Does Influenza Vaccination Reduce the Risk of Contracting COVID-19?
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Statistical Analysis
3. Ethical Considerations
4. Results
4.1. Relationship between Vaccination against Flu and SARS-CoV-2 Infection
4.2. SARS-CoV-2 Infection Levels According to Flu Risk Group and Vaccination against Flu
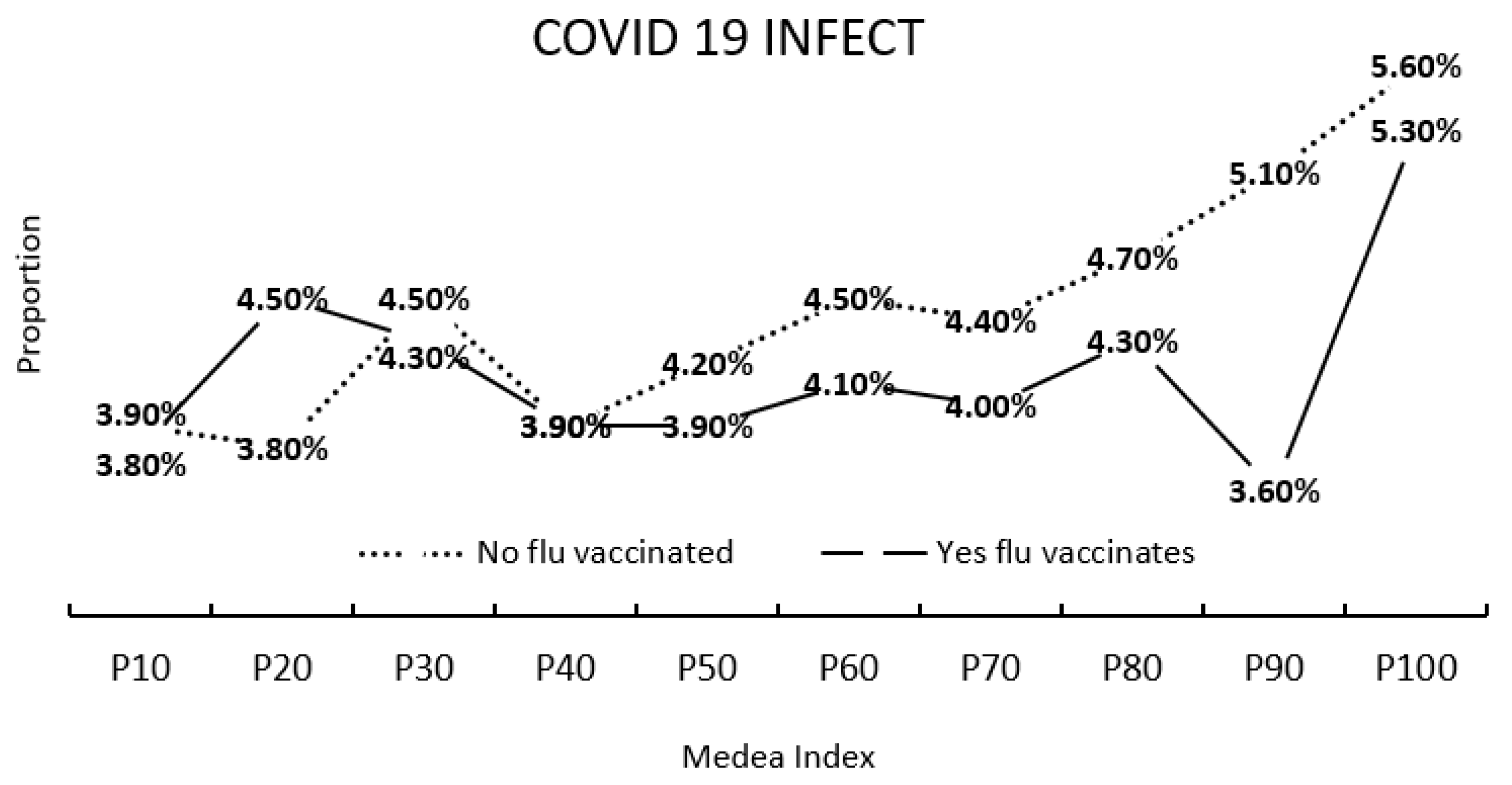
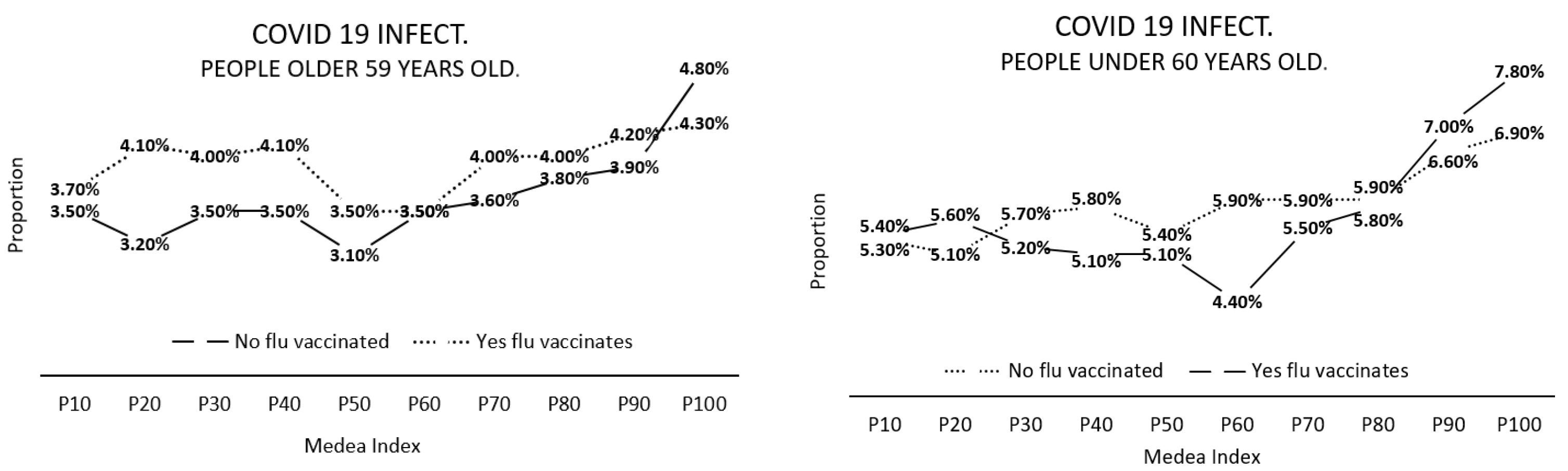
4.3. Relationships between the MEDEA Deprivation Index, Flu Vaccination and SARS-CoV-2 Infection
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhang, X.; Chen, X.; Zhang, Z.; Roy, A.; Shen, Y. Strategies to trace back the origin of COVID-19. J. Infect. 2020, 80, e39–e40. [Google Scholar] [CrossRef] [PubMed]
- Tatar, M.; Shoorekchali, J.M.; Faraji, M.R.; Wilson, F.A. International COVID-19 vaccine inequality amid the pandemic: Perpetuating a global crisis? J. Glob. Health 2021, 11, 03086. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.F. Social and economic inequality in coronavirus disease 2019 vaccination coverage across Illinois counties. Sci. Rep. 2021, 11, 18443. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G. COVID-19: Stigmatising the unvaccinated is not justified. Lancet Infect. Dis. 2021, 398, 1871. [Google Scholar] [CrossRef]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2021, 22, 183–195. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. An Agency of the European Union. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 7 September 2021).
