Infrarenal Remains Infrarenal—EVAR Suitability of Small AAA Is Rarely Compromised despite Morphological Changes during Surveillance
Abstract
1. Introduction
2. Materials and Methods
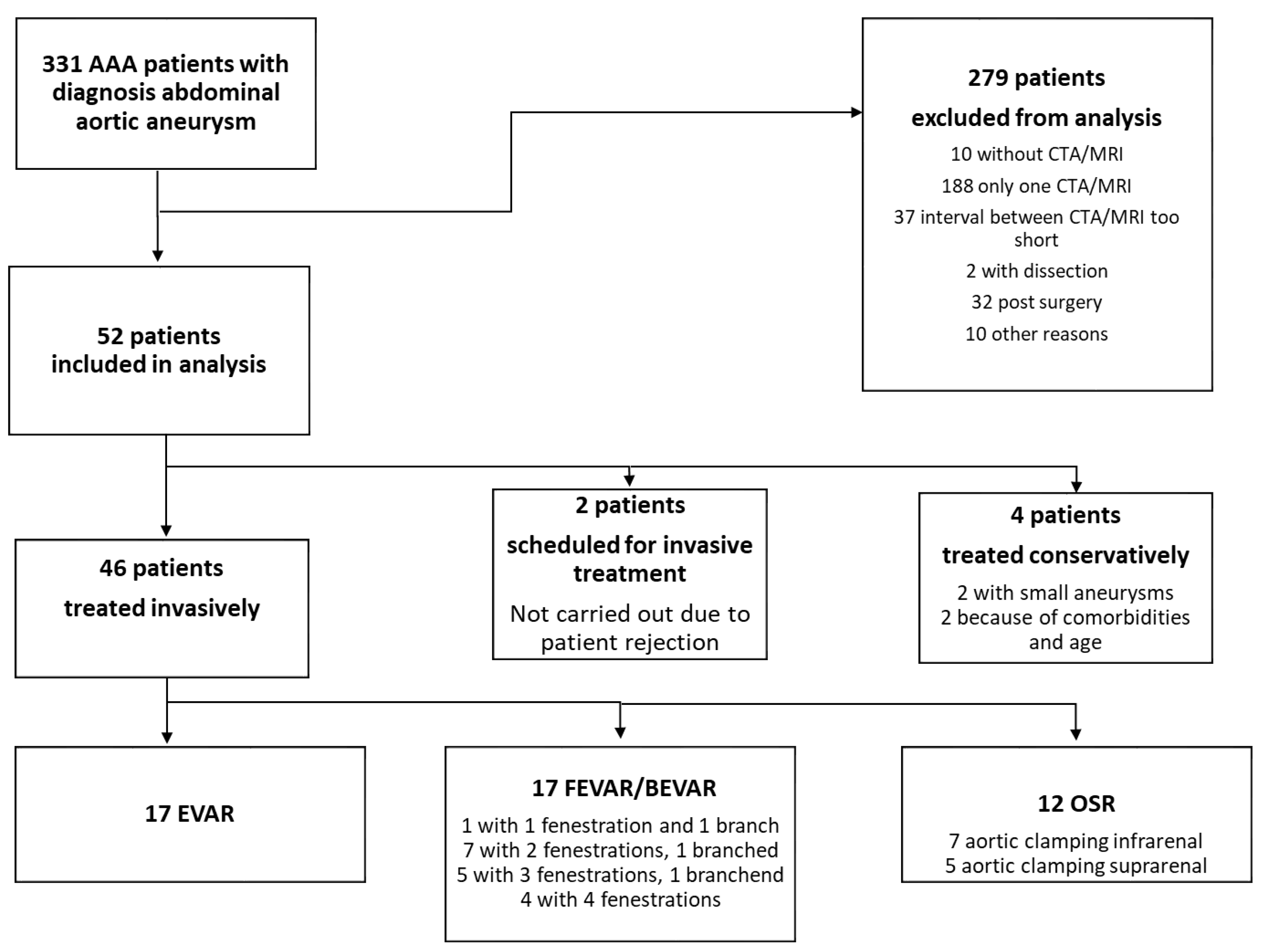
2.1. Definitions and Inclusion Criteria
2.2. Exclusion Criteria
2.3. Statistical Analysis
3. Results
3.1. Analysis of Morphological Parameters
3.2. Correlation Analysis
3.3. Types of Operative Therapy
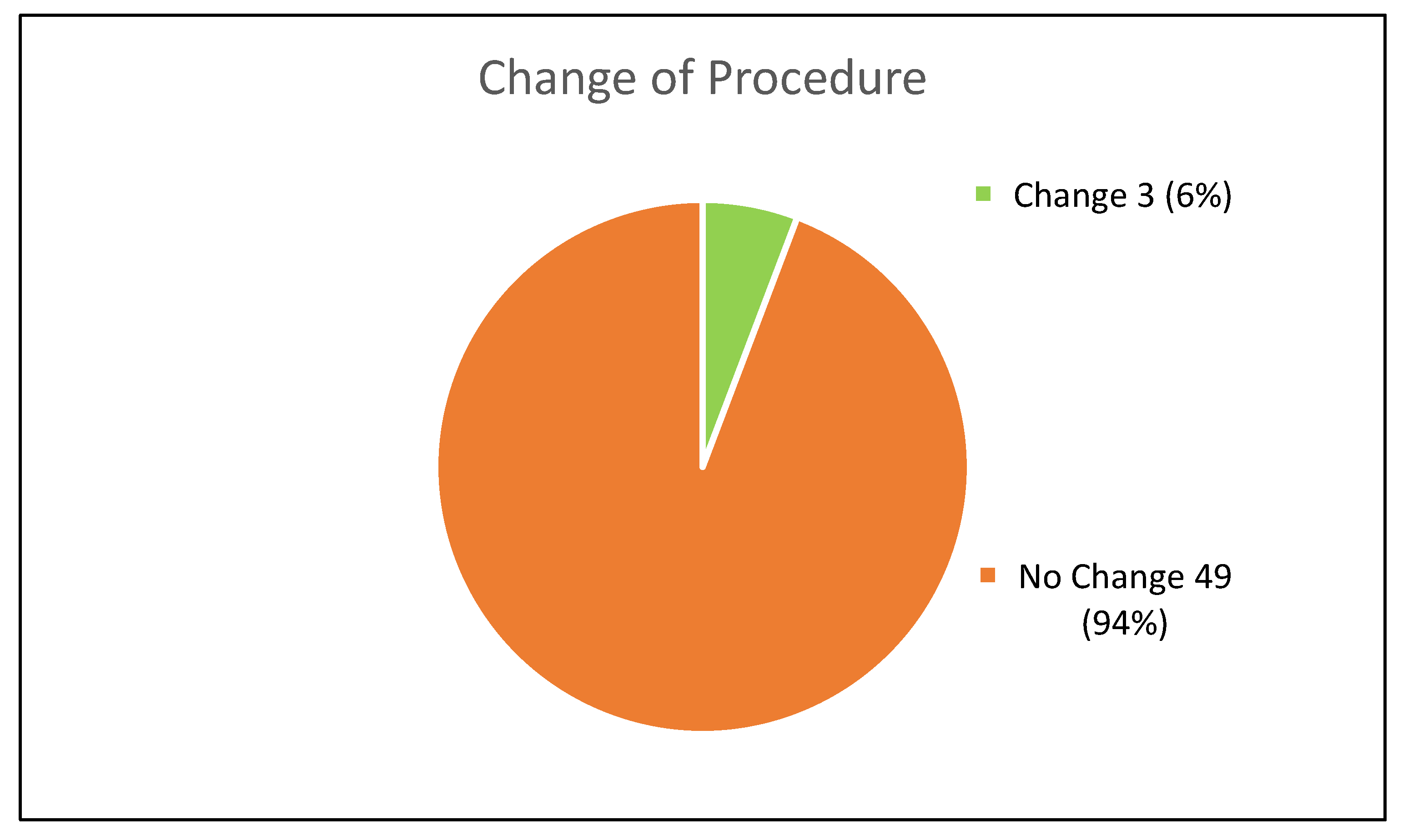
3.4. Procedural Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; Herwaarden, J.v.; Karkos, C.; Koelemay, M.; et al. European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Patel, R.; Sweeting, M.J.; Powell, J.T.; Greenhalgh, R.M. EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): A randomised controlled trial. Lancet 2016, 388, 2366–2374. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Muard, H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef] [PubMed]
- Debus, E.S.; Heidemann, F.; Gross-Fengels, W.; Mahlmann, A.; Muhl, E.; Pfister, K.; Roth, S.; Stroszczynski, C.; Walther, A.; Weiss, N.; et al. Kurzfassung S3-Leitlinie zu Screening, Diagnostik, Therapie und Nachsorge des Bauchaortenaneurysmas. Gefässchirurgie 2018, 23, 432–451. [Google Scholar] [CrossRef]
- van Rijswijk, R.E.; Jebbink, E.G.; Zeebregts, C.J.; Reijnen, M. A systematic review of anatomic predictors of abdominal aortic aneurysm remodeling after endovascular repair. J. Vasc. Surg. 2022, 75, 1777–1785. [Google Scholar] [CrossRef]
- Pini, R.; Faggioli, G.; Indelicato, G.; Gallitto, E.; Mascoli, C.; Abualhin, M.; Stella, A.; Gargiulo, M. Anatomical Predictors of Flared Limb Complications in Endovascular Aneurysm Repair. J. Endovasc. Ther. 2019, 26, 550–555. [Google Scholar] [CrossRef]
- Wortmann, M.; Bischoff, M.S.; Hakimi, M.; Böckler, D. How to overcome challenging iliac artery anatomy in endovascular repair for AAA. J. Cardiovasc. Surg. (Torino) 2018, 59, 4–13. [Google Scholar] [CrossRef]
