Circadian Blood Pressure Profile in Pediatric Patients with Primary Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Ethical Issues
2.3. Clinical Parameters
2.4. Laboratory Tests
2.5. Blood Pressure Measurements and Dipping Status
2.6. Echocardiography and assessment of Properties of Arteries
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics and Biochemical Parameters
3.2. Blood Pressure and Markers of Hypertension-Mediated Organ Damage
3.3. Determinants of BP Dipping and Association between BP Dipping and HMOD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flynn, J.T.; Daniels, S.R.; Hayman, L.L.; Maahs, D.M.; McCrindle, B.W.; Mitsnefes, M.; Zachariah, J.P.; Urbina, E.M. Update: Ambulatory blood pressure monitoring in children and adolescents: A scientific statement from the American Heart Association. Hypertension 2014, 63, 1116–1135. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Y.; Melgarejo, J.D.; Thijs, L.; Zhang, Z.Y.; Boggia, J.; Wei, F.F.; Hansen, T.W.; Asayama, K.; Ohkubo, T.; Jeppesen, J.; et al. Association of Office and Ambulatory Blood Pressure with Mortality and Cardiovascular Outcomes. Jama 2019, 322, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Palatini, P.; Verdecchia, P.; Beilin, L.J.; Eguchi, K.; Imai, Y.; Kario, K.; Ohkubo, T.; Pierdomenico, S.D.; Saladini, F.; Schwartz, J.E.; et al. Association of Extreme Nocturnal Dipping with Cardiovascular Events Strongly Depends on Age. Hypertension 2020, 75, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Hoshide, S.; Mizuno, H.; Kabutoya, T.; Nishizawa, M.; Yoshida, T.; Abe, H.; Katsuya, T.; Fujita, Y.; Okazaki, O.; et al. Nighttime Blood Pressure Phenotype and Cardiovascular Prognosis: Practitioner-Based Nationwide JAMP Study. Circulation 2020, 142, 1810–1820. [Google Scholar] [CrossRef]
- Gavriilaki, M.; Anyfanti, P.; Nikolaidou, B.; Lazaridis, A.; Gavriilaki, E.; Douma, S.; Gkaliagkousi, E. Nighttime dipping status and risk of cardiovascular events in patients with untreated hypertension: A systematic review and meta-analysis. J. Clin. Hypertens. 2020, 22, 1951–1959. [Google Scholar] [CrossRef]
- Hermida, R.C.; Crespo, J.J.; Domínguez-Sardiña, M.; Otero, A.; Moyá, A.; Ríos, M.T.; Sineiro, E.; Castiñeira, M.C.; Callejas, P.A.; Pousa, L.; et al. Bedtime hypertension treatment improves cardiovascular risk reduction: The Hygia Chronotherapy Trial. Eur Heart J 2020, 41, 4565–4576. [Google Scholar] [CrossRef]
- Cilsal, E. In newly diagnosed hypertensive children, increased arterial stiffness and reduced heart rate variability were associated with a non-dipping blood pressure pattern. Rev. Port. Cardiol. 2020, 39, 331–338. [Google Scholar] [CrossRef]
- Wu, H.; Shi, L.; Lin, Y.; Zheng, T. The Correlation Between ABPM Parameters and Left Ventricular Hypertrophy in Pediatric Essential Hypertension. Front. Pediatr. 2022, 10, 896054. [Google Scholar] [CrossRef]
