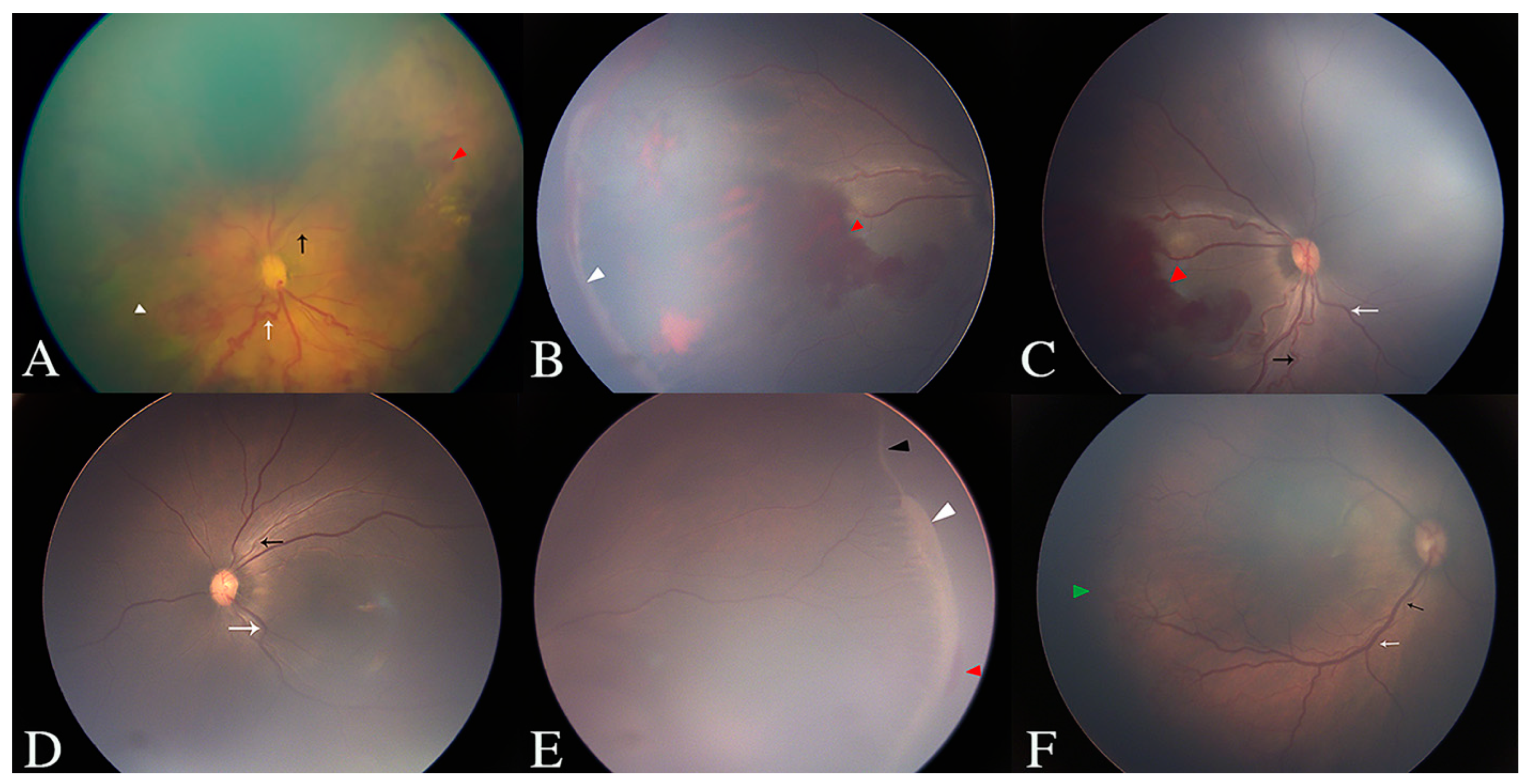
1. Introduction
Retinopathy of prematurity (ROP) is a retinovascular disease characterized by retinal ischemia, aberrant angiogenesis, fibrovascular proliferation, and progressive vitreoretinal traction [
1]. It is a major cause of blindness in premature-birth infants. To describe the fundus change of ROP in detail and to optimize management, three concentric zones are used to describe the location of the disease, including zone I (a circle with a radius that extends from the optic disc to twice the distance from the center of the optic disc to the center of the macula), zone II (the area from the edge of zone I to the nasalora serrata), and zone III (the residual crescent of the retina anterior to zone II). In addition, five stages are graded to describe the severity of disease, including stage 1 (demarcation line), stage 2 (ridge), stage 3 (extraretinal fibrovascular proliferation), stage 4 (partial retinal detachment), and stage 5 (total retinal detachment). Lastly, plus disease is used to describe the increased venous dilation and arteriolar tortuosity of the posterior retinal vessels [
2].