- Schuurmann, R.C.L.; van Noort, K.; Overeem, S.P.; Ouriel, K.; Jordan, W.D.; Muhs, B.E., Jr.; Mannetje, Y.; Reijnen, M.; Fioole, B.; Ünlü, Ç.; et al. Aortic Curvature Is a Predictor of Late Type Ia Endoleak and Migration After Endovascular Aneurysm Repair. J. Endovasc. Ther. 2017, 24, 411–417. [Google Scholar] [CrossRef]
- Thompson, S.G.; Brown, L.C.; Sweeting, M.J.; Bown, M.J.; Kim, L.G.; Glover, M.J.; Buxton, M.; Powell, J. Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: Implications for surveillance intervals and their cost-effectiveness. Health Technol. Assess 2013, 17, 1–118. [Google Scholar] [CrossRef]
- Cronenwett, J.L.; Johnston, K.W. The United Kingdom Small Aneurysm Trial: Implications for surgical treatment of abdominal aortic aneurysms. J. Vasc. Surg. 1999, 29, 191–194. [Google Scholar] [CrossRef]
- Lederle, F.A.; Johnson, G.R.; Wilson, S.E.; Ballard, D.J.; Jordan, W.D.; Blebea, J., Jr. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA 2002, 287, 2968–2972. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Wilson, S.E.; Johnson, G.R.; Reinke, D.B.; Littooy, F.N.; Acher, C.W.; Ballard, D.J.; Messina, L.M.; Gordon, I.L.; Chute, E.P.; et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N. Engl. J. Med. 2002, 346, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.T.; Gotensparre, S.M.; Sweeting, M.J.; Brown, L.C.; Fowkes, F.G.; Thompson, S.G. Rupture rates of small abdominal aortic aneurysms: A systematic review of the literature. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [PubMed]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.C.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): Executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J. Am. Coll. Cardiol. 2006, 47, 1239–1312. [Google Scholar] [PubMed]
- Brown, P.M.; Zelt, D.T.; Sobolev, B. The risk of rupture in untreated aneurysms: The impact of size, gender, and expansion rate. J. Vasc. Surg. 2003, 37, 280–284. [Google Scholar] [CrossRef]