- Chang, J.C.; Xiao, R.; Meyers, K.E.; Mercer-Rosa, L.; Natarajan, S.S.; Weiss, P.F.; Knight, A.M. Nocturnal blood pressure dipping as a marker of endothelial function and subclinical atherosclerosis in pediatric-onset systemic lupus erythematosus. Arthritis Res. Ther. 2020, 22, 129. [Google Scholar] [CrossRef]
- Seeman, T.; Hradský, O.; Gilík, J. Nocturnal blood pressure non-dipping is not associated with increased left ventricular mass index in hypertensive children without end-stage renal failure. Eur. J. Pediatr. 2016, 175, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef] [PubMed]
- Kułaga, Z.; Litwin, M.; Tkaczyk, M.; Palczewska, I.; Zajączkowska, M.; Zwolińska, D.; Krynicki, T.; Wasilewska, A.; Moczulska, A.; Morawiec-Knysak, A.; et al. Polish 2010 growth references for school-aged children and adolescents. Eur. J. Pediatr. 2011, 170, 599–609. [Google Scholar] [CrossRef] [PubMed]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.; McCallin, T.; Martinez, J.; Chacko, S.; Yusuf, S. Hyperlipidemia. Pediatrics Rev. 2020, 41, 393–402. [Google Scholar] [CrossRef]
- Feig, D.I.; Kang, D.H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef]
- Skrzypczyk, P.; Ofiara, A.; Szyszka, M.; Dziedzic-Jankowska, K.; Sołtyski, J.; Pańczyk-Tomaszewska, M. Vitamin D in children with primary hypertension. Arter. Hypertens. 2018, 22, 127–134. [Google Scholar] [CrossRef]
- Skrzypczyk, P.; Ozimek, A.; Ofiara, A.; Szyszka, M.; Sołtyski, J.; Stelmaszczyk-Emmel, A.; Górska, E.; Pańczyk-Tomaszewska, M. Markers of endothelial injury and subclinical inflammation in children and adolescents with primary hypertension. Cent. Eur. J. Immunol. 2019, 44, 253–261. [Google Scholar] [CrossRef]
- Kułaga, Z.; Litwin, M.; Grajda, A.; Kułaga, K.; Gurzkowska, B.; Góźdź, M.; Pan, H. Oscillometric blood pressure percentiles for Polish normal-weight school-aged children and adolescents. J. Hypertens. 2012, 30, 1942–1954. [Google Scholar] [CrossRef]
- Skrzypczyk, P.; Okarska-Napierała, M.; Pietrzak, R.; Pawlik, K.; Waścińska, K.; Werner, B.; Pańczyk-Tomaszewska, M. NT-proBNP as a Potential Marker of Cardiovascular Damage in Children with Chronic Kidney Disease. J. Clin. Med. 2021, 10, 4344. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Daniels, S.R.; Devereux, R.B.; Meyer, R.A.; Roman, M.J.; de Divitiis, O.; Alderman, M.H. Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. J. Am. Coll. Cardiol. 1992, 20, 1251–1260. [Google Scholar] [CrossRef]
- Khoury, P.R.; Mitsnefes, M.; Daniels, S.R.; Kimball, T.R. Age-specific reference intervals for indexed left ventricular mass in children. J. Am. Soc. Echocardiogr. 2009, 22, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczyk, P.; Przychodzień, J.; Mizerska-Wasiak, M.; Kuźma-Mroczkowska, E.; Okarska-Napierała, M.; Górska, E.; Stelmaszczyk-Emmel, A.; Demkow, U.; Pańczyk-Tomaszewska, M. Renalase in Children with Glomerular Kidney Diseases. Adv. Exp. Med. Biol. 2017, 1021, 81–92. [Google Scholar] [CrossRef]
