Evaluation of a New Echocardiographic Tool for Cardiac Output Monitoring: An Experimental Study on A Controlled Hemorrhagic Shock Model in Anesthetized Piglets
Abstract
:1. Introduction
2. Methods
2.1. Materials
- −
- COth and COman: CO calculated with a manually measured sub-aortic velocity time index (VTIman) and LVOT;
- −
- COth and COauto: CO calculated with VTIauto and manually measured LVOT;
- −
- COF and stroke volume (SV) measured by thermodilution;
- −
- VTIman and SV measured by thermodilution;
- −
- VTIauto and SV measured by thermodilution;
- −
- VTIman and COth;
- −
- VTIauto and COth.
2.2. Animal Preparation
2.3. Experimental Protocol and Measurement Times
2.4. Echocardiography
2.5. Statistical Analysis
3. Results
3.1. Animals and Compliance to the Protocol
3.2. Measures
3.3. Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.L.; et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Marik, P.E.; Teboul, J.-L. Prediction of fluid responsiveness: An update. Ann. Intensive Care 2016, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Weil, M.H. Fluid challenge revisited. Crit. Care Med. 2006, 34, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Michard, F.; Boussat, S.; Chemla, D.; Anguel, N.; Mercat, A.; Lecarpentier, Y.; Richard, C.; Pinsky, M.R.; Teboul, J.L. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am. J. Respir. Crit. Care Med. 2000, 162, 134–138. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Rhodes, A.; Perel, A.; Martin, G.S.; Della Rocca, G.; Vallet, B.; Pinsky, M.R.; Hofer, C.K.; Teboul, J.-L.; De Boode, W.-P.; et al. Clinical review: Update on hemodynamic monitoring—A consensus of 16. Crit. Care 2011, 15, 229. [Google Scholar] [CrossRef]
