Prognostic Value of Preoperative Systemic Inflammatory Parameters in Advanced Gastric Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Clinicopathological Data
2.2. Preoperative Systemic Inflammatory Parameters
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Included Patients
3.2. Difference among Preoperative Systemic Inflammatory Parameters
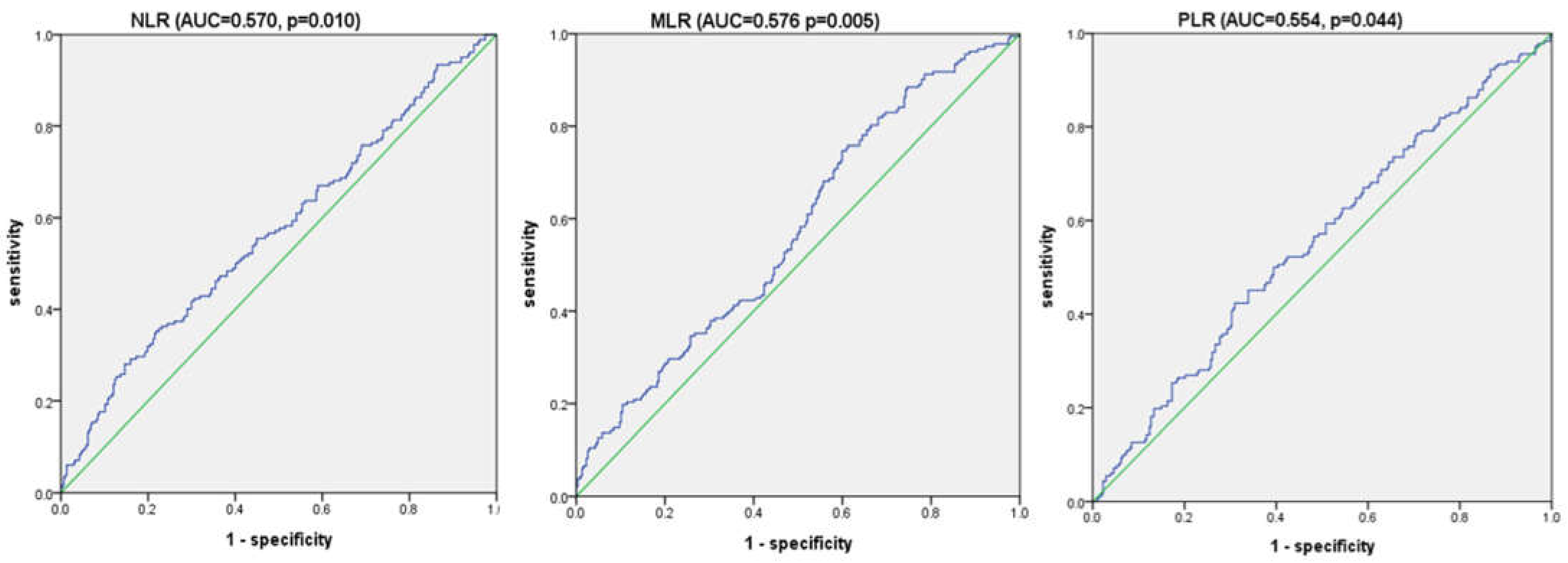
3.3. Receiver Operating Characteristic Curve Analysis for Overall Survival
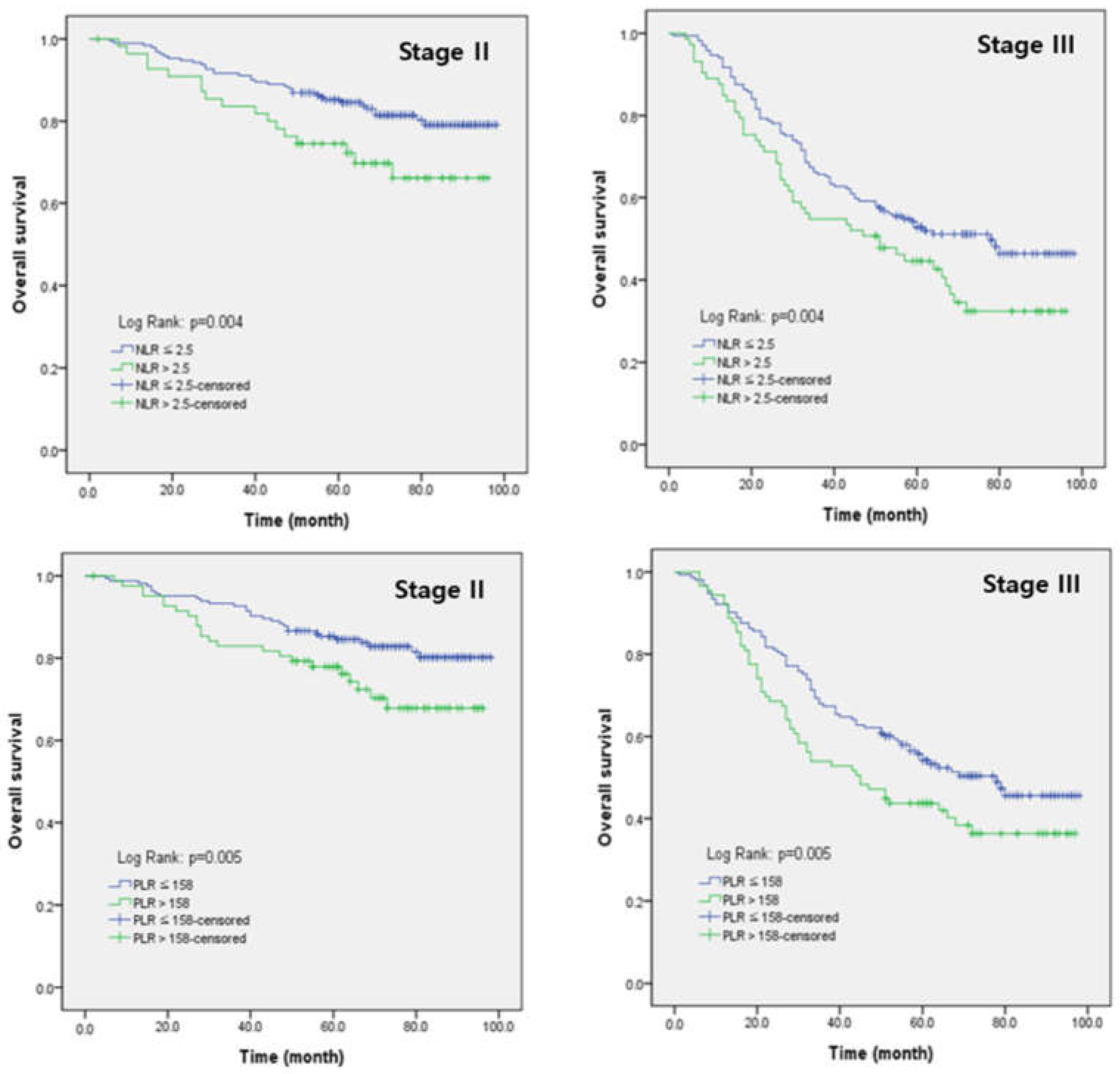
3.4. Univariate and Multivariate Analysis of Clinicopathological Factors for Overall Survival
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Information Committee of the Korean Gastric Cancer Association. Korean Gastric Cancer Association-Led Nationwide Survey on Surgically Treated Gastric Cancers in 2019. J. Gastric Cancer 2021, 21, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean Practice Guideline for Gastric Cancer 2018: An Evidence-based, Multi-disciplinary Approach. J. Gastric Cancer 2019, 19, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Röcken, C.; Behrens, H.M. Validating the prognostic and discriminating value of the TNM-classification for gastric cancer—A critical appraisal. Eur. J. Cancer 2015, 51, 577–586. [Google Scholar] [CrossRef]
- Feng, F.; Zheng, G.; Wang, Q.; Liu, S.; Liu, Z.; Xu, G.; Wang, F.; Guo, M.; Lian, X.; Zhang, H. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. 2018, 18, 148. [Google Scholar] [CrossRef]
- Guner, A.; Kim, S.Y.; Yu, J.E.; Min, I.K.; Roh, Y.H.; Roh, C.; Seo, W.J.; Cho, M.; Choi, S.; Choi, Y.Y.; et al. Parameters for Predicting Surgical Outcomes for Gastric Cancer Patients: Simple Is Better Than Complex. Ann. Surg. Oncol. 2018, 25, 3239–3247. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Takiguchi, N.; Kainuma, O.; Soda, H.; Ikeda, A.; Cho, A.; Miyazaki, A.; Gunji, H.; Yamamoto, H.; Nagata, M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer 2010, 13, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Fujii, T.; Yabusaki, N.; Murotani, K.; Iwata, N.; Kanda, M.; Tanaka, C.; Nakayama, G.; Sugimoto, H.; Koike, M.; et al. Clinical Implication of Inflammation-Based Prognostic Score in Pancreatic Cancer: Glasgow Prognostic Score Is the Most Reliable Parameter. Medicine 2016, 95, e3582. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.X.; Su, Z.J.; Zhang, J.H.; Wang, C.R.; Ke, S.Y. A comparison of the prognostic value of preoperative inflammation-based scores and TNM stage in patients with gastric cancer. OncoTargets Ther. 2015, 8, 1375–1385. [Google Scholar] [CrossRef]
- Cheng, C.B.; Zhang, Q.X.; Zhuang, L.P.; Sun, J.W. Prognostic value of lymphocyte-to-C-reactive protein ratio in patients with gastric cancer after surgery: A multicentre study. Jpn. J. Clin. Oncol. 2020, 50, 1141–1149. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Inoue, H.; Kosuga, T.; Kubota, T.; Konishi, H.; Shiozaki, A.; Okamoto, K.; Fujiwara, H.; Otsuji, E. Significance of a preoperative systemic immune-inflammation index as a predictor of postoperative survival outcomes in gastric cancer. World J. Surg. Oncol. 2021, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, R.; Inagawa, S.; Sano, N.; Tadano, S.; Adachi, S.; Yamamoto, M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur. J. Surg. Oncol. 2018, 44, 607–612. [Google Scholar] [CrossRef]
- Chen, X.D.; Mao, C.C.; Wu, R.S.; Zhang, W.T.; Lin, J.; Sun, X.W.; Chi, C.H.; Lou, N.; Wang, P.F.; Shen, X.; et al. Use of the combination of the preoperative platelet-to-lymphocyte ratio and tumor characteristics to predict peritoneal metastasis in patients with gastric cancer. PLoS ONE 2017, 12, e0175074. [Google Scholar] [CrossRef]
- Kim, E.Y.; Lee, J.W.; Yoo, H.M.; Park, C.H.; Song, K.Y. The Platelet-to-Lymphocyte Ratio Versus Neutrophil-to-Lymphocyte Ratio: Which is Better as a Prognostic Factor in Gastric Cancer? Ann. Surg. Oncol. 2015, 22, 4363–4370. [Google Scholar] [CrossRef]
- Pan, Y.C.; Jia, Z.F.; Cao, D.H.; Wu, Y.H.; Jiang, J.; Wen, S.M.; Zhao, D.; Zhang, S.L.; Cao, X.Y. Preoperative lymphocyte-to-monocyte ratio (LMR) could independently predict overall survival of resectable gastric cancer patients. Medicine 2018, 97, e13896. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, S.; Song, J.H.; Choi, S.; Cho, M.; Kwon, I.G.; Son, T.; Kim, H.I.; Cheong, J.H.; Hyung, W.J.; et al. Prognostic significance of body mass index and prognostic nutritional index in stage II/III gastric cancer. Eur. J. Surg. Oncol. 2020, 46 Pt A, 620–625. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Suzuki, S.; Akiyoshi, T.; Oba, K.; Otsuka, F.; Tominaga, T.; Nagasaki, T.; Fukunaga, Y.; Ueno, M. Comprehensive Comparative Analysis of Prognostic Value of Systemic Inflammatory Biomarkers for Patients with Stage II/III Colon Cancer. Ann. Surg. Oncol. 2020, 27, 844–852. [Google Scholar] [CrossRef]
- Roxburgh, C.S.; McMillan, D.C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010, 6, 149–163. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, K.; Guo, M.; Long, J.; Liu, L.; Liu, C.; Xu, J.; Ni, Q.; Luo, G.; Yu, X. Prognostic Value of the CRP/Alb Ratio, a Novel Inflammation-Based Score in Pancreatic Cancer. Ann. Surg. Oncol. 2017, 24, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhu, J.; Zhao, L.; Mai, K.; Ye, J.; Huang, S.; Zhao, Y. Lymphocyte to monocyte ratio and neutrophil to lymphocyte ratio are superior inflammation-based predictors of recurrence in patients with hepatocellular carcinoma after hepatic resection. J. Surg. Oncol. 2017, 115, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Angin, Y.S.; Yildirim, M.; Dasiran, F.; Okan, I. Could lymphocyte to C-reactive protein ratio predict the prognosis in patients with gastric cancer? ANZ J. Surg. 2021, 91, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, N.; Wang, S.; Guo, J.; Song, X.; Qi, Y.; Qiu, W.; Lv, J. Prognostic significance of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in patients with metastatic gastric cancer. Medicine 2020, 99, e19405. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wang, S.; Wang, S.; Huang, L. Elevated baseline circulating platelet-to-lymphocyte ratio and survival in initial stage IV gastric cancer patients: A meta-analysis. PLoS ONE 2022, 17, e0265897. [Google Scholar] [CrossRef]