Type I ROP is characterized as any stage of ROP with plus disease (+), stage 3 ROP in zone I, or stages 2 or 3 ROP + in zone II [
3]. It was proven that early treatment should be given to these kinds of ROP to avoid an unfavorable outcome. Until now, several studies have proven that anti-vascular endothelial growth factor (VEGF) therapy could induce the regression of ROP lesions and promote the normal angiogenesis in type I ROP [
4,
5,
6]. Based on these results, we hypothesized that the intraocular VEGF level might be associated with the severity of type 1 ROP. To test our hypothesis, we measured the VEGF level in the aqueous humor collected from infants with type I ROP and analyzed the relationship between the aqueous VEGF level and the severity of ROP.
2. Methods
This retrospective case series involved infants who were diagnosed with type I ROP in Beijing Children’s Hospital (affiliated with Capital Medical University) within the period from February 2018 to February 2021. All of the infants received intravitreal anti-VEGF therapy (ranibizumab, 0.025–0.05 mL/0.25–0.5 g) (Lucentis®, Novartis Ophthalmics, Basel, Switzerland) as the first-line treatment. Infants who had other treatments (e.g., laser photocoagulation, cryotherapy) before anti-VEGF treatment were excluded from the study. The general characteristics of the infants, such as the gestational age (GA), birth weight (BW), and the infant’s postmenstrual age (PMA) at the time of examination, were recorded. The study was conducted in accordance with the Declaration of Helsinki. Institutional review board approval was obtained.
The involved infants underwent an ROP screening examination according to the procedure described in the previous study [
7]. The ocular findings, such as the location of vascularization, the stage of acute disease, and the plus disease spectrum, were recorded in accordance with the International Classification of ROP, Third Edition (ICROP3) [
8]. The artery tortuosity in zone I was graded as mild (tortuosity angle > 90°), moderate (tortuosity angle = 90°), and severe (tortuosity angle < 90°). The venous tortuosity in zone I was also graded based on the same criteria. If more than one grade of vessel tortuosity was present in the same eye, only the most severe grade was taken into analysis. The severity of ROP was categorized into three groups: aggressive retinopathy of prematurity (A-ROP; posterior location of retinopathy and prominence of plus disease), threshold ROP (T-ROP; at least 5 continuous or 8 cumulative hours of stage 3 ROP in zone 1 or 2, with plus disease), and type 1 pre-threshold ROP (P-T-1; zone 1 any-stage ROP with plus disease, zone 1 stage 3 ROP with or without plus disease, and zone 2 stage 2 or 3 ROP with plus disease) [
3,
8].
The aqueous humor was obtained before the intravitreal injection of an anti-VEGF agent. About 50–100 μL of aqueous humor was obtained through paracentesis using a 29-gauge needle. The aqueous VEGF level was measured using the Becton Dickinson Cytometric Bead Array bead-based immunoassay (Human VEGF Flex Set, No. 558336, BD Bioscience, San Jose, CA), following the manufacturer’s instructions. Briefly, VEGF capture beads were incubated with calibrators (standards ranging from 0 to 2500 pg/mL) or the aqueous humor in the dark. Anti-VEGF phycoerythrin-labeled antibody was then added and the mixture was subsequently incubated in the dark for 2 h. After washing, two-color flow cytometry analysis was performed, and data were acquired and analyzed using the Becton Dickinson FCAP Array software. The VEGF concentrations in the aqueous humor from the patients and controls were determined from the standard curves.