- Bobbia, X.; Zieleskiewicz, L.; Pradeilles, C.; Hudson, C.; Muller, L.; Claret, P.G.; Leone, M.; de La Coussaye, J.-E.; Winfocus France Group. The clinical impact and prevalence of emergency point-of-care ultrasound: A prospective multicenter study. Anaesth. Crit. Care Pain Med. 2017, 36, 383–389. [Google Scholar] [CrossRef]
- Muller, L.; Toumi, M.; Bousquet, P.-J.; Riu-Poulenc, B.; Louart, G.; Candela, D.; Zoric, L.; Suehs, C.; de La Coussaye, J.-E.; Molinari, N.; et al. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: The mini-fluid challenge study. Anesthesiology 2011, 115, 541–547. [Google Scholar] [CrossRef]
- Blancas, R.; Martínez-González, Ó.; Ballesteros, D.; Núñez, A.; Luján, J.; Rodríguez-Serrano, D.; Hernández, A.; Martínez-Díaz, C.; Parra, C.M.; Matamala, B.L.; et al. Lack of correlation between left ventricular outflow tract velocity time integral and stroke volume index in mechanically ventilated patients. Med. Intensiva 2019, 43, 73–78. [Google Scholar] [CrossRef]
- Maeder, M.T.; Karapanagiotidis, S.; Dewar, E.M.; Kaye, D.M. Accuracy of Echocardiographic Cardiac Index Assessment in Subjects with Preserved Left Ventricular Ejection Fraction. Echocardiography 2015, 32, 1628–1638. [Google Scholar] [CrossRef]