- Ulug, P.; Powell, J.T.; Martinez, M.A.; Ballard, D.J.; Filardo, G. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst. Rev. 2020, 7, Cd001835. [Google Scholar]
- Propper, B.W.; Rasmussen, T.E.; Jones, W.T.; Gifford, S.M.; Burkhardt, G.E.; Clouse, W.D. Temporal changes of aortic neck morphology in abdominal aortic aneurysms. J. Vasc. Surg. 2010, 51, 1111–1115. [Google Scholar] [CrossRef][Green Version]
- Welborn, M.B., 3rd; Yau, F.S.; Modrall, J.G.; Lopez, J.A.; Floyd, S.; Valentine, R.J.; Clagett, G.P. Endovascular repair of small abdominal aortic aneurysms: A paradigm shift? Vasc, Endovascular Surg. 2005, 39, 381–391. [Google Scholar] [CrossRef]
- Yau, F.S.; Rosero, E.B.; Clagett, G.P.; Valentine, R.J.; Modrall, G.J.; Arko, F.R.; Timaran, C.H. Surveillance of small aortic aneurysms does not alter anatomic suitability for endovascular repair. J. Vasc. Surg. 2007, 45, 96–100. [Google Scholar] [CrossRef][Green Version]
- Panthofer, A.M.; Olson, S.L.; Rademacher, B.L.; Grudzinski, J.K.; Chaikof, E.L.; Matsumura, J.S. Anatomic eligibility for endovascular aneurysm repair preserved over 2 years of surveillance. J. Vasc. Surg. 2021, 74, 1527–1536.e1. [Google Scholar] [CrossRef] [PubMed]
- Ouriel, K.; Tanquilut, E.; Greenberg, R.K.; Walker, E. Aortoiliac morphologic correlations in aneurysms undergoing endovascular repair. J. Vasc. Surg. 2003, 38, 323–328. [Google Scholar] [CrossRef]
- Boyd, A.J. Intraluminal thrombus: Innocent bystander or factor in abdominal aortic aneurysm pathogenesis? JVS Vasc. Sci. 2021, 2, 159–169. [Google Scholar] [CrossRef]
- Manenti, A.; Farinetti, A.; Manco, G.; Mattioli, A.V. Intraluminal Thrombus and Abdominal Aortic Aneurysm Complications. Ann. Vasc. Surg. 2022, 83, e11–e12. [Google Scholar] [CrossRef]
- Etienne, H.; Journe, C.; Rouchaud, A.; Senemaud, J.; Louedec, L.; Pellenc, Q.; Coscas, R.; Gouya, L.; Dupont, S.; Michel, J.-B. Persistence of Intraluminal Thrombus Makes Saccular Aneurysm More Biologically Active than Fusiform in an Experimental Rat Model. J. Vasc. Res. 2020, 57, 164–176. [Google Scholar] [CrossRef]