- Skrzypczyk, P.; Stelmaszczyk-Emmel, A.; Szyszka, M.; Ofiara, A.; Pańczyk-Tomaszewska, M. Circulating calcification inhibitors are associated with arterial damage in pediatric patients with primary hypertension. Pediatr. Nephrol. 2021, 36, 2371–2382. [Google Scholar] [CrossRef]
- Reusz, G.S.; Cseprekal, O.; Temmar, M.; Kis, E.; Cherif, A.B.; Thaleb, A.; Fekete, A.; Szabó, A.J.; Benetos, A.; Salvi, P. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension 2010, 56, 217–224. [Google Scholar] [CrossRef]
- Doyon, A.; Kracht, D.; Bayazit, A.K.; Deveci, M.; Duzova, A.; Krmar, R.T.; Litwin, M.; Niemirska, A.; Oguz, B.; Schmidt, B.M.; et al. Carotid artery intima-media thickness and distensibility in children and adolescents: Reference values and role of body dimensions. Hypertension 2013, 62, 550–556. [Google Scholar] [CrossRef]
- Pludowski, P.; Litwin, M.; Sladowska, J.; Antoniewicz, J.; Niemirska, A.; Wierzbicka, A.; Lorenc, R.S. Bone mass and body composition in children and adolescents with primary hypertension: Preliminary data. Hypertension 2008, 51, 77–83. [Google Scholar] [CrossRef]
- Litwin, M.; Kułaga, Z. Obesity, metabolic syndrome, and primary hypertension. Pediatr. Nephrol. 2021, 36, 825–837. [Google Scholar] [CrossRef]
- Gupta-Malhotra, M.; Banker, A.; Shete, S.; Hashmi, S.S.; Tyson, J.E.; Barratt, M.S.; Hecht, J.T.; Milewicz, D.M.; Boerwinkle, E. Essential hypertension vs. secondary hypertension among children. Am. J. Hypertens. 2015, 28, 73–80. [Google Scholar] [CrossRef]
- Symonides, B.; Jędrusik, P.; Artyszuk, L.; Gryboś, A.; Dziliński, P.; Gaciong, Z. Different diagnostic criteria significantly affect the rates of hypertension in 18-year-old high school students. Arch. Med. Sci. 2010, 6, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Obrycki, Ł.; Feber, J.; Brzezińska, G.; Litwin, M. Evolution of isolated systolic hypertension with normal central blood pressure in adolescents-prospective study. Pediatr. Nephrol. 2021, 36, 361–371. [Google Scholar] [CrossRef]
- Reffo, E.; Stritoni, V.; Di Salvo, G. Inflammatory syndrome in children associated with COVID-19 complicated by acute myocardial infarction. Eur. Heart J. 2021, 42, 2136. [Google Scholar] [CrossRef] [PubMed]