- Bobbia, X.; Muller, L.; Claret, P.-G.; Vigouroux, L.; Perez-Martin, A.; de La Coussaye, J.-E.; Lefrant, J.; Louart, G.; Roger, C.; Markarian, T. A New Echocardiographic Tool for Cardiac Output Evaluation: An Experimental Study. Shock 2018, 52, 1. [Google Scholar] [CrossRef]
- Grau-Mercier, L.; Coisy, F.; Markarian, T.; Muller, L.; Roger, C.; Lefrant, J.-Y.; Claret, P.-G.; Bobbia, X. Can blood loss be assessed by echocardiography? An experimental study on a controlled hemorrhagic shock model in piglets. J. Trauma Acute Care Surg. 2022, 92, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Zieleskiewicz, L.; Claret, P.-G.; Muller, L.; de La Coussaye, J.E.; Lefrant, J.Y.; Schuster, I.; Roger, C.; Bobbia, X. Global longitudinal strain changes during hemorrhagic shock: An experimental study. Turk. J. Emerg. Med. 2020, 20, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Temporelli, P.L.; Scapellato, F.; Eleuteri, E.; Imparato, A.; Giannuzzi, P. Doppler echocardiography in advanced systolic heart failure: A noninvasive alternative to Swan-Ganz catheter. Circ. Heart Fail. 2010, 3, 387–394. [Google Scholar] [CrossRef]
- Truijen, J.; Westerhof, B.E.; Kim, Y.-S.; Stok, W.J.; de Mol, B.A.; Preckel, B.; Hollmann, M.W.; van Lieshout, J.J. The effect of haemodynamic and peripheral vascular variability on cardiac output monitoring: Thermodilution and non-invasive pulse contour cardiac output during cardiothoracic surgery. Anaesthesia 2018, 73, 1489–1499. [Google Scholar] [CrossRef]
