] [CrossRef]
- Freisinger, E.; Malyar, N.M.; Reinecke, H.; Lawall, H. Impact of diabetes on outcome in critical limb ischemia with tissue loss: A large-scaled routine data analysis. Cardiovasc. Diabetol. 2017, 16, 41. [Google Scholar] [CrossRef]
- Malyar, N.; Furstenberg, T.; Wellmann, J.; Meyborg, M.; Luders, F.; Gebauer, K.; Bunzemeier, H.; Roeder, N.; Reinecke, H. Recent trends in morbidity and in-hospital outcomes of in-patients with peripheral arterial disease: A nationwide population-based analysis. Eur. Heart J. 2013, 34, 2706–2714. [Google Scholar] [CrossRef]
- Kreutzburg, T.; Peters, F.; Riess, H.C.; Hischke, S.; Marschall, U.; Kriston, L.; L’Hoest, H.; Sedrakyan, A.; Debus, E.S.; Behrendt, C.A. Editor’s Choice-Comorbidity Patterns Among Patients with Peripheral Arterial Occlusive Disease in Germany: A Trend Analysis of Health Insurance Claims Data. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 59–66. [Google Scholar] [CrossRef]
- Langner, I.; Ohlmeier, C.; Zeeb, H.; Haug, U.; Riedel, O. Individual mortality information in the German Pharmacoepidemiological Research Database (GePaRD): A validation study using a record linkage with a large cancer registry. BMJ Open 2019, 9, e028223. [Google Scholar] [CrossRef]
- Hoffmann, F.; Icks, A. Structural differences between health insurance funds and their impact on health services research: Results from the Bertelsmann Health-Care Monitor. Gesundheitswesen 2012, 74, 291–297. [Google Scholar] [CrossRef]
- Peters, F.; Kreutzburg, T.; Kuchenbecker, J.; Debus, E.S.; Marschall, U.; L’Hoest, H.; Behrendt, C.A. A retrospective cohort study on the provision and outcomes of pharmacological therapy after revascularization for peripheral arterial occlusive disease: A study protocol. BMJ Surg. Interv. Health Technol. 2020, 2, e000020. [Google Scholar] [CrossRef]
- Reinecke, H.; Unrath, M.; Freisinger, E.; Bunzemeier, H.; Meyborg, M.; Luders, F.; Gebauer, K.; Roeder, N.; Berger, K.; Malyar, N.M. Peripheral arterial disease and critical limb ischaemia: Still poor outcomes and lack of guideline adherence. Eur. Heart J. 2015, 36, 932–938. [Google Scholar] [CrossRef]
- Fontaine, R.; Kim, M.; Kieny, R. Surgical treatment of peripheral circulation disorders. Helv. Chir. Acta 1954, 21, 499–533. [Google Scholar]
- van Walraven, C.; Austin, P.C.; Jennings, A.; Quan, H.; Forster, A.J. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med. Care 2009, 47, 626–633. [Google Scholar] [CrossRef]
- Aalen, O. Nonparametric estimation of partial transition probabilities in multiple decrement models. Ann. Stat. 1978, 6, 534–545. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sorensen, H.T.; von Elm, E.; Langan, S.M.; Committee, R.W. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef]
- Swart, E.; Gothe, H.; Geyer, S.; Jaunzeme, J.; Maier, B.; Grobe, T.G.; Ihle, P. Gute Praxis Sekundärdatenanalyse (GPS): Leitlinien und Empfehlungen. Gesundheitswesen 2015, 77, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Chronic Lower Extremity Ischemia and Its Association with the Frailty Syndrome in Patients with Diabetes. Int. J. Environ. Res. Public Health 2020, 17, 9339. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Pathogenesis and Clinical Significance of In-Stent Restenosis in Patients with Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 11970. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, R.J.; Brownrigg, J.R.; Andros, G.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Mills, J.L.; Reekers, J.; Shearman, C.P.; Zierler, R.E.; et al. International Working Group on the Diabetic Foot. Effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral artery disease: A systematic review. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 136–144. [Google Scholar] [CrossRef]
- Thiruvoipati, T.; Kielhorn, C.E.; Armstrong, E.J. Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World J. Diabetes 2015, 6, 961–969. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 69, e71–e126. [Google Scholar] [CrossRef]