- Nchimi, A.; Courtois, A.; El Hachemi, M.; Touat, Z.; Drion, P.; Withofs, N.; Warnock, G.; Bahri, M.-A.; Dogné, J.-M.; Cheramy-Bien, J.-P.; et al. Multimodality imaging assessment of the deleterious role of the intraluminal thrombus on the growth of abdominal aortic aneurysm in a rat model. Eur. Radiol. 2016, 26, 2378–2386. [Google Scholar] [CrossRef]
- Nana, P.; Spanos, K.; Dakis, K.; Brodis, A.; Kouvelos, G. Imaging Predictive Factors of Abdominal Aortic Aneurysm Growth. J. Clin. Med. 2021, 10, 1917. [Google Scholar] [CrossRef]
- Martufi, G.; Lindquist Liljeqvist, M.; Sakalihasan, N.; Panuccio, G.; Hultgren, R.; Roy, J.; Gasser, T.C. Local Diameter, Wall Stress, and Thrombus Thickness Influence the Local Growth of Abdominal Aortic Aneurysms. J. Endovasc. Ther. 2016, 23, 957–966. [Google Scholar] [CrossRef]
- Domonkos, A.; Staffa, R.; Kubicek, L. Effect of intraluminal thrombus on growth rate of abdominal aortic aneurysms. Int. Angiol. 2019, 38, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Horvat, N.; Virag, L.; Karsaj, I. Mechanical role of intraluminal thrombus in aneurysm growth: A computational study. Biomech. Model. Mechanobiol. 2021, 20, 1819–1832. [Google Scholar] [CrossRef]
- Haller, S.J.; Crawford, J.D.; Courchaine, K.M.; Bohannan, C.J.; Landry, G.J.; Moneta, G.L.; Azarbal, A.F.; Rugonyi, S. Intraluminal thrombus is associated with early rupture of abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 1051–1058.e1. [Google Scholar] [CrossRef] [PubMed]
- Kontopodis, N.; Pantidis, D.; Dedes, A.; Daskalakis, N.; Ioannou, C.V. The-Not So-Solid 5.5 cm Threshold for Abdominal Aortic Aneurysm Repair: Facts, Misinterpretations, and Future Directions. Front. Surg. 2016, 3, 1. [Google Scholar] [CrossRef]
- Koncar, I.B.; Nikolic, D.; Milosevic, Z.; Ilic, N.; Dragas, M.; Sladojevic, M.; Markovic, M.; Filipovic, N.; Davidovic, L. Morphological and Biomechanical Features in Abdominal Aortic Aneurysm with Long and Short Neck-Case-Control Study in 64 Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2017, 45, 223–230. [Google Scholar] [CrossRef]