- Yamada, T.; Tsutsui, M.; Sugo, Y.; Sato, T.; Akazawa, T.; Sato, N.; Yamashita, K.; Ishihara, H.; Takeda, J. Multicenter study verifying a method of noninvasive continuous cardiac output measurement using pulse wave transit time: A comparison with intermittent bolus thermodilution cardiac output. Anesth. Analg. 2012, 115, 82–87. [Google Scholar] [CrossRef]
- Kööbi, T.; Kaukinen, S.; Turjanmaa, V.M. Cardiac output can be reliably measured noninvasively after coronary artery bypass grafting operation. Crit. Care Med. 1999, 27, 2206–2211. [Google Scholar] [CrossRef]
- Distante, A.; Moscarelli, E.; Rovai, D.; L’Abbate, A. Monitoring of changes in cardiac output by transcutaneous aortovelography, a non-invasive Doppler technique: Comparison with thermodilution. J. Nucl. Med. Allied. Sci. 1980, 24, 171–175. [Google Scholar]
- Mercado, P.; Maizel, J.; Beyls, C.; Titeca-Beauport, D.; Joris, M.; Kontar, L.; Riviere, A.; Bonef, O.; Soupison, T.; Tribouilloy, C.; et al. Transthoracic echocardiography: An accurate and precise method for estimating cardiac output in the critically ill patient. Crit. Care 2017, 21, 136. [Google Scholar] [CrossRef]
- Feissel, M.; Michard, F.; Mangin, I.; Ruyer, O.; Faller, J.P.; Teboul, J.L. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest 2001, 119, 867–873. [Google Scholar] [CrossRef]
- Shiran, A.; Adawi, S.; Ganaeem, M.; Asmer, E. Accuracy and reproducibility of left ventricular outflow tract diameter measurement using transthoracic when compared with transesophageal echocardiography in systole and diastole. Eur. J. Echocardiogr. 2009, 10, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