Statistical analyses were carried out using the SPSS software for Windows (version 28.0; IBM-SPSS, Chicago, IL, USA). The normality of data was tested using the Kolmogorov–Smirnov test. Spearman’s correlation coefficient was used to analyze the correlation between the GA, BW, and PMA of included infants and the aqueous VEGF level. The partial correlation coefficient was used to analyze the correlation between the ocular findings of ROP (the location of ROP lesions, the stage of ROP lesions, and the grade of vessel tortuosity in zone I) and the aqueous VEGF level. Multiple linear regression analysis was further used to determine the relationship between the above-mentioned variables and the aqueous VEGF level. The one-way ANOVA analysis was used for the comparison of aqueous VEGF levels among different ROP groups. All of the p-values less than 0.05 were considered statistically significant, and all of the tests were two-sided.
3. Results
Forty-nine infants (88 eyes) were included in this study. The GA ranged from 25.7 to 33.9 weeks, with a mean GA of 29.1 ± 1.8 weeks and a median GA of 29 weeks. The BW ranged from 760 to 2800 g, with a mean BW of 1300 ± 376 g and a median BW of 1255 g. The PMA ranged from 33.1 to 47 weeks, with a mean PMA of 39.9 ± 3.3 weeks and a median PMA of 40.1 weeks. The mean aqueous VEGF level of all involved eyes was 278.2 ± 492.1 pg/mL (range: 1.3–3824.1 pg/mL), with a median value of 128.9 pg/mL. Detailed results of the fundus examinations, including the vessel tortuosity in zone I, and the location and stage of the ROP lesions, are listed in
Table 1 and shown in
Figure 1. Data of the corresponding aqueous VEGF levels are also listed in
Table 1.
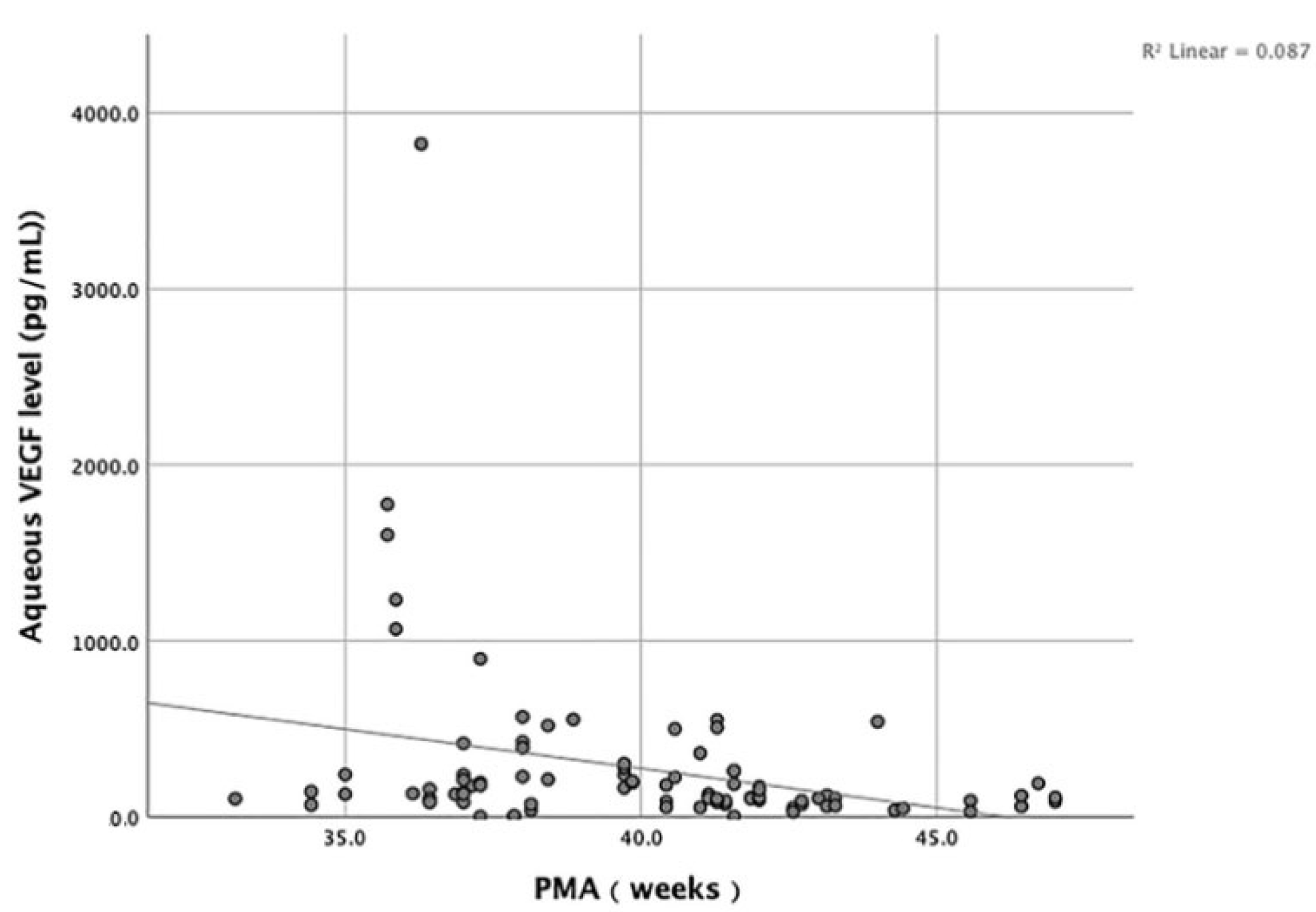
We analyzed the correlation between the GA, BW, and PMA of included infants and the aqueous VEGF level. The results show that no significant correlation existed between the GA, BW, and the aqueous VEGF level (
p > 0.05). On the contrary, the PMA was negatively correlated with the aqueous VEGF level (