- Peters, F.; Kreutzburg, T.; Rieß, H.C.; Heidemann, F.; Marschall, U.; L’Hoest, H.; Debus, E.S.; Sedrakyan, A.; Behrendt, C.A. Editor’s Choice-Optimal Pharmacological Treatment of Symptomatic Peripheral Arterial Occlusive Disease and Evidence of Female Patient Disadvantage: An Analysis of Health Insurance Claims Data. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 421–429. [Google Scholar] [CrossRef]
- Cea Soriano, L.; Soriano-Gabarro, M.; Garcia Rodriguez, L.A. Validation of low-dose aspirin prescription data in The Health Improvement Network: How much misclassification due to over-the-counter use? Pharmacoepidemiol. Drug Saf. 2016, 25, 392–398. [Google Scholar] [CrossRef][Green Version]
- Hageman, S.H.J.; de Borst, G.J.; Dorresteijn, J.A.N.; Bots, M.L.; Westerink, J.; Asselbergs, F.W.; Visseren, F.L.J. Cardiovascular risk factors and the risk of major adverse limb events in patients with symptomatic cardiovascular disease. Heart 2020, 106, 1686–1692. [Google Scholar] [CrossRef]
- Catalano, M.; Born, G.; Peto, R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: Randomized, double-blind trial. J. Intern. Med. 2007, 261, 276–284. [Google Scholar] [CrossRef]
- Ardeshna, D.; Khare, S.; Jagadish, P.S.; Bhattad, V.; Cave, B.; Khouzam, R.N. The dilemma of aspirin resistance in obese patients. Ann. Transl. Med. 2019, 7, 404. [Google Scholar] [CrossRef]
- Stansby, G.; Mister, R.; Fowkes, G.; Roughton, M.; Nugara, F.; Brittenden, J.; Bradbury, A.; Ashley, S.; Shearman, C.; Hannon, R.; et al. High risk of peripheral arterial disease in the United Kingdom: 2-year results of a prospective registry. Angiology 2011, 62, 111–118. [Google Scholar] [CrossRef]
- Capodanno, D.; Bhatt, D.L.; Eikelboom, J.W.; Fox, K.A.A.; Geisler, T.; Michael Gibson, C.; Gonzalez-Juanatey, J.R.; James, S.; Lopes, R.D.; Mehran, R.; et al. Dual-pathway inhibition for secondary and tertiary antithrombotic prevention in cardiovascular disease. Nature reviews. Cardiology 2020, 17, 242–257. [Google Scholar] [CrossRef]
- Debus, E.S.; Nehler, M.R. The Voyager PAD Trial-New Path for Post-revascularisation PAD Patients. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 699–700. [Google Scholar] [CrossRef]
- Behrendt, C.A.; Peters, F.; Mani, K. The Swinging Pendulum of Evidence: Is There a Reality Behind Results from Randomised Trials and Real World Data? Lessons Learned from the Paclitaxel Debate. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 510–511. [Google Scholar] [CrossRef]
- Behrendt, C.-A.; Kreutzburg, T.; Nordanstig, J.; Twine, C.; Marschall, U.; Kakkos, S.; Aboyans, V.; Peters, F. The OAC3-PAD Risk Score Predicts Major Bleeding Events on Year after Hospitalisation for Peripheral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2022, in press. [CrossRef]
- Kreutzburg, T.; Peters, F.; Kuchenbecker, J.; Marschall, U.; Lee, R.; Kriston, L.; Debus, E.S.; Behrendt, C.A. Editor’s Choice-The GermanVasc Score: A Pragmatic Risk Score Predicts Five Year Amputation Free Survival in Patients with Peripheral Arterial Occlusive Disease. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 248–256. [Google Scholar] [CrossRef]
- Peters, F.; Behrendt, C.-A. External validation of the OAC3-PAD risk score to predict major bleeding events using the prospective GermanVasc cohort study. Eur. J. Vasc. Endovasc. Surg. 2022, in press. [CrossRef]
- Sigvant, B.; Kragsterman, B.; Falkenberg, M.; Hasvold, P.; Johansson, S.; Thuresson, M.; Nordanstig, J. Contemporary cardiovascular risk and secondary preventive drug treatment patterns in peripheral artery disease patients undergoing revascularization. J. Vasc. Surg. 2016, 64, 1009–1017.e3. [Google Scholar] [CrossRef]
| Total (N = 80,426) | CLTI (N = 40,004) | IC (N = 40,422) | |
|---|---|---|---|
| Age, years, mean (SD) | 72.74 (11.26) | 75.98 (11.37) | 69.53 (10.19) |
| Female sex, n (%) | 37,258 (46.3) | 19,478 (48.7) | 17,780 (44.0) |