- Yu, P.; Zhu, J.; Zhang, Z.; Han, Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J. Infect. Dis. 2020, 221, 1757–1761. [Google Scholar] [CrossRef]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. Available online: https://www.nejm.org/doi/full/10.1056/NEJMc2001468 (accessed on 21 December 2021). [CrossRef]
- Keilman, L.J. Seasonal Flu (Flu). Nurs. Clin. N. Am. 2019, 54, 227–243. [Google Scholar] [CrossRef]
- Coleman, B.L.; Fadel, S.; Fitzpatrick, T.; Thomas, S.-M. Risk factors for serious outcomes associated with flu illness in high- versus low- and middle-income countries: Systematic literature review and meta-analysis. Flu Other Respir. Viruses 2018, 12, 22–29. [Google Scholar] [CrossRef]
- World Health Organization. Vaccines against flu. Relev. Epidemiol. Hebd. 2012, 87, 461–476. [Google Scholar]
- Loerbroks, A.; Stock, C.; Bosch, J.A.; Litaker, D.G.; Apfelbacher, C.J. Flu vaccination coverage among high-risk groups in 11 European countries. Eur. J. Public Health 2012, 22, 562–568. [Google Scholar] [CrossRef]
- Costantino, C.; Vitale, F. Flu vaccination in high-risk groups: A revision of existing guidelines and rationale for an evidence-based preventive strategy. J. Prev. Med. Hyg. 2016, 57, E13–E18. [Google Scholar] [PubMed]
- Mereckiene, J.; Cotter, S.; Weber, J.T.; Nicoll, A.; D’Ancona, F.; Lopalco, P.L.; Johansen, K.; Wasley, A.M.; Jorgensen, P.; Lévy-Bruhl, D.; et al. Flu A(H1N1)pdm09 vaccination policies and coverage in Europe. Eurosurveillance 2012, 17, 20064. Available online: https://www.eurosurveillance.org/content/10.2807/ese.17.04.20064-en (accessed on 21 December 2021). [CrossRef] [PubMed]
- Rizzo, C.; Rezza, G.; Ricciardi, W. Strategies in recommending flu vaccination in Europe and US. Hum. Vaccines Immunother. 2018, 14, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Khanijahani, A.; Iezadi, S.; Gholipour, K.; Azami-Aghdash, S.; Naghibi, D. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int. J. Equity Health 2021, 20, 248. [Google Scholar] [CrossRef]
- Martins, L.D.; da Silva, I.; Batista, W.V.; de Fátima Andrade, M.; de Freitas, E.D.; Martins, J.A. How socio-economic and atmospheric variables impact COVID-19 and flu outbreaks in tropical and subtropical regions of Brazil. Environ. Res. 2020, 191, 110184. [Google Scholar] [CrossRef]
- Mendelson, M. Could enhanced flu and pneumococcal vaccination programs help limit the potential damage from SARS-CoV-2 to fragile health systems of southern hemisphere countries this winter? Int. J. Infect. Dis. 2020, 94, 32–33. [Google Scholar] [CrossRef]
- Li, Q.; Tang, B.; Bragazzi, N.L.; Xiao, Y.; Wu, J. Modeling the impact of mass flu vaccination and public health interventions on COVID-19 epidemics with limited detection capability. Math. Biosci. 2020, 325, 108378. [Google Scholar] [CrossRef]
- Pan American Health Organization. The Immunization Program. In The Context of the COVID-19 Pandemic. Version 2: 24 April 2020; Pan American Health Organization (PAHO): Washington, DC, USA, 2020. [Google Scholar]
- World Health Organization. United Nations Children’s Fund. In Immunization in the Context of COVID-19 Pandemic: Frequently Asked Questions (FAQ), 16 April 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Venkatakrishnan, A.; Anand, P.; Lenehan, P.; Suratekar, R.; Raghunathan, B.; Niesen, M.J.; Soundararajan, V. Omicron variant of SARS-CoV-2 harbors a unique insertion mutation of putative viral or human genomic origin. OSF Prepr. 2021. [Google Scholar] [CrossRef]
- Vila-Córcoles, A.; Ochoa-Gondar, O.; Satué-Gracia, E.M.; Torrente-Fraga, C.; Gomez-Bertomeu, F.; Vila-Rovira, A.; Hospital-Guardiola, I.; De Diego-Cabanes, C.; Bejarano-Romero, F.; Basora-Gallisà, J. Influence of prior comorbidities and chronic medications use on the risk of COVID-19 in adults: A population-based cohort study in Tarragona, Spain. BMJ Open 2020, 10, e041577. [Google Scholar] [CrossRef]
- Amato, M.; Werba, J.P.; Frigerio, B.; Coggi, D.; Sansaro, D.; Ravani, A.; Ferrante, P.; Veglia, F.; Tremoli, E.; Baldassarre, D. Relationship between Flu Vaccination Coverage Rate and COVID-19 Outbreak: An Italian Ecological Study. Vaccines 2020, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Del Riccio, M.; Lorini, C.; Bonaccorsi, G.; Paget, J.; Caini, S. The Association between Flu Vaccination and the Risk of SARS-CoV-2 Infection, Severe Illness, and Death: A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 7870. [Google Scholar] [CrossRef] [PubMed]
- Noale, M.; Trevisan, C.; Maggi, S.; Incalzi, R.A.; Pedone, C.; Di Bari, M.; Adorni, F.; Jesuthasan, N.; Sojic, A.; Galli, M.; et al. The Association between Flu and Pneumococcal Vaccinations and SARS-Cov-2 Infection: Data from the EPICOVID19 Web-Based Survey. Vaccines 2020, 8, 471. [Google Scholar] [CrossRef]
- Pawlowski, C.; Puranik, A.; Bandi, H.; Venkatakrishnan, A.J.; Agarwal, V.; Kennedy, R.; O’Horo, J.C.; Gores, G.J.; Williams, A.W.; Halamka, J.; et al. Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. Sci. Rep. 2021, 11, 4741. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Berjón, M.F.; Borrell, C.; Cano-Serral, G.; Esnaola, S. Construcción de un índice de privación a partir de datos censales en grandes ciudades españolas (Proyecto MEDEA). Gac Sanit. 2008, 22, 179–187. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 16 December 2021).