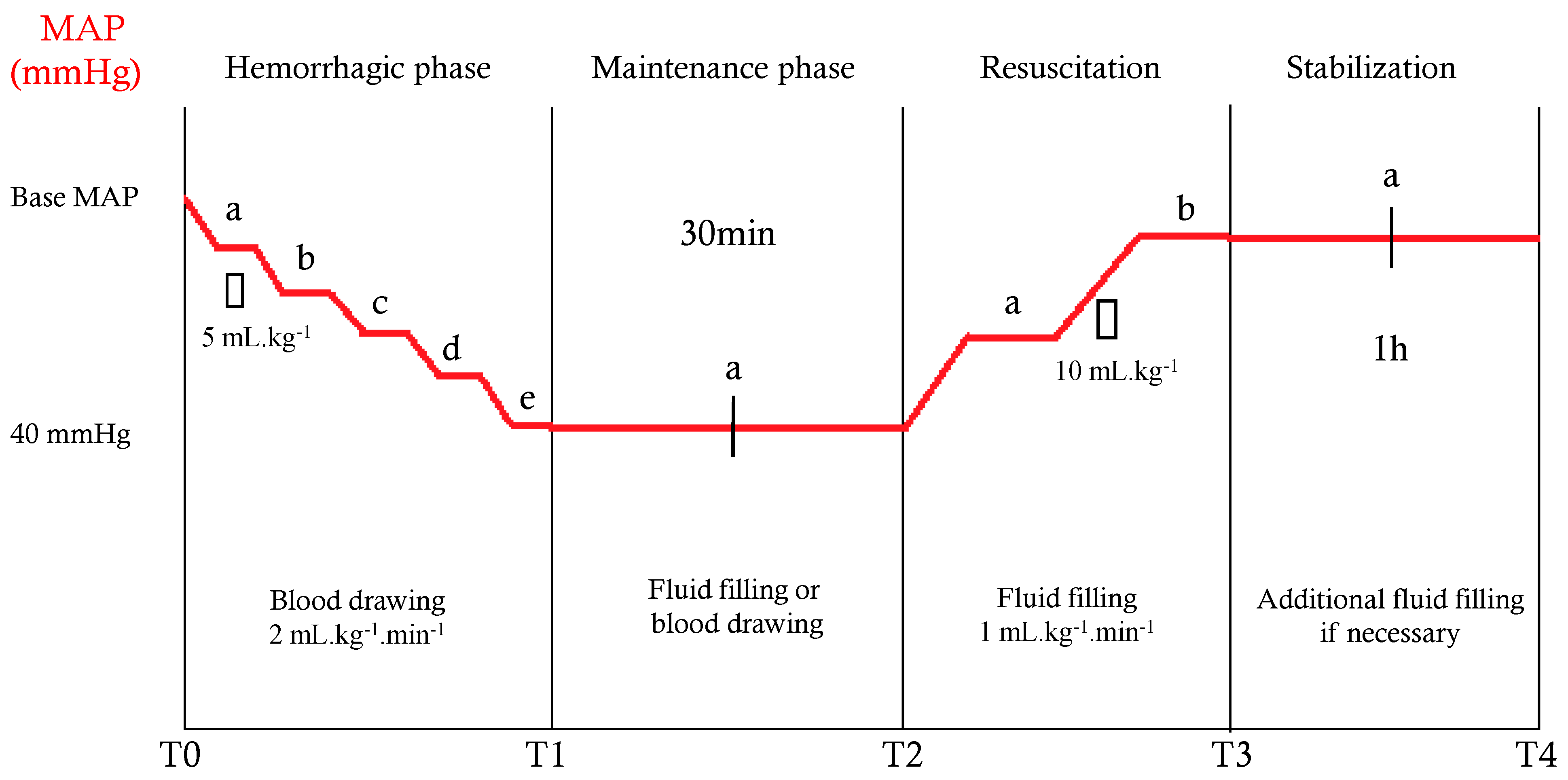
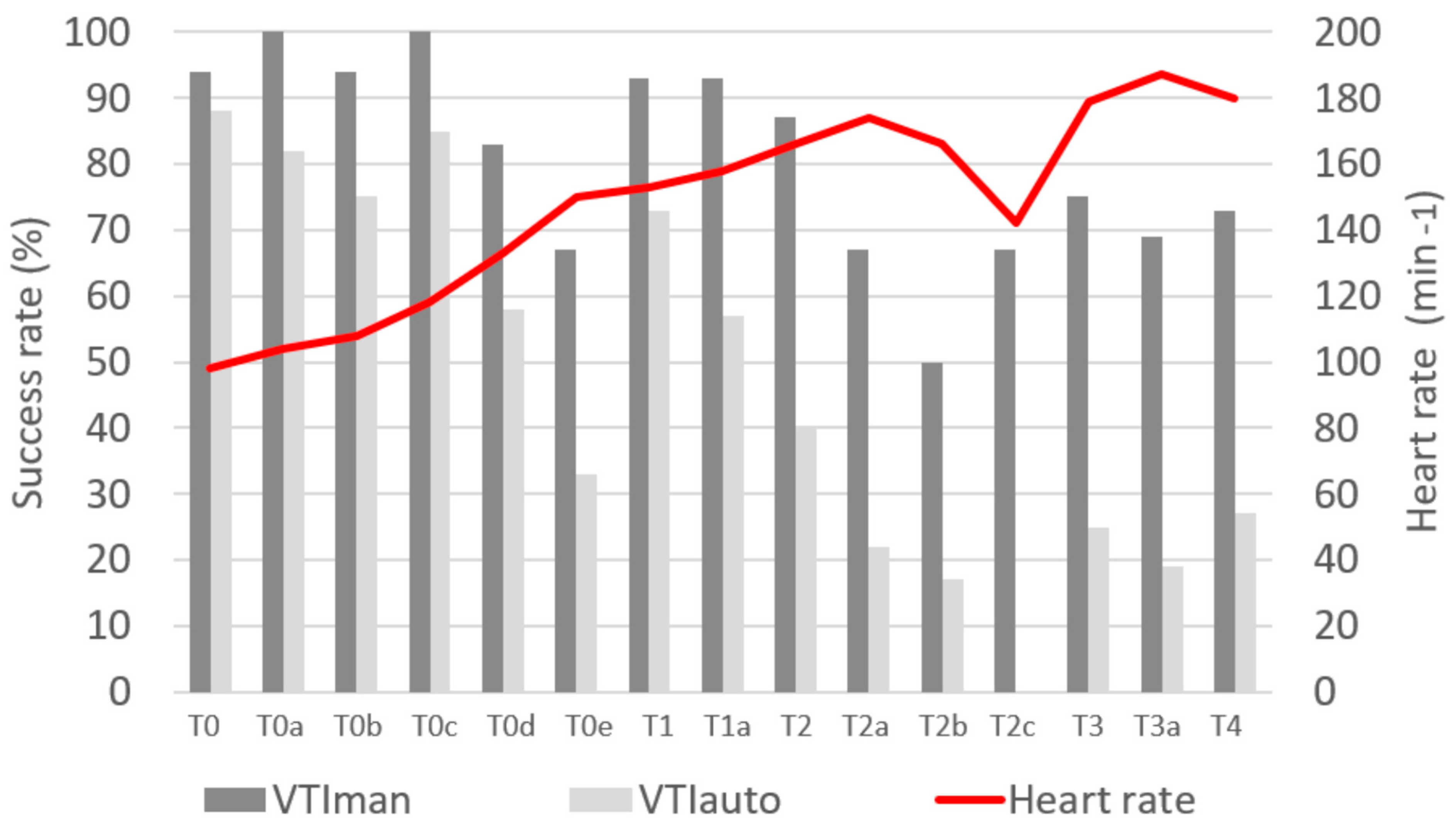
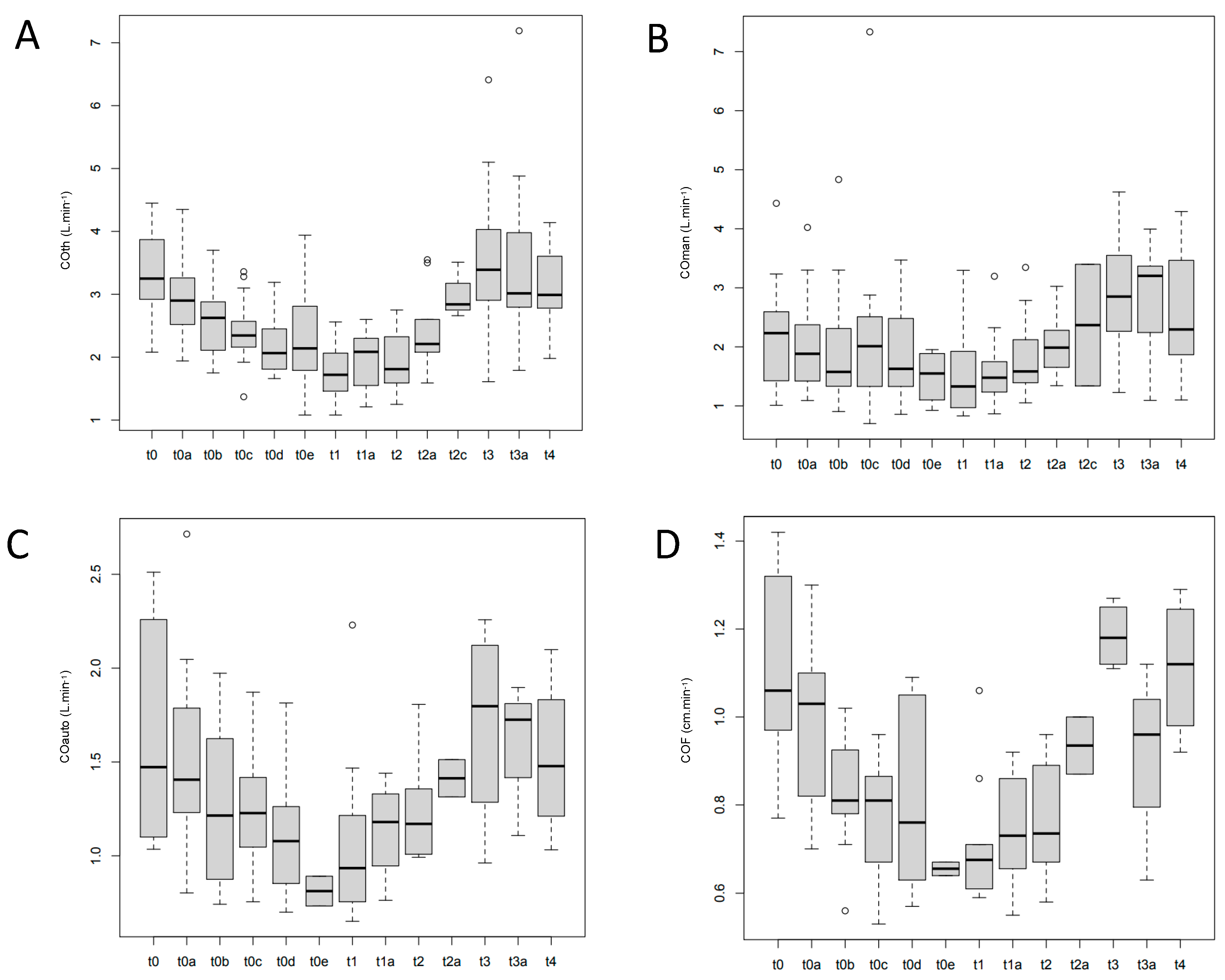

| Median arterial pressure (mmHg) | |
| T0 | 73 [69; 80] |
| T1 | 39 [36; 40] |
| T2 | 41 [39; 43] |
| T3 | 80 [68; 88] |
| T4 | 67 [62; 72] |
| Heart rate (min–1) | |
| T0 | 102 [80; 120] |
| T1 | 163 [138; 172] |
| T2 | 190 [166; 194] |
| T3 | 172 [169; 202] |
| T4 | 180 [162; 201] |
| COth (L.min–1) | |
| T0 | 3.15 [2.99; 3.59] |
| T1 | 1.63 [1.42; 2.02] |
| T2 | 1.87 [1.66; 2.30] |
| T3 | 3.42 [2.51; 4.89] |
| T4 | 2.80 [2.58; 3.90] |
| Hemoglobin (g.dL–1) | |
| T0 | 9.5 [8.8; 9.9] |
| T1 | 8.2 [7.5; 8.9] |
| T2 | 7.5 [6.9; 8.3] |
| T3 | 6.9 [4.3; 7.6] |
| T4 | 6.5 [5.5; 7.8] |
| pH | |
| T0 | 7.45 [7.39; 7.48] |
| T1 | 7.44 [7.39; 7.47] |
| T2 | 7.36 [7.07; 7.42] |
| T3 | 7.32 [7.27; 7.38] |
| T4 | 7.35 [7.34; 7.40] |
| T0–T1 volume of hemorrhage (mL.kg–1) | −25 [−33; −14] |
| T1–T2 volume for maintenance (mL.kg–1) | 2 [−6; 5] |
| T2–T3 resuscitation fluid volume (mL.kg–1) | 16 [13; 33] |
| T3–T4 volume for stabilization (mL.kg–1) | 3 [0; 11] |
| Measure 1 | Measure 2 | Correlation Coefficient [95%CI] between Measures 1 and 2 | p |
|---|---|---|---|
| COth | COF | 0.73 [0.62; 0.81] | <0.01 |
| COman | 0.66 [0.56; 0.74] | <0.01 | |
| COauto | 0.73 [0.63; 0.81] | <0.01 | |
| VTIman | 0.54 [0.42; 0.64] | <0.01 | |
| VTIauto | 0.45 [0.28; 0.59] | <0.01 | |
| SV | COF | 0.57 [0.41; 0.69] | <0.01 |
| VTIman | 0.70 [0.59; 0.75] | <0.01 | |