- Charfeddine, S.; Yousfi, C.; Maalej, B.; Triki, F.; Abid, L.; Kammoun, S. Acute myocardial infarction in a child with nephrotic syndrome. Rev. Port. Cardiol. 2021, 40, 457.e451–457.e454. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.C.; El-Said, H.; Tremoulet, A.H.; Friedman, K.; Gordon, J.B.; Newburger, J.W. Management of Myocardial Infarction in Children with Giant Coronary Artery Aneurysms after Kawasaki Disease. J. Pediatr. 2020, 221, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.; Trelewicz, J.; Wawer, Z.; Antoniewicz, J.; Wierzbicka, A.; Rajszys, P.; Grenda, R. Intima-media thickness and arterial elasticity in hypertensive children: Controlled study. Pediatr. Nephrol. 2004, 19, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.; Niemirska, A.; Sladowska-Kozlowska, J.; Wierzbicka, A.; Janas, R.; Wawer, Z.T.; Wisniewski, A.; Feber, J. Regression of target organ damage in children and adolescents with primary hypertension. Pediatr. Nephrol. 2010, 25, 2489–2499. [Google Scholar] [CrossRef]
- Kavey, R.E. Left ventricular hypertrophy in hypertensive children and adolescents: Predictors and prevalence. Curr. Hypertens. Rep 2013, 15, 453–457. [Google Scholar] [CrossRef]
- Birkenhäger, A.M.; van den Meiracker, A.H. Causes and consequences of a non-dipping blood pressure profile. Neth. J. Med. 2007, 65, 127–131. [Google Scholar]
- Flynn, J.T. Differentiation between primary and secondary hypertension in children using ambulatory blood pressure monitoring. Pediatrics 2002, 110, 89–93. [Google Scholar] [CrossRef]
- Skrzypczyk, P.; Pańczyk-Tomaszewska, M.; Roszkowska-Blaim, M. 24-godzinny pomiar ciśnienia tętniczego u dzieci z nadciśnieniem tętniczym pierwotnym i wtórnym do miąższowych chorób nerek. Med. Og. Nauk. Zdr. 2013, 19, 49–54. [Google Scholar]
- Jędzura, A.; Adamczyk, P.; Bjanid, O.; Świętochowska, E.; Roszkowska-Bjanid, D.; Baraniecka, A.; Banaszak, B.; Plesiński, K.; Morawiec-Knysak, A.; Ziora, K.; et al. Non-dipping status and selected adipokines concentration in children with primary arterial hypertension. Clin. Exp. Hypertens. 2017, 39, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Macumber, I.R.; Weiss, N.S.; Halbach, S.M.; Hanevold, C.D.; Flynn, J.T. The Association of Pediatric Obesity with Nocturnal Non-Dipping on 24-Hour Ambulatory Blood Pressure Monitoring. Am. J. Hypertens. 2016, 29, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Tadic, M.; Sala, C.; Gherbesi, E.; Grassi, G.; Mancia, G. Blood Pressure Non-Dipping and Obstructive Sleep Apnea Syndrome: A Meta-Analysis. J. Clin. Med. 2019, 8, 1367. [Google Scholar] [CrossRef] [PubMed]
- Bankir, L.; Bochud, M.; Maillard, M.; Bovet, P.; Gabriel, A.; Burnier, M. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension 2008, 51, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Libianto, R.; Moran, J.; O’Callaghan, C.; Baqar, S.; Chen, A.X.; Baker, S.T.; Clarke, M.; MacIsaac, R.J.; Jerums, G.; Ekinci, E.I. Relationship between urinary sodium-to-potassium ratio and ambulatory blood pressure in patients with diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 2018, 45, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Sala, C.; Tadic, M.; Rescaldani, M.; Grassi, G.; Mancia, G. Non-Dipping Pattern and Subclinical Cardiac Damage in Untreated Hypertension: A Systematic Review and Meta-Analysis of Echocardiographic Studies. Am. J. Hypertens. 2015, 28, 1392–1402. [Google Scholar] [CrossRef]