p = 0.001, R= −0.346) (
Figure 2).
According to the severity of ROP, 17 eyes, 11 eyes, and 60 eyes were categorized as A-ROP, T-ROP, and P-T-1, respectively. The mean aqueous VEGF level was 747.5 ± 976.1 pg/mL in the A-ROP group, 268.0 ± 199.0 pg/mL in the T-ROP group, and 147.1 ± 105.3 pg/mL in the P-T-1 group, with a median value of 425.8 pg/mL, 212.0 pg/mL, and 110.0 pg/mL, respectively. Significant differences were found among the three groups (p = 0.000). Further pairwise comparison showed that the aqueous VEGF level in the A-ROP group was significantly higher than those in the T-ROP group (p = 0.006) and in the P-T-1 group (p = 0.000). However, no significant difference in aqueous VEGF level was found between the T-ROP and P-T-1 groups (p = 0.402).
The zone, stage and the plus disease spectrum were important parameters to evaluate the severity of ROP and could have relevance to the aqueous VEGF level. To avoid potential bias, we used the partial correlation coefficient to analyze the relationship between these parameters and the aqueous VEGF level. The results show that the aqueous VEGF level was negatively correlated with the zone of ROP lesions (p = 0.000, r = −0.480) as well as with the stage of ROP lesions (p = 0.013, r = −0.268). The aqueous VEGF level was positively correlated with the venous tortuosity in zone I (p = 0.015 r = 0.263) and had no relevance with the artery tortuosity in zone I (p = 0.178).
According to the multiple linear regression analysis, the zone of ROP lesions, the stage of ROP lesions, and the grade of venous tortuosity in zone I significantly affected the aqueous VEGF level. The variable that most positively affected the aqueous VEGF level was the grade of venous tortuosity in zone I. On the contrary, the variable that most negatively affected the aqueous VEGF level was the zone of ROP lesions. The multiple linear regression analysis results of the variables affecting the aqueous VEGF level are presented in
Table 2.