- Conlon, A.; Ashur, C.; Washer, L.; Eagle, K.A.; Bowman, M.A.H. Impact of the flu vaccine on COVID-19 infection rates and severity. Am. J. Infect. Control 2021, 49, 694–700. [Google Scholar] [CrossRef]
- Debisarun, P.A.; Gössling, K.L.; Bulut, O.; Kilic, G.; Zoodsma, M.; Liu, Z.; Oldenburg, M.; Rüchel, N.; Zhang, B.; Xu, C.-J.; et al. Induction of trained immunity by flu vaccination-impact on COVID-19. PLOS Pathog. 2021, 17, e1009928. [Google Scholar] [CrossRef]
- WHO. Recommended Composition of Influenza Virus Vaccines for Use in the 2020–2021 Northern Hemisphere Influenza Season. Available online: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2020-2021-northern-hemisphere-influenza-seasonnorthern-hemisphere-influenza-season (accessed on 20 October 2021).
- Huang, K.; Lin, S.-W.; Sheng, W.-H.; Wang, C.-C. Influenza vaccination and the risk of COVID-19 infection and severe illness in older adults in the United States. Sci. Rep. 2021, 11, 11025. [Google Scholar] [CrossRef]
- Fine, P.E.M.; Chen, R.T. Confounding in Studies of Adverse Reactions to Vaccines. Am. J. Epidemiology 1992, 136, 121–135. [Google Scholar] [CrossRef]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Ssentongo, P.; Ssentongo, A.E.; Heilbrunn, E.S.; Ba, D.M.; Chinchilli, V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0238215. [Google Scholar] [CrossRef] [PubMed]