- Halloran, B.G.; Davis, V.A.; McManus, B.M.; Lynch, T.G.; Baxter, B.T. Localization of aortic disease is associated with intrinsic differences in aortic structure. J. Surg. Res. 1995, 59, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Gallitto, E.; Gargiulo, M.; Faggioli, G.; Pini, R.; Mascoli, C.; Freyrie, A.; Ancetti, S.; Stella, A. Impact of iliac artery anatomy on the outcome of fenestrated and branched endovascular aortic repair. J. Vasc. Surg. 2017, 66, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Sangha, G.S.; Busch, A.; Acuna, A.; Berman, A.G.; Phillips, E.H.; Trenner, M.; Eckstein, H.-H.; Maegdefessel, L.; Goergen, C.J. Effects of Iliac Stenosis on Abdominal Aortic Aneurysm Formation in Mice and Humans. J. Vasc. Res. 2019, 56, 217–229. [Google Scholar] [CrossRef]
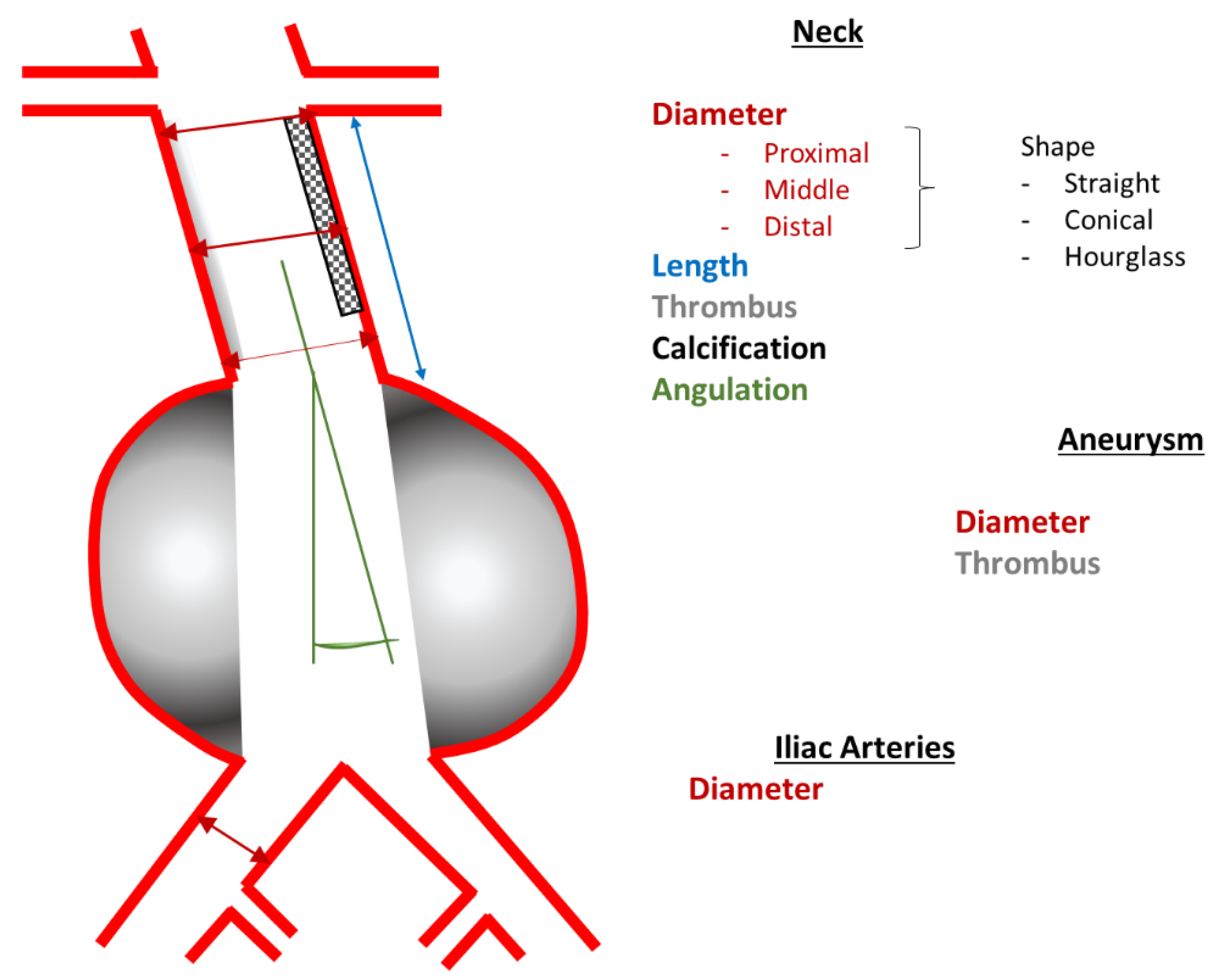
| Proximal Landing Zone, i.e., Aneurysm Neck | Shape | straight, conical, hourglass, kinked |
| Diameter [mm]: | outer wall to outer wall
| |
| Length [mm] | distal renal artery to aneurysm | |
| Morphology |
| |
| Angulation [°] | between neck and aneurysm | |
| Aneurysm | Type |
|
| Maximum diameter [mm] | outer wall to outer wall | |
| Thrombus | area in percent of the cross-section [%] | |
| Distal Landing Zone, i.e., Common Iliac Arteries | Maximum diameter [mm] | outer wall to outer wall |
| Age | Mean ± SD (Years) |
| First CTA/MRI 1 | 70 ± 8 |
| Last CTA/MRI 1 | 73 ± 8 |
| Sex | Patient number [n (%)] |
| Male | 46 (88) |
| Female | 6 (12) |
| Comorbidities | |
| Arterial hypertension | 37 (71) |
| Coronary heart disease | 34 (65) |
| Peripheral arterial occlusive disease | 17 (33) |
| Cerebrovascular pathologies | 11 (21) |
| First CTA/MRI 1 | Last CTA/MRI 1 | |
|---|---|---|
| Mean ± SD [mm] | Mean ± SD [mm] | |
| Maximum aneurysm diameter | 47.7 ± 9.3 | 56.3 ± 11.6 |
| patient number [n (%)] | patient number [n (%)] | |
| Aneurysm type | ||