- Narayan, O.; Cameron, J.D. Ambulatory blood pressure monitoring and dipping status in predicting left ventricular hypertrophy. J. Hypertens. 2014, 32, 1962–1963. [Google Scholar] [CrossRef]
- Abdalla, M.; Caughey, M.C.; Tanner, R.M.; Booth, J.N., 3rd; Diaz, K.M.; Anstey, D.E.; Sims, M.; Ravenell, J.; Muntner, P.; Viera, A.J.; et al. Associations of Blood Pressure Dipping Patterns with Left Ventricular Mass and Left Ventricular Hypertrophy in Blacks: The Jackson Heart Study. J. Am. Heart. Assoc. 2017, 6, e004847. [Google Scholar] [CrossRef]
- Rodrigues, J.C.L.; Amadu, A.M.; Ghosh Dastidar, A.; Harries, I.; Burchell, A.E.; Ratcliffe, L.E.K.; Hart, E.C.; Hamilton, M.C.K.; Paton, J.F.R.; Nightingale, A.K.; et al. Noctural dipping status and left ventricular hypertrophy: A cardiac magnetic resonance imaging study. J. Clin. Hypertens. 2018, 20, 784–793. [Google Scholar] [CrossRef]
- Shinohata, R.; Nakatsu, T.; Yuki, Y.; Nishitani, A.; Mashima, K.; Toyonaga, S.; Ogawa, H.; Hirohata, S.; Usui, S.; Kitawaki, T.; et al. Association of augmentation index of radial pressure wave form with diurnal variation pattern of blood pressure in untreated patients with essential hypertension. J. Hypertens. 2008, 26, 535–543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, J.S.; Shin, J.H.; Park, J.B.; Choi, D.J.; Youn, H.J.; Park, C.G.; Kwan, J.; Ahn, Y.; Kim, D.W.; Rim, S.J.; et al. Relationship between arterial stiffness and circadian pattern of blood pressure. Medicine 2019, 98, e14953. [Google Scholar] [CrossRef] [PubMed]
- Karadag, B.; Ozyigit, T.; Serindag, Z.; Ilhan, A.; Ozben, B. Blood pressure profile is associated with microalbuminuria and retinopathy in hypertensive nondiabetic patients. Wien Klin. Wochenschr. 2018, 130, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Oliveras, A.; Armario, P.; Martell-Clarós, N.; Ruilope, L.M.; de la Sierra, A. Urinary albumin excretion is associated with nocturnal systolic blood pressure in resistant hypertensives. Hypertension 2011, 57, 556–560. [Google Scholar] [CrossRef]
- Bakhoum, C.Y.; Vuong, K.T.; Carter, C.E.; Gabbai, F.B.; Ix, J.H.; Garimella, P.S. Proteinuria and nocturnal blood pressure dipping in hypertensive children and adolescents. Pediatr. Res. 2021, 90, 876–881. [Google Scholar] [CrossRef]
| Dipping Pattern | Night Drop in Blood Pressure (Difference between Day and Night Compared to Day) |
|---|---|
| Extreme dipper | ≥20% |
| Dipper | ≥10% but <20% |
| Non-dipper | ≥0% but <10% |
| Reverse dipper | <0% |
| Parameter | Value in the Study Group n or Mean ± SD (IQR) |
|---|---|
| Number of patients (n) | 112 |