4. Discussion
The pathogenesis of ROP was divided into two phases. In phase I, the delayed physiological retinal vascular development results in a peripheral avascular area, and in phase II, intravitreal angiogenesis occurs at the junction of the avascularized and vascularized retinas [
9]. Vascular endothelial growth factor (VEGF) is the principal mediator of pathological angiogenesis [
10]. It is downregulated in phase I when a premature-birth infant is exposed to a relatively high-oxygen environment after birth, and is upregulated by an avascularized retina in phase II [
11]. Based on these theories, we measured the aqueous VEGF level in type 1 ROP and analyzed the relationship between the aqueous VEGF level and the severity of retinopathy of prematurity.
The GA and BW are two major risk factors for the occurrence of ROP [
12]. In the CRYO-ROP cohort, each 100 g increase in BW decreased the odds of reaching the threshold ROP by 27%, and each weekly increase in GA decreased the odds of reaching the threshold disease by 19% [
13]. In our study, the GA and BW did not correlate with the aqueous VEGF level. One possible explanation for this was that the aqueous humor sample was not collected soon enough after infant birth. Medical interventions after birth might have added bias to the correlation analysis. Another possible explanation was that the GA and BW influenced the development and progression of ROP via other physiopathologic mechanisms. For example, several inflammatory parameters in plasma, including interleukin (IL)-8, monocyte chemoattractant protein (MCP)-1, alkaline phosphatase (AP), and IL-1β, were proven to be correlated with GA and BW in preterm infants [
14]. The role of inflammation in the development of ROP was also proven in both the plasma and the intraocular fluid [
15,
16].
In our study, the aqueous VEGF level was the highest in the A-ROP group, followed by those of the T-ROP and P-T-1 groups, which could explain the most aggressive retinopathy in A-ROP. A-ROP is the most severe form of type 1 ROP, and it can rapidly progress to tractional retinal detachment (RD). Nedime et al. [
17], in a study involving 15 eyes with A-ROP, administered intravitreal injections of ranibizumab (0.25 mg/0.025 mL). All eyes developed plus disease and received retreatment during the follow-up visit. Tong et al. [
18] reported a case series of 160 eyes diagnosed with A-ROP and that had received an intravitreal injection of ranibizumab (0.3 mg/0.03 mL). The RD occurrence rate was 21.6%, and the rate of multiple intravitreal injections was 65.5%. Based on our finding that the aqueous VEGF level in the A-ROP group was much higher than those in the T-ROP and P-T-1 groups, we propose that the conventional dose of intravitreal anti-VEGF agent might be insufficient to neutralize the intraocular VEGF in eyes with A-ROP, and thus cause the rate of reactivation to remain high even after administering timely anti-VEGF treatment. Since the neurodevelopmental outcomes in preterm infants after intravitreal injection of anti-VEGF agents remains unclear [
19,
20,
21,
22], the optimal dose of intravitreal anti-VEGF agent for A-ROP infants still needs to be investigated in future studies.
Our study shows that the aqueous VEGF level in the T-ROP group was higher than in the P-T-1 group, although no significant difference was found. This result is consistent with the study of Lyu et al. [
16] in 2018. The results of the CRYO-ROP study show an approximate 50% risk of retinal detachment in T-ROP if not treated [
23]. In the ETROP study, early treatment of pre-threshold type 1 ROP was also recommended for a better visual prognosis and retinal structure [
3]. Our VEGF level findings in the T-ROP and P-T-1 groups further verified the necessity of early treatment for T-ROP and P-T-1 on the molecular level.
We analyzed the relationship between the aqueous VEGF level and the fundus changes of ROP, including the morphological change in the posterior retinal vessels, and the location and stage of ROP lesions. Our results show that eyes with ROP lesions closer to the posterior region had a higher aqueous VEGF level, which proved the more severe disease of zone I ROP. It was notable that eyes with a higher stage of ROP lesion had a lower aqueous VEGF level, which did not seem to conform with the natural course of ROP. The possible reason for this was that we only involved type I ROP in this study, which meant that eyes with stage I ROP had zone I disease and the most severe plus disease, simultaneously, which caused the selection bias. We also found that it was the venous tortuosity, but not the artery tortuosity, which positively correlated with the aqueous VEGF level. In another study of central retinal vein occlusion, the aqueous VEGF concentration was also proven to be significantly correlated with the venous tortuosity [
24]. We propose that retinal venous tortuosity is correlated with the degree of circulatory disorder and retinal ischemia, and could further be used as an indicator to identify eyes with more severe ROP.