- Qian, C.; Cai, R.; Zhang, W.; Wang, J.; Hu, X.; Zhang, Y.; Jiang, B.; Yuan, H.; Liu, F. Neutrophil-Lymphocyte Ratio and Circulating Tumor Cells Counts Predict Prognosis in Gastrointestinal Cancer Patients. Front. Oncol. 2021, 11, 710704. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, S.; Liu, Y.; Zhai, L.; Sun, X. Prognostic value of systemic inflammatory markers in ovarian Cancer: A PRISMA-compliant meta-analysis and systematic review. BMC Cancer 2018, 18, 443. [Google Scholar] [CrossRef]
- Heras, P.; Hatzopoulos, A.; Kritikos, N.; Kritikos, K. Platelet count and tumor progression in gastric cancer patients. Scand. J. Gastroenterol. 2010, 45, 1005–1006. [Google Scholar] [CrossRef]
- Yu, P.C.; Long, D.; Liao, C.C.; Zhang, S. Association between density of tumor-infiltrating lymphocytes and prognoses of patients with gastric cancer. Medicine 2018, 97, e11387. [Google Scholar] [CrossRef]
- Lee, J.S.; Won, H.S.; Sun, S.; Hong, J.H.; Ko, Y.H. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: A systematic review and meta-analysis. Medicine 2018, 97, e11769. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 489) | |
|---|---|---|
| Age | <65 | 296 (60.5%) |
| ≥65 | 193 (39.5%) | |
| Gender | Male | 334 (68.3%) |
| Female | 155 (31.7%) | |
| BMI | Mean ± SD | 23.5 ± 3.5 |
| Tumor location | Upper | 110 (22.5%) |
| Middle | 208 (42.5%) | |
| Lower | 171 (35.0%) | |
| Tumor size (cm) | Mean ± SD | 5.93 ± 3.3 |
| Differentiation | Differentiated | 99 (20.2%) |
| Undifferentiated | 390 (79.8%) | |
| T stage | 1/2 | 110 (22.5%) |
| 3/4 | 379 (77.5%) | |
| N stage | 0/1 | 227 (46.4%) |
| 2/3 | 262 (53.6%) | |
| TNM stage | II | 247 (50.5%) |
| III | 242 (49.5%) | |
| Operation | ||
| Extent of resection | STG | 309 (63.2%) |
| TG | 180 (36.8%) | |
| Approach | Laparoscopy | 118 (24.1%) |
| Open | 371 (75.9%) | |
| LND | <D2 | 54 (11.0%) |
| ≥D2 | 435 (89.0%) | |
| Harvested lymph nodes | Mean ± SD | 50.0 ± 16.1 |
| Complications | No | 402 (82.2%) |
| Yes | 87 (17.8%) | |
| Hospital stay | Mean ± SD | 9.71 ± 10.0 |
| Adjuvant chemotherapy | No | 59 (12.1%) |
| Yes | 430 (87.9%) | |
| Survival | Survivor | 307 (62.8%) |
| Non-survivor | 182 (37.2%) |
| Variables | Survivor (n = 307) | Non-Survivor (n = 182) | p-Value |
|---|---|---|---|
| WBC (103/uL) | 6.61 ± 1.91 | 6.47 ± 1.90 | 0.427 |
| Neutrophil (103/uL) | 3.95 ± 1.54 | 3.97 ± 1.53 | 0.891 |