| Age (years) | 14.7 ± 2.1 (13.8–16.8) |
| Boys / Girls | 79/33 |
| BMI Z-score | 1.36 ± 0.95 (0.77–2.04) |
| Duration of hypertension (months) | 14.6 ± 21.5 (3–14) |
| Duration of pregnancy (weeks) | 38.6 ± 2.8 (38–40) |
| Birth weight (g) | 3242 ± 636 (2920–3710) |
| eGFR acc. Schwartz formula (mL/min/1.73 m2) | 98 ± 20.5 (83.5–113.2) |
| Uric acid (mg/dL) | 5.9 ± 1.3 (5.2–6.8) |
| Sodium (mmol/L) | 142.7 ± 2.0 (141–144) |
| Potassium (mmol/L) | 4.5 ± 0.3 (4.3–4.7) |
| Total cholesterol (mg/dL) | 156.8 ± 25.9 (137–171) |
| LDL cholesterol (mg/dL) | 84.6 ± 22.4 (68–99) |
| HDL cholesterol (mg/dL) | 50.7 ± 13.2 (41–55) |
| Triglycerides (mg/dL) | 107.2 ± 52.3 (67–141) |
| Urinary albumin excretion (mg/24 h) | 34.2 ± 8.1 (5.3–20.7) |
| Urinary sodium excretion (mmol/kg/24 h) | 2.2 ± 1.2 (1.5–3.0) |
| Urinary potassium excretion (mmol/kg/24 h) * | 0.8 ± 0.3 (0.6–0.9) |
| Parameter | Study Group ± SD (IQR) |
|---|---|
| Peripheral office SBP [mmHg] | 133.9 ± 10.8 (126–143) |
| Peripheral office SBP Z-score | 1.68 ± 0.81 (1.06–2.20) |
| Peripheral office DBP [mmHg] | 79.3 ± 11.1 (73–85) |
| Peripheral office DBP Z-score | 2.01 ± 1.46 (1.17–2.48) |
| Central office SBP [mmHg] | 112.0 ± 9.3 (106–118) |
| Central office DBP [mmHg] | 81.0 ± 11.2 (74–87) |
| Central office MAP [mmHg] | 95.9 ± 10.0 (89–101) |
| 24 h ambulatory SBP [mmHg] | 133.2 ± 6.8 (129–138) |
| 24 h ambulatory DBP [mmHg] | 72.6 ± 6.6 (68–77) |
| 24 h ambulatory MAP [mmHg] | 92.7 ± 5.9 (88–97) |
| 24 h ambulatory MAP Z-score | 1.6 ± 1.3 (0.9–2.1) |
| 24 h ambulatory HR [bpm] | 79.6 ± 11.2 (72–89) |
| activity ambulatory SBP [mmHg] | 137.0 ± 7.3 (132–142) |
| activity ambulatory DBP [mmHg] | 75.8 ± 7.5 (71–80) |
| activity ambulatory MAP [mmHg] | 95.3 ± 10.9 (91–100) |
| activity ambulatory HR [bpm] | 83.4 ± 12.0 (76–93) |
| resting ambulatory SBP [mmHg] | 121.9 ± 8.6 (116–128) |
| resting ambulatory DBP [mmHg] | 63.1 ± 7.1 (59–67) |
| resting ambulatory MAP [mmHg] | 82.7 ± 6.9 (79–87) |
| resting ambulatory HR [bpm] | 68.9 ± 12.0 (62–76) |
| Systolic DIP [%] | 10.9 ± 5.9 (7.3–14.8) |
| Diastolic DIP [%] | 16.2 ± 8.5 (11.6–21.2) |
| Parameter | Study Group ± SD (IQR) |
|---|---|
| ECHO IVSd [mm] | 7.6 ± 1.8 (6–9) |
| ECHO LVPWd [mm] | 7.8 ± 1.7 (7–9) |
| ECHO LVEDd [mm] | 48.7 ± 5.3 (46–53) |
| ECHO LVM [g] | 168.3 ± 60.0 (121.6–205.2) |
| ECHO LVMI [g/m2.7] | 40.8 ± 11.4 (32.5–47.7) |
| aPWV [m/s] | 5.4 ± 0.9 (4.7–5.8) |
| aPWV Z-score | −0.01 ± 1.09 (−0.76–0.68) |
| AP [mm Hg] | −1.6 ± 4.9 (−4.0–0.0) |
| AIx [%] | −5.4 ± 14.7 (−13.7–1.0) |
| AIx75HR [%] | −2.9 ± 13.2 (−11.3–4.0) |
| Buckberg SEVR [%] | 165.5 ± 42.3 (134–194) |
| cIMT [mm] | 0.46 ± 0.07 (0.40–0.50) |
| cIMT Z-score | 1.28 ± 1.41 (0.16–2.29) |
| Analyzed Parameter | r | p |
|---|---|---|
| SBP DIP [%] vs. weight Z-score | −0.191 | 0.043 |