In summary, we measured the aqueous VEGF level of type 1 ROP and found that the aqueous VEGF level in A-ROP was the highest in type I ROP. The location of the ROP lesions and the venous tortuosity in zone I correlated with the aqueous VEGF level, and could indicate the severity of ROP.
Our study had some limitations. First, its retrospective design introduced a potential selection bias. Second, the dilation of the posterior retinal vessels was not analyzed due to the lack of image analysis methods used. Third, the aqueous sample was collected only before the first intravitreal injection, and the variation in the aqueous VEGF level was not investigated. In the future, we intend to conduct studies with prospective design and different doses of intravitreal anti-VEGF agent to determine the optimal dose for A-ROP. Furthermore, the concentration of VEGF and other inflammatory cytokines in the intraocular fluid sample should be measured to further explore the interaction among these cytokines and their effect on the progression of ROP.
Author Contributions
T.L., Y.P., L.L. (Lili Liu) and M.H. collected and analyzed the data; Z.Q. and T.L. drafted the manuscript; Z.Q., Y.C., C.Z. and C.P. participated in the laboratory examination and investigation; Y.T., L.L. (Li Li) and N.L. revised the manuscript and gave final approval for the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81670883).
Institutional Review Board Statement
The ethics committee of the Beijing Children’s Hospital approved this study and informed consent was obtained from the guardians of the involved infants. Ethic code number: [2021]-E-073-R.
Informed Consent Statement
All authors confirm that the publication of the work is approved by all co-authors. The work has not been published before and is not under consideration for publication elsewhere. Written consent to publish this information was obtained from the study participants.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tran, K.D.; Cernichiaro-Espinosa, L.A.; Berrocal, A.M. Management of Retinopathy of Prematurity—Use of Anti-VEGF Therapy. Asia Pac. J. Ophthalmol. 2018, 7, 56–62. [Google Scholar]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch. Ophthalmol. 2003, 121, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Mintz-Hittner, H.A.; Kuffel, R.R. Intravitreal injection of bevacizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina 2008, 28, 831–838. [Google Scholar] [CrossRef]
- Mintz-Hittner, H.A.; Kennedy, K.A.; Chuang, A.Z.; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 2011, 364, 603–615. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chae, J.B.; Yang, S.J.; Yoon, Y.H.; Kim, J.G. Effects of intravitreal bevacizumab and laser in retinopathy of prematurity therapy on the development of peripheral retinal vessels. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1257–1262. [Google Scholar] [CrossRef]
- Wu, C.; Petersen, R.A.; VanderVeen, D.K. RetCam imaging for retinopathy of prematurity screening. J. AAPOS 2006, 10, 107–111. [Google Scholar]
- Chiang, M.F.; Quinn, G.E.; Fielder, A.R.; Ostmo, S.R.; Chan, P.R.V.; Berrocal, A.; Binenbaum, G.; Blair, M.; Campbell, J.P.; Capone, A., Jr.; et al. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology 2021, 128, e51–e68. [Google Scholar]
- Hartnett, M.E.; Penn, J.S. Mechanisms and Management of Retinopathy of Prematurity. N. Engl. J. Med. 2012, 367, 2515–2526. [Google Scholar] [CrossRef]
- Chen, J.; Smith, L.E.H. Retinopathy of prematurity. Angiogenesis 2007, 10, 133–140. [Google Scholar]