- Liotta, G.; Marazzi, M.C.; Orlando, S.; Palombi, L. Is social connectedness a risk factor for the spreading of COVID-19 among older adults? The Italian paradox. PLoS ONE 2020, 15, e0233329. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.E.; Brown, C.S.; Nguyen-Van-Tam, J.S. Flu in long-term care facilities. Influenza Other Respir. Viruses 2017, 11, 356–366. [Google Scholar] [CrossRef]
- Jordan, R.E.; Hawker, J.I. Flu in elderly people in care homes. BMJ 2006, 333, 1229–1230. [Google Scholar] [CrossRef][Green Version]
- Monto, A.S.; Hornbuckle, K.; Ohmit, S.E. Flu Vaccine Effectiveness among Elderly Nursing Home Residents: A Cohort Study. Am. J. Epidemiol. 2001, 154, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Mena, G.E.; Martinez, P.P.; Mahmud, A.S.; Marquet, P.A.; Buckee, C.O.; Santillana, M. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science 2021, 28, 372. [Google Scholar] [CrossRef] [PubMed]
- Riou, J.; Panczak, R.; Althaus, C.L.; Junker, C.; Perisa, D.; Schneider, K.; Criscuolo, N.G.; Low, N.; Egger, M. Socioeconomic position and the COVID-19 care cascade from testing to mortality in Switzerland: A population-based analysis. Lancet Public Health 2021, 6, e683–e691. [Google Scholar] [CrossRef]
- Hawkins, R.; Charles, E.; Mehaffey, J. Socio-economic status and COVID-19–related cases and fatalities. Public Health 2020, 189, 129–134. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Tang, W.; Hatef, E.; Kitchen, C.; Weiner, J.P.; Kharrazi, H. Differential impact of mitigation policies and socioeconomic status on COVID-19 prevalence and social distancing in the United States. BMC Public Health 2021, 21, 1140. [Google Scholar] [CrossRef]
- Morrissey, K.; Spooner, F.; Salter, J.; Shaddick, G. Area level deprivation and monthly COVID-19 cases: The impact of government policy in England. Soc. Sci. Med. 2021, 289, 114413. [Google Scholar] [CrossRef]
- Plotkin, S.A. Updates on immunologic correlates of vaccine-induced protection. Vaccine 2020, 38, 2250–2257. [Google Scholar] [CrossRef] [PubMed]
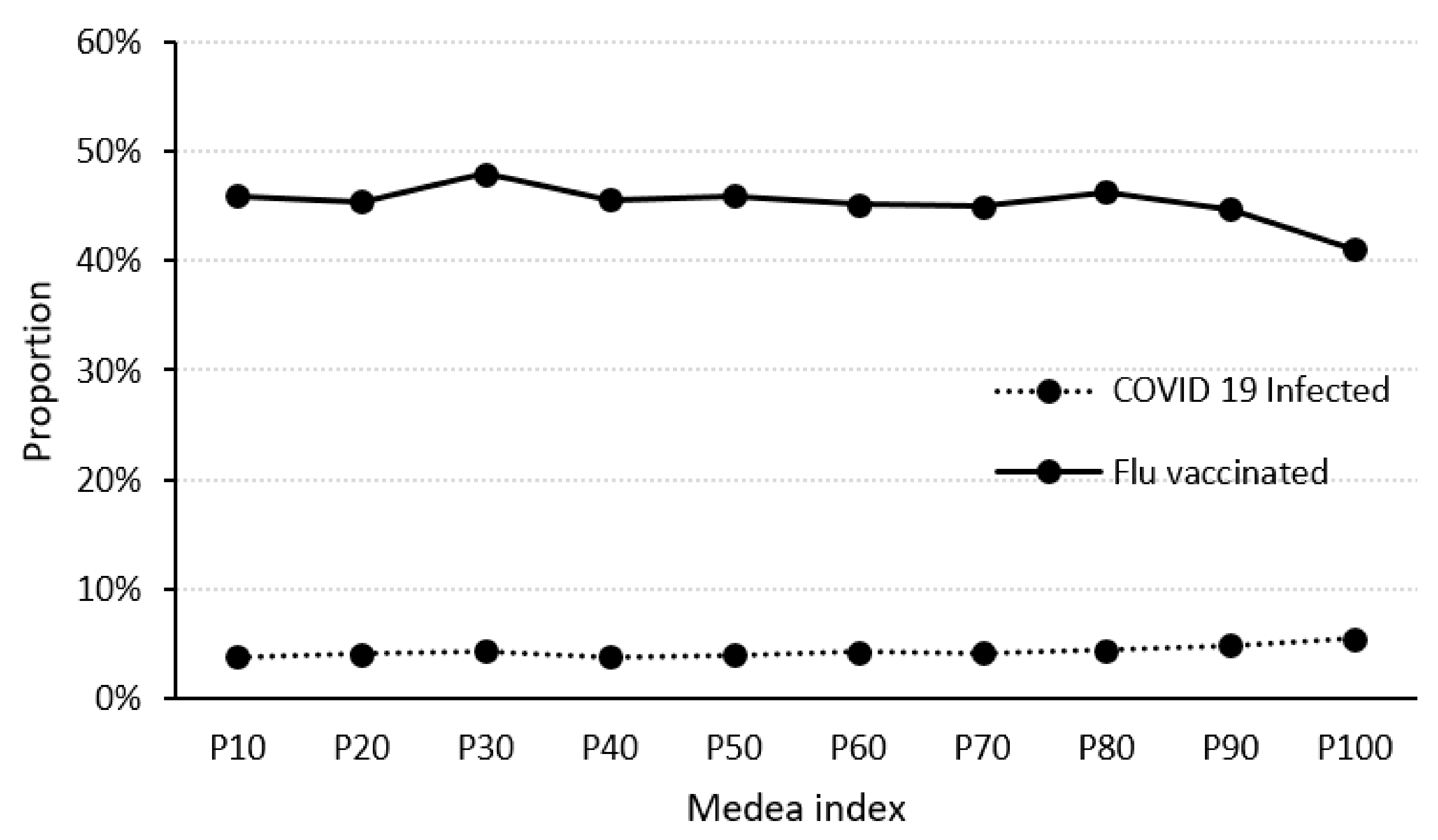
| Age | Sample Size | Vaccinated against Flu (%) | Vaccinated Women (%) |
|---|---|---|---|
| 0–14 | 8348 | 49.4 | 48 |
| 15–20 | 5578 | 21.3 | 23.2 |
| 21–30 | 12,540 | 16.1 | 21 |
| 31–40 | 18,991 | 21.9 | 27.2 |
| 41–50 | 31,466 | 25 | 28.1 |
| 51–60 | 58,278 | 31.5 | 33.9 |
| 61–70 | 122,390 | 43.1 | 43.6 |
| 71–80 | 100,797 | 58 | 56.8 |
| >80 | 71,149 | 61.9 | 59.8 |
| Total | 429,537 | 44.95 | 56.8 |
| Probability of SARS-CoV-2 Infection | p-Value | ||
|---|---|---|---|
| No FVS | Yes FVS | ||
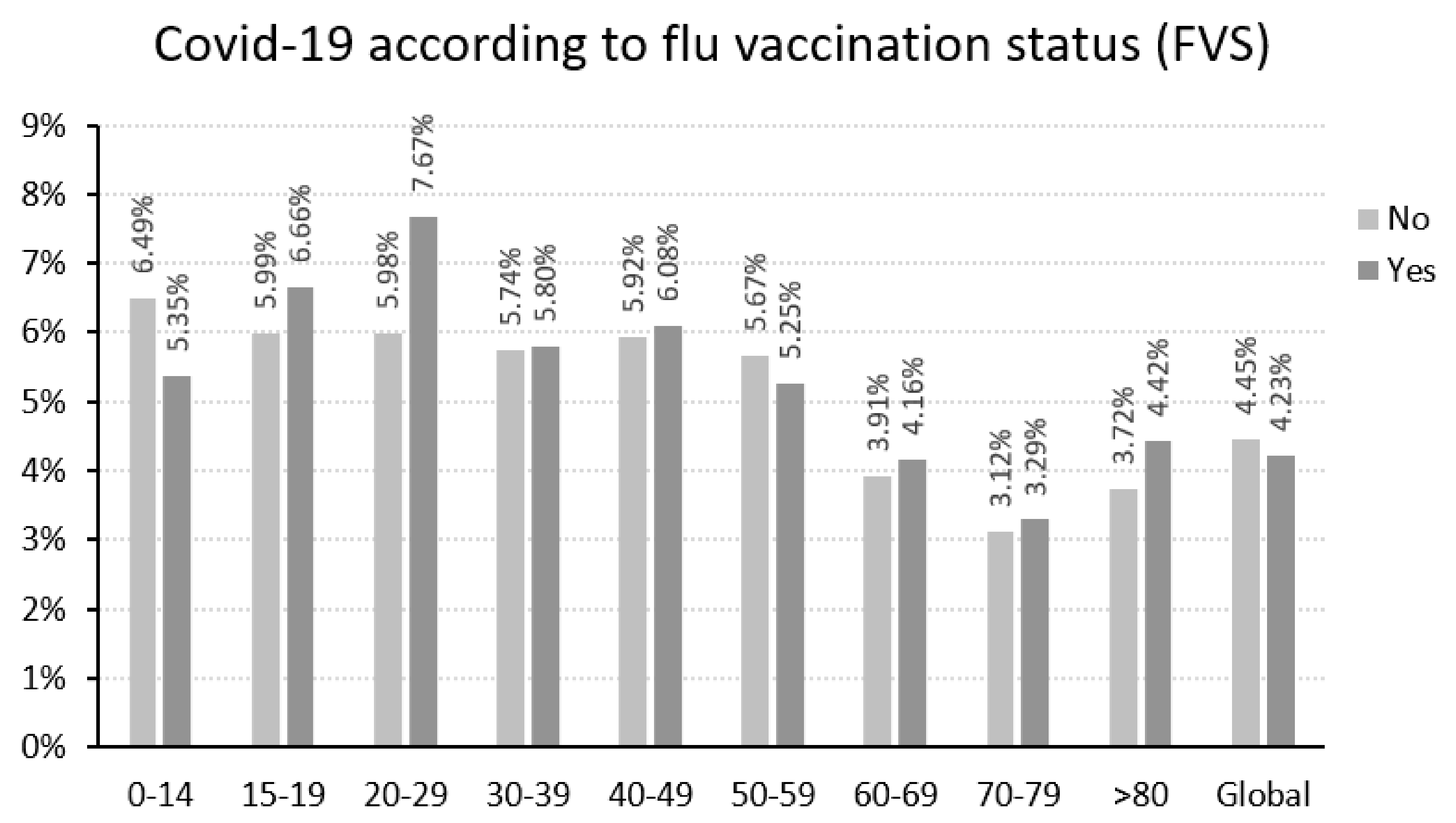
| Total population | 4.45% | 4.23% | <0.001 |
| <60 years old | 5.83% | 5.70% | 0.3585 |
| ≥60 years old | 3.62% | 3.72% | <0.001 |
| Risk Factor | Sample Size | Proportion of People Contracting COVID-19 Not FVS/FVS (p-Value) | Relative Risk | ||