| van Walraven Score > 9, n (%) | 32,539 (40.5) | 22,260 (55.6) | 10,279 (25.4) |
| No invasive treatment at index, n (%) | 17,488 (21.7) | 11,635 (29.1) | 5853 (14.5) |
| Percutaneous endovascular intervention, n (%) | 39,382 (49.0) | 14,929 (37.3) | 24,453 (60.5) |
| Open surgical repair, n (%) | 19,658 (24.4) | 9668 (24.2) | 9990 (24.7) |
| Primary amputation only, n (%) | 3898 (4.8) | 3772 (9.4) | 126 (0.3) |
| Congestive heart failure, n (%) | 23,516 (29.2) | 16,272 (40.7) | 7244 (17.9) |
| Cardiac arrhythmias, n (%) | 23,987 (29.8) | 16,174 (40.4) | 7813 (19.3) |
| Valvular disease, n (%) | 10,117 (12.6) | 6783 (17.0) | 3334 (8.2) |
| Hypertension, n (%) | 66,233 (82.4) | 33,976 (84.9) | 32,257 (79.8) |
| Diabetes (complicated), n (%) | 23,154 (28.8) | 16,547 (41.4) | 6607 (16.3) |
| Renal failure, n (%) | 25,940 (32.3) | 17,239 (43.1) | 8701 (21.5) |
| Liver disease, n (%) | 4057 (5.0) | 2554 (6.4) | 1503 (3.7) |
| Solid tumour or metastatic cancer, n (%) | 6421 (8.0) | 3594 (9.0) | 2827 (7.0) |
| Dyslipidaemia, n (%) | 39,371 (49.0) | 18,158 (45.4) | 21,213 (52.5) |
| History of coronary artery disease, n (%) | 29,873 (37.1) | 16,255 (40.6) | 13,618 (33.7) |
| Prior myocardial infarction, n (%) | 12,084 (15.0) | 6737 (16.8) | 5347 (13.2) |
| Prior stroke or TIA, n (%) | 8088 (10.1) | 5336 (13.3) | 2752 (6.8) |
| History of bleeding, n (%) | 20,182 (25.1) | 13,543 (33.9) | 6639 (16.4) |
| Subgroup/Cohort | Medication | 12 Months Prior, Observed | 6 Months after, Estimated, 95% CI |
|---|---|---|---|
| Total cohort (N = 80,426) | SAPT, n (%) | 20,594 (25.6) | 37.9 (37.6–38.3) |
| DAPT, n (%) | 3330 (4.1) | 14.8 (14.6–15.1) | |
| VKA, n (%) | 7317 (9.1) | 7.5 (7.3–7.7) | |
| DOAC, n (%) | 3166 (3.9) | 4.3 (4.1–4.4) | |
| DPI, n (%) | 3116 (3.9) | 7.4 (7.2–7.6) | |
| No therapy, n (%) | 42,903 (53.3) | 28.1 # | |
| CLTI (N = 40,004) | SAPT, n (%) | 9828 (24.6) | 34.3 (33.9–34.8) |
| DAPT, n (%) | 1456 (3.6) | 12.3 (11.9–12.6) | |
| VKA, n (%) | 5105 (12.8) | 10.3 (10.0–10.7) | |
| DOAC, n (%) | 2120 (5.3) | 6.1 (5.9–6.4) | |
| DPI, n (%) | 2013 (5.0) | 9.3 (9–9.6) | |
| No therapy, n (%) | 19,482 (48.7) | 27.7 # | |
| IC (N = 40,422) | SAPT, n (%) | 10,766 (26.6) | 41.3 (40.8–41.8) |
| DAPT, n (%) | 1874 (4.6) | 17.2 (16.9–17.6) | |
| VKA, n (%) | 2212 (5.5) | 5.0 (4.8–5.2) | |
| DOAC, n (%) | 1046 (2.6) | 2.7 (2.5–2.8) | |
| DPI, n (%) | 1103 (2.7) | 5.6 (5.4–5.8) | |
| No therapy, n (%) | 23,421 (57.9) | 28.2 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, F.; Kuchenbecker, J.; Acar, L.; Marschall, U.; L’Hoest, H.; Lareyre, F.; Spanos, K.; Behrendt, C.-A. Antithrombotic Treatment Patterns of Patients with Symptomatic Peripheral Arterial Occlusive Disease in Germany: Evidence from Health Insurance Claims Data. J. Clin. Med. 2022, 11, 5455. https://doi.org/10.3390/jcm11185455
Peters F, Kuchenbecker J, Acar L, Marschall U, L’Hoest H, Lareyre F, Spanos K, Behrendt C-A. Antithrombotic Treatment Patterns of Patients with Symptomatic Peripheral Arterial Occlusive Disease in Germany: Evidence from Health Insurance Claims Data. Journal of Clinical Medicine. 2022; 11(18):5455. https://doi.org/10.3390/jcm11185455
Chicago/Turabian StylePeters, Frederik, Jenny Kuchenbecker, Laura Acar, Ursula Marschall, Helmut L’Hoest, Fabien Lareyre, Konstantinos Spanos, and Christian-Alexander Behrendt. 2022. "Antithrombotic Treatment Patterns of Patients with Symptomatic Peripheral Arterial Occlusive Disease in Germany: Evidence from Health Insurance Claims Data" Journal of Clinical Medicine 11, no. 18: 5455. https://doi.org/10.3390/jcm11185455
APA StylePeters, F., Kuchenbecker, J., Acar, L., Marschall, U., L’Hoest, H., Lareyre, F., Spanos, K., & Behrendt, C.-A. (2022). Antithrombotic Treatment Patterns of Patients with Symptomatic Peripheral Arterial Occlusive Disease in Germany: Evidence from Health Insurance Claims Data. Journal of Clinical Medicine, 11(18), 5455. https://doi.org/10.3390/jcm11185455