| with neck | 35 (67) | 33 (63) |
| “no neck“ | 17 (33) | 19 (36) |
| Neck shape | ||
| straight | 28 (54) | 26 (50) |
| Conical | 2 (4) | 3 (6) |
| Hourglass | 1 (2) | 1 (2) |
| Bended | 4 (8) | 5 (10) |
| Neck thrombus present | 31 (60) | 32 (63) |
| Increased | N.A. 2 | 16 (31) |
| Unchanged | N.A. 2 | 6 (12) |
| Decreased | N.A. 2 | 9 (17) |
| New | N.A. 2 | 1 (2) |
| Aneurysm thrombus present | 46 (88) | 47 (90) |
| Increased | N.A. 2 | 32 (61) |
| Unchanged | N.A. 2 | 5 (10) |
| Decreased | N.A. 2 | 9 (17) |
| New | N.A. 2 | 1 (2) |
| Angulation | ||
| Increased | N.A. 2 | 41 (79) |
| Unchanged | N.A. 2 | 3 (6) |
| Decreased | N.A. 2 | 8 (15) |
| Diameter common iliac arteries > 20 mm | ||
| Overall | 18 (35) | 18 (35) |
| Unilateral | 10 (19) | 9 (17) |
| Bilateral | 8 (15) | 9 (17) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, C.; Bülow, T.; Gombert, A.; Kalder, J.; Keschenau, P.R. Infrarenal Remains Infrarenal—EVAR Suitability of Small AAA Is Rarely Compromised despite Morphological Changes during Surveillance. J. Clin. Med. 2022, 11, 5319. https://doi.org/10.3390/jcm11185319
Becker C, Bülow T, Gombert A, Kalder J, Keschenau PR. Infrarenal Remains Infrarenal—EVAR Suitability of Small AAA Is Rarely Compromised despite Morphological Changes during Surveillance. Journal of Clinical Medicine. 2022; 11(18):5319. https://doi.org/10.3390/jcm11185319
Chicago/Turabian StyleBecker, Corinna, Tanja Bülow, Alexander Gombert, Johannes Kalder, and Paula Rosalie Keschenau. 2022. "Infrarenal Remains Infrarenal—EVAR Suitability of Small AAA Is Rarely Compromised despite Morphological Changes during Surveillance" Journal of Clinical Medicine 11, no. 18: 5319. https://doi.org/10.3390/jcm11185319
APA StyleBecker, C., Bülow, T., Gombert, A., Kalder, J., & Keschenau, P. R. (2022). Infrarenal Remains Infrarenal—EVAR Suitability of Small AAA Is Rarely Compromised despite Morphological Changes during Surveillance. Journal of Clinical Medicine, 11(18), 5319. https://doi.org/10.3390/jcm11185319