| SBP DIP [%] vs. BMI Z-score | −0.242 | 0.010 |
| SBP DIP [%] vs. urinary potassium excretion * [mmol/kg/24 h] | 0.702 | <0.001 |
| DBP DIP [%] vs. urinary potassium excretion * [mmol/kg/24 h] | 0.540 | 0.011 |
| Analyzed Parameter | r | p |
|---|---|---|
| SBP DIP [%] vs. AoSBP [mm Hg] | −0.144 | 0.252 |
| DBP DIP [%] vs. AoSBP [mm Hg] | −0.032 | 0.801 |
| SBP DIP [%] vs. LVMI [g/m2.7] | −0.395 | 0.006 |
| DBP DIP [%] vs. LVMI [g/m2.7] | −0.139 | 0.350 |
| SBP DIP [%] vs. aPWV Z-score | 0.071 | 0.584 |
| DBP DIP [%] vs. aPWV Z-score | 0.080 | 0.537 |
| SBP DIP [%] vs. AIx75HR [%] | 0.206 | 0.108 |
| DBP DIP [%] vs. AIx75HR [%] | 0.367 | 0.003 |
| SBP DIP [%] vs. cIMT Z-score | 0.150 | 0.235 |
| DBP DIP [%] vs. cIMT Z-score | 0.169 | 0.182 |
| SBP DIP vs. urinary albumin excretion [mg/24 h] | 0.058 | 0.573 |
| DBP DIP vs. urinary albumin excretion [mg/24 h] | −0.036 | 0.724 |
| Parameter | Non-Dippers | Dippers | Extreme Dippers | p |
|---|---|---|---|---|
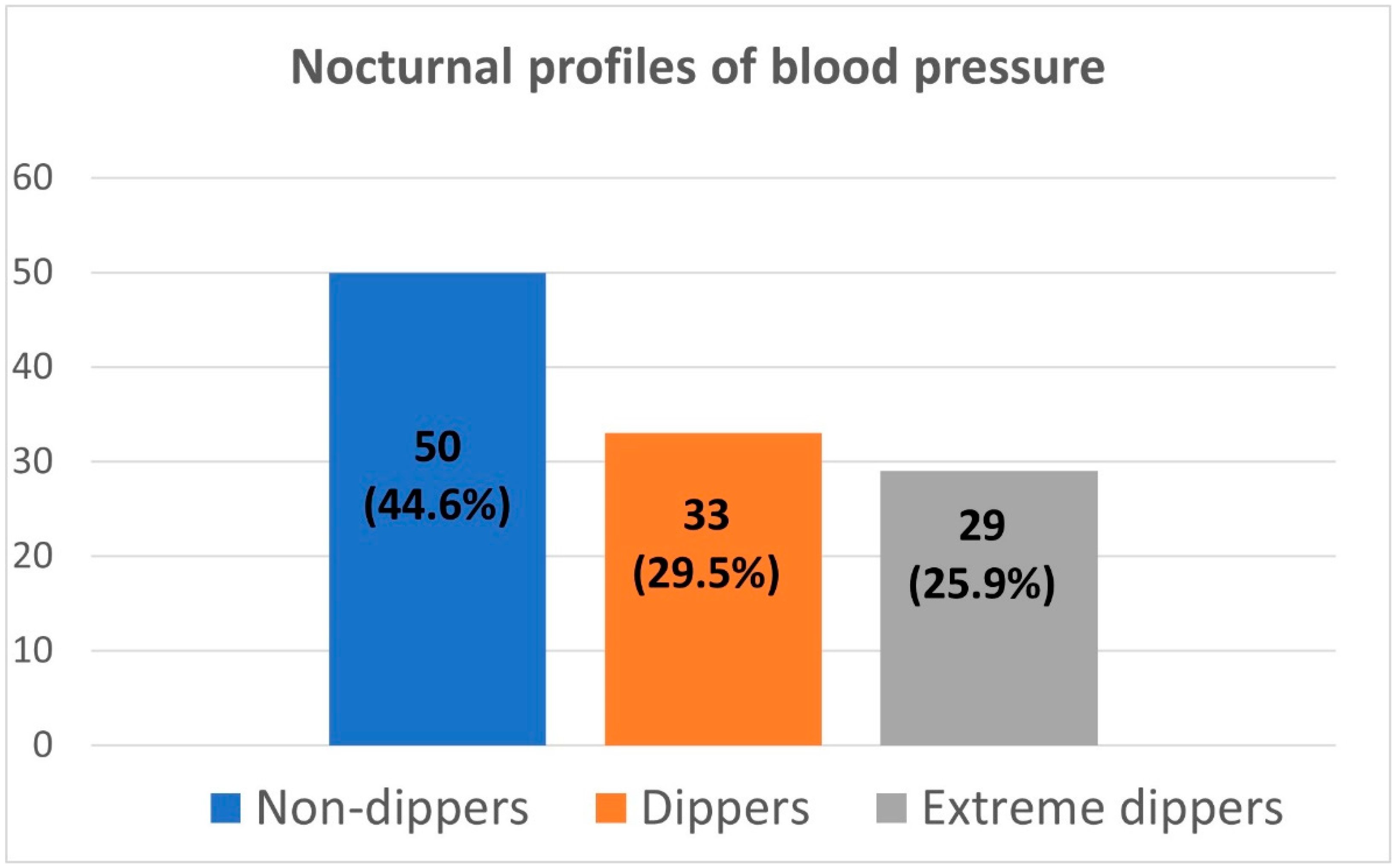
| Number of patients | 50 | 33 | 29 | - |
| Age [years] | 14.7 ± 2.5 | 14.9 ± 2.6 | 14.6 ± 3.3 | 0.910 |
| Sex (boys/girls) | 32/18 | 27/6 | 20/9 | 0.516 |
| BMI Z-score | 1.5 ± 1.1 | 1.2 ± 0.9 | 1.3 ± 0.9 | 0.298 |
| Duration of hypertension [month] | 15.6 ± 24.8 | 16.8 ± 22.8 | 10.5 ± 12.6 | 0.531 |
| Duration of pregnancy [weeks] | 37.9 ± 3.7 | 39.5 ± 1.4 | 38.3 ± 2.2 | 0.287 |
| Birth weight [g] | 3102 ± 676 | 3328 ± 660 | 3396 ± 499 | 0.436 |
| eGFR [mL/min/1.73 m2] | 99.4 ± 21.1 | 95.2 ± 17.0 | 98.7 ± 23.0 | 0.662 |
| Uric acid [mg/dL] | 6.0 ± 1.4 | 5.9 ± 1.2 | 5.8 ± 1.3 | 0.894 |
| Sodium [mmol/L] | 142.7 ± 1.9 | 142.9 ± 1.7 | 142.4 ± 2.4 | 0.624 |
| Potassium [mmol/L] | 4.5 ± 0.3 | 4.6 ± 0.4 | 4.4 ± 0.3 | 0.149 |
| Total cholesterol [mg/dL] | 152.9 ± 22.3 | 158.9 ± 25.5 | 161.1 ± 31.7 | 0.364 |
| LDL cholesterol [mg/dL] | 83.6 ± 21.4 | 81.4 ± 21.4 | 89.8 ± 25.2 | 0.360 |
| HDL cholesterol [mg/dL] | 48.5 ± 12.6 | 54.8 ± 15.9 | 50.2 ± 9.9 | 0.119 |
| Triglycerides [mg/dL] | 107.0 ± 48.8 | 107.3 ± 61.2 | 107.3 ± 49.1 | 1.000 |
| Urinary albumin excretion [mg/24 h] | 53.5 ± 11.2 | 10.4 ± 7.2 | 31.2 ± 5.9 | 0.083 |
| Urinary sodium excretion [mmol/kg/24 h] | 2.3 ± 1.2 | 2.2 ± 1.0 | 2.4 ± 1.3 | 0.846 |
| Urinary potassium excretion [mmol/kg/24 h] * | 0.6 ± 0.2 | 1.0 ± 0.4 | 1.2 ± 0.2 | <0.001 1 |
| Peripheral office SBP [mmHg] | 136.7 ± 10.5 | 132.3 ± 9.9 | 131.1 ± 9.9 | 0.183 |
| Peripheral office SBP Z-score | 2.00 ± 0.95 | 1.44 ± 0.65 | 1.49 ± 0.53 | 0.113 |
| Peripheral office DBP [mmHg] | 82.6 ± 12.7 | 76.8 ± 8.0 | 76.8 ± 10.9 | 0.108 |
| Peripheral office DBP Z-score | 2.62 ± 1.57 | 1.47 ± 1.01 | 1.82 ± 1.78 | 0.075 |
| Central office SBP [mmHg] | 114.8 ± 9.8 | 109.9 ± 7.6 | 110.0 ± 9.9 | 0.117 |
| Central office DBP [mmHg] | 84.4 ± 12.9 | 78.4 ± 8.2 | 78.6 ± 10.9 | 0.112 |