| VTIauto | 0.73 [0.63; 0.81] | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markarian, T.; Grau-Mercier, L.; Occelli, C.; Ajavon, F.; Claret, P.-G.; Coisy, F.; Bobbia, X. Evaluation of a New Echocardiographic Tool for Cardiac Output Monitoring: An Experimental Study on A Controlled Hemorrhagic Shock Model in Anesthetized Piglets. J. Clin. Med. 2022, 11, 5420. https://doi.org/10.3390/jcm11185420
Markarian T, Grau-Mercier L, Occelli C, Ajavon F, Claret P-G, Coisy F, Bobbia X. Evaluation of a New Echocardiographic Tool for Cardiac Output Monitoring: An Experimental Study on A Controlled Hemorrhagic Shock Model in Anesthetized Piglets. Journal of Clinical Medicine. 2022; 11(18):5420. https://doi.org/10.3390/jcm11185420
Chicago/Turabian StyleMarkarian, Thibaut, Laura Grau-Mercier, Céline Occelli, Florian Ajavon, Pierre-Géraud Claret, Fabien Coisy, and Xavier Bobbia. 2022. "Evaluation of a New Echocardiographic Tool for Cardiac Output Monitoring: An Experimental Study on A Controlled Hemorrhagic Shock Model in Anesthetized Piglets" Journal of Clinical Medicine 11, no. 18: 5420. https://doi.org/10.3390/jcm11185420
APA StyleMarkarian, T., Grau-Mercier, L., Occelli, C., Ajavon, F., Claret, P.-G., Coisy, F., & Bobbia, X. (2022). Evaluation of a New Echocardiographic Tool for Cardiac Output Monitoring: An Experimental Study on A Controlled Hemorrhagic Shock Model in Anesthetized Piglets. Journal of Clinical Medicine, 11(18), 5420. https://doi.org/10.3390/jcm11185420