| Lymphocyte (103/uL) | 2.07 ± 0.62 | 1.88 ± 0.63 | 0.001 |
| Monocyte (103/uL) | 0.43 ± 0.16 | 0.44 ± 0.19 | 0.463 |
| Platelet (103/uL) | 278.1 ± 89.3 | 262.4 ± 68.8 | 0.030 |
| Albumin (g/dL) | 4.44 ± 0.40 | 4.46 ± 0.40 | 0.771 |
| NLR | 2.00 ± 0.85 | 2.30 ± 1.11 | 0.003 |
| MLR | 0.22 ± 0.09 | 0.25 ± 0.13 | 0.002 |
| PLR | 144.4 ± 59.6 | 154.0 ± 60.8 | 0.086 |
| PNI | 24.2 ± 7.7 | 24.3 ± 7.6 | 0.879 |
| Variables | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Age(year) | ≥65 | 1.658 (1.240–2.218) | 0.001 | 1.715 (1.265–2.324) | 0.001 |
| Tumor size(cm) | ≥5.45 | 1.877 (1.394–2.527) | 0.000 | 1.433 (1.055–1.945) | 0.021 |
| Depth of invasion | ≥T3 | 2.067 (1.355–3.153) | 0.001 | 2.349 (1.520–3.629) | 0.000 |
| LN metastasis | ≥N2 | 2.540 (1.844–3.498) | 0.000 | 2.633 (1.896–3.657) | 0.000 |
| LND | <D2 | 1.185 (0.727–1.925) | 0.499 | ||
| Adjuvant Chemotherapy | yes | 0.590 (0.387–0.898) | 0.014 | 0.602 (0.396–0.914) | 0.017 |
| NLR | >2.5 | 1.708 (1.258–2.318) | 0.001 | 1.459 (1.070–1.989) | 0.017 |
| PLR | >158 | 1.553 (1.157–2.085) | 0.003 | 1.504 (1.115–2.029) | 0.008 |
| MLR | >0.2 | 1.315 (0.970–1.783) | 0.078 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.G.; Eom, B.W.; Yoon, H.; Kim, Y.-W.; Ryu, K.W. Prognostic Value of Preoperative Systemic Inflammatory Parameters in Advanced Gastric Cancer. J. Clin. Med. 2022, 11, 5318. https://doi.org/10.3390/jcm11185318
Kim SG, Eom BW, Yoon H, Kim Y-W, Ryu KW. Prognostic Value of Preoperative Systemic Inflammatory Parameters in Advanced Gastric Cancer. Journal of Clinical Medicine. 2022; 11(18):5318. https://doi.org/10.3390/jcm11185318
Chicago/Turabian StyleKim, Sung Gon, Bang Wool Eom, Hongman Yoon, Young-Woo Kim, and Keun Won Ryu. 2022. "Prognostic Value of Preoperative Systemic Inflammatory Parameters in Advanced Gastric Cancer" Journal of Clinical Medicine 11, no. 18: 5318. https://doi.org/10.3390/jcm11185318
APA StyleKim, S. G., Eom, B. W., Yoon, H., Kim, Y.-W., & Ryu, K. W. (2022). Prognostic Value of Preoperative Systemic Inflammatory Parameters in Advanced Gastric Cancer. Journal of Clinical Medicine, 11(18), 5318. https://doi.org/10.3390/jcm11185318