| Central office MAP [mmHg] | 98.7 ± 11.3 | 93.2 ± 8.0 | 94.4 ± 9.4 | 0.121 |
| 24 h ambulatory SBP [mmHg] | 132.6 ± 7.0 | 133.9 ± 7.0 | 133.6 ± 6.2 | 0.678 |
| 24 h ambulatory DBP [mmHg] | 72.3 ± 6.5 | 71.7 ± 6.1 | 74.1 ± 7.2 | 0.335 |
| 24 h ambulatory MAP [mmHg] | 5.1 ± 0.7 | 5.5 ± 1.1 | 5.1 ± 0.7 | 0.510 |
| 24 h ambulatory MAP Z-score | −0.2 ± 0.9 | 0.1 ± 1.2 | −0.2 ± 0.9 | 0.253 |
| 24 h ambulatory HR [bpm] | 81.3 ± 10.9 | 76.2 ± 10.9 | 80.5 ± 11.5 | 0.112 |
| ECHO LVMI [g/m2.7] | 44.5 ± 12.5 | 35.6 ± 10.1 | 37.9 ± 7.7 | 0.049 2 |
| aPWV [m/s] | 5.2 ± 0.7 | 5.6 ± 1.1 | 5.3 ± 0.9 | 0.213 |
| aPWV Z-score | −0.18 ± 0.92 | 0.17 ± 1.21 | 0.01 ± 1.23 | 0.527 |
| AIxHR75 [%] | −4.1 ± 13.7 | −5.1 ± 12.7 | 3.1 ± 12.1 | 0.027 1 |
| cIMT [mm] | 0.44 ± 0.07 | 0.46 ± 0.08 | 0.46 ± 0.05 | 0.484 |
| cIMT Z-score | 1.00 ± 1.36 | 1.37 ± 1.57 | 1.64 ± 1.24 | 0.357 |
| HMOD | BP Dipping | p | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|---|
| Left ventricular hypertrophy | SBP DIP | 0.061 | 0.896 | 0.799–1.005 |
| Left ventricular hypertrophy | DBP DIP | 0.326 | 0.966 | 0.900–1.036 |
| Abnormal aPWV | SBP DIP | 0.894 | 1.011 | 0.858–1.191 |
| Abnormal aPWV | DBP DIP | 0.622 | 1.027 | 0.924–1.141 |
| Abnormal AIx75HR | SBP DIP | 0.034 | 1.122 | 1.009–1.249 |
| Abnormal AIx75HR | DBP DIP | 0.015 | 1.095 | 1.017–1.177 |
| Abnormal cIMT | SBP DIP | 0.150 | 1.071 | 0.976–1.175 |
| Abnormal cIMT | DBP DIP | 0.359 | 1.029 | 0.968–1.092 |
| Abnormal UAE | SBP DIP | 0.304 | 0.956 | 0.878–1.041 |
| Abnormal UAE | DBP DIP | 0.448 | 0.978 | 0.922–1.037 |
| Any HMOD | SBP DIP | 0.408 | 1.028 | 0.963–1.097 |
| Any HMOD | DBP DIP | 0.258 | 1.027 | 0.981–1.075 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szyszka, M.; Skrzypczyk, P.; Ofiara, A.; Wabik, A.M.; Pietrzak, R.; Werner, B.; Pańczyk-Tomaszewska, M. Circadian Blood Pressure Profile in Pediatric Patients with Primary Hypertension. J. Clin. Med. 2022, 11, 5325. https://doi.org/10.3390/jcm11185325
Szyszka M, Skrzypczyk P, Ofiara A, Wabik AM, Pietrzak R, Werner B, Pańczyk-Tomaszewska M. Circadian Blood Pressure Profile in Pediatric Patients with Primary Hypertension. Journal of Clinical Medicine. 2022; 11(18):5325. https://doi.org/10.3390/jcm11185325
Chicago/Turabian StyleSzyszka, Michał, Piotr Skrzypczyk, Anna Ofiara, Anna Maria Wabik, Radosław Pietrzak, Bożena Werner, and Małgorzata Pańczyk-Tomaszewska. 2022. "Circadian Blood Pressure Profile in Pediatric Patients with Primary Hypertension" Journal of Clinical Medicine 11, no. 18: 5325. https://doi.org/10.3390/jcm11185325
APA StyleSzyszka, M., Skrzypczyk, P., Ofiara, A., Wabik, A. M., Pietrzak, R., Werner, B., & Pańczyk-Tomaszewska, M. (2022). Circadian Blood Pressure Profile in Pediatric Patients with Primary Hypertension. Journal of Clinical Medicine, 11(18), 5325. https://doi.org/10.3390/jcm11185325