- Enríquez, A.B.; Avery, R.L.; Baumal, C.R. Update on Anti-Vascular Endothelial Growth Factor Safety for Retinopathy of Prematurity. Asia Pac. J. Ophthalmol. 2020, 9, 358–368. [Google Scholar]
- Kim, S.J.; Port, A.D.; Swan, R.; Campbell, J.P.; Chan, R.V.P.; Chiang, M.F. Retinopathy of Prematurity: A Review of Risk Factors and their Clinical Significance. Surv. Ophthalmol. 2018, 63, 618–637. [Google Scholar] [PubMed]
- Schaffer, D.B.; Palmer, E.A.; Plotsky, D.F.; Metz, H.S.; Flynn, J.T.; Tung, B.; Hardy, R.J. Prognostic Factors in the Natural Course of Retinopathy of Prematurity. Ophthalmology 1993, 100, 230–237. [Google Scholar] [CrossRef]
- Rodríguez-Benítez, M.V.; Gámez-Belmonte, R.; Gil-Campos, M.; Hernández-Chirlaque, C.; Bouzas, P.R.; Sánchez de Medina, F.; Martínez-Augustin, O. Premature Birth Infants Present Elevated Inflammatory Markers in the Meconium. Front. Pediatr. 2021, 8, 627475. [Google Scholar]
- Silveira, R.C.; Fortes Filho, J.B.; Procianoy, R.S. Assessment of the contribution of cytokine plasma levels to detect retinopathy of prematurity in very low birth weight infants. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1297–1301. [Google Scholar] [CrossRef]
- Lyu, J.; Zhang, Q.; Jin, H.; Xu, Y.; Chen, C.; Ji, X.; Zhang, X.; Rao, Y.; Zhao, P. Aqueous cytokine levels associated with severity of type 1 retinopathy of prematurity and treatment response to ranibizumab. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1469–1477. [Google Scholar] [CrossRef]
- Sahinoglu-Keskek, N.; Akkoyun, I.; Torer, B. Favorable outcomes in the treatment of aggressive posterior retinopathy of prematurity. Eur. J. Ophthalmol. 2021, 31, 179–183. [Google Scholar] [CrossRef]
- Tong, Q.; Yin, H.; Zhao, M.; Li, X.; Yu, W. Outcomes and prognostic factors for aggressive posterior retinopathy of prematurity following initial treatment with intravitreal ranibizumab. BMC Ophthalmol. 2018, 18, 150. [Google Scholar]
- Araz-Ersan, B.; Kir, N.; Tuncer, S.; Aydinoglu-Candan, O.; Yildiz-Inec, D.; Akdogan, B.; Ekici, B.; Demirel, A.; Ozmen, M. Preliminary anatomical and neurodevelopmental outcomes of intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. Curr. Eye Res. 2015, 40, 585–591. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Huang, Y.S.; Huang, C.Y.; Hsu, J.F.; Shih, C.P.; Hwang, Y.S.; Yao, T.C.; Lai, C.C.; Wu, W.C. Neurodevelopmental Outcomes after Intravitreal Bevacizumab Therapy for Retinopathy of Prematurity: A Prospective Case-Control Study. Ophthalmology 2019, 126, 1567–1577. [Google Scholar] [CrossRef]
- Lien, R.; Yu, M.H.; Hsu, K.H.; Liao, P.J.; Chen, Y.P.; Lai, C.C.; Wu, W.C. Neurodevelopmental Outcomes in Infants with Retinopathy of Prematurity and Bevacizumab Treatment. PLoS ONE 2016, 11, e0148019. [Google Scholar]
- Natarajan, G.; Shankaran, S.; Nolen, T.L.; Sridhar, A.; Kennedy, K.A.; Hintz, S.R.; Phelps, D.L.; DeMauro, S.B.; Carlo, W.A.; Gantz, M.G.; et al. Neurodevelopmental Outcomes of Preterm Infants with Retinopathy of Prematurity by Treatment. Pediatrics 2019, 144, e20183537. [Google Scholar] [CrossRef]
- Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch. Ophthalmol. 1988, 106, 471–479. [CrossRef]
- Yasuda, S.; Kachi, S.; Kondo, M.; Ueno, S.; Kaneko, H.; Terasaki, H. Significant Correlation between Retinal Venous Tortuosity and Aqueous Vascular Endothelial Growth Factor Concentration in Eyes with Central Retinal Vein Occlusion. PLoS ONE 2015, 10, e0134267. [Google Scholar]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).