|---|---|---|---|---|---|
| All | >59 Years Old | All | >59 Years Old | ||
| Chronic cardiovascular diseases | 231,853 | 4.6/4 (<0.001) | 3.9/3.8 (0.248) | 0.87 | 0.97 |
| Digestive problems | 8699 | 5.1/4.6 (0.2699) | 4.2/4.3 (0.8851) | 0.90 | 1.02 |
| Mellitus diabetes | 75,986 | 5/4.5 (<0.001) | 4.5/4.2 (0.0615) | 0.90 | 0.93 |
| Pregnant women | 3688 | 5.8/6.5 (0.3799) | 1.12 | ||
| Immunodeficiencies | 6882 | 5.2/4.5 (0.2094) | 3.2/4 (0.2416) | 0.87 | 1.25 |
| Malformations | 1135 | 3.5/4.8 (0.2971) | 4.2/2.3 (0.4831) | 1.37 | 0.55 |
| Chronic kidney disease | 34,597 | 4.7/4.3 (0.0457) | 4.6/4.2 (0.0967) | 0.91 | 0.91 |
| Neoplasms | 4926 | 4.9/4.1 (0.1535) | 4/4 (0.9901) | 0.84 | 1.00 |
| Neurological diseases | 6525 | 4.5/4.7 (0.8261) | 4.8/4.7 (0.8706) | 1.04 | 0.98 |
| Chronic lung diseases | 92,187 | 5.4/4.8 (<0.001) | 4.2/4.4 (0.4177) | 0.89 | 1.05 |
| Hematologic disorders | 6477 | 5.2/4.3 (0.0826) | 4.2/3.8 (0.5704) | 0.83 | 0.90 |
| Organ transplant | 1668 | 6.1/5.4 (0.5284) | 5.1/4.7 (0.7882) | 0.89 | 0.92 |
| HIV infection | 2245 | 2.8/3.2 (0.6069) | 2.3/2.8 (0.7066) | 1.14 | |
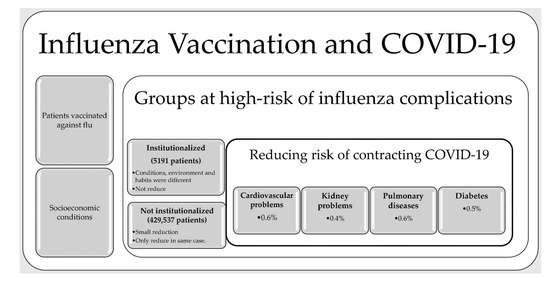
| Linving in a nursing home | 5191 | ------ | 6.6/12.6 (<0.001) | 1.91 | |
| Older than 60 years without other RF. | 98,570 | 3.3/3.4 (0.3701) | 1.03 | ||
| Any age with RF different from the average for their age | 330,967 | 4.9/4.2 (<0.001) | 3.9/3.8 (0.1139) | 0.86 | 0.97 |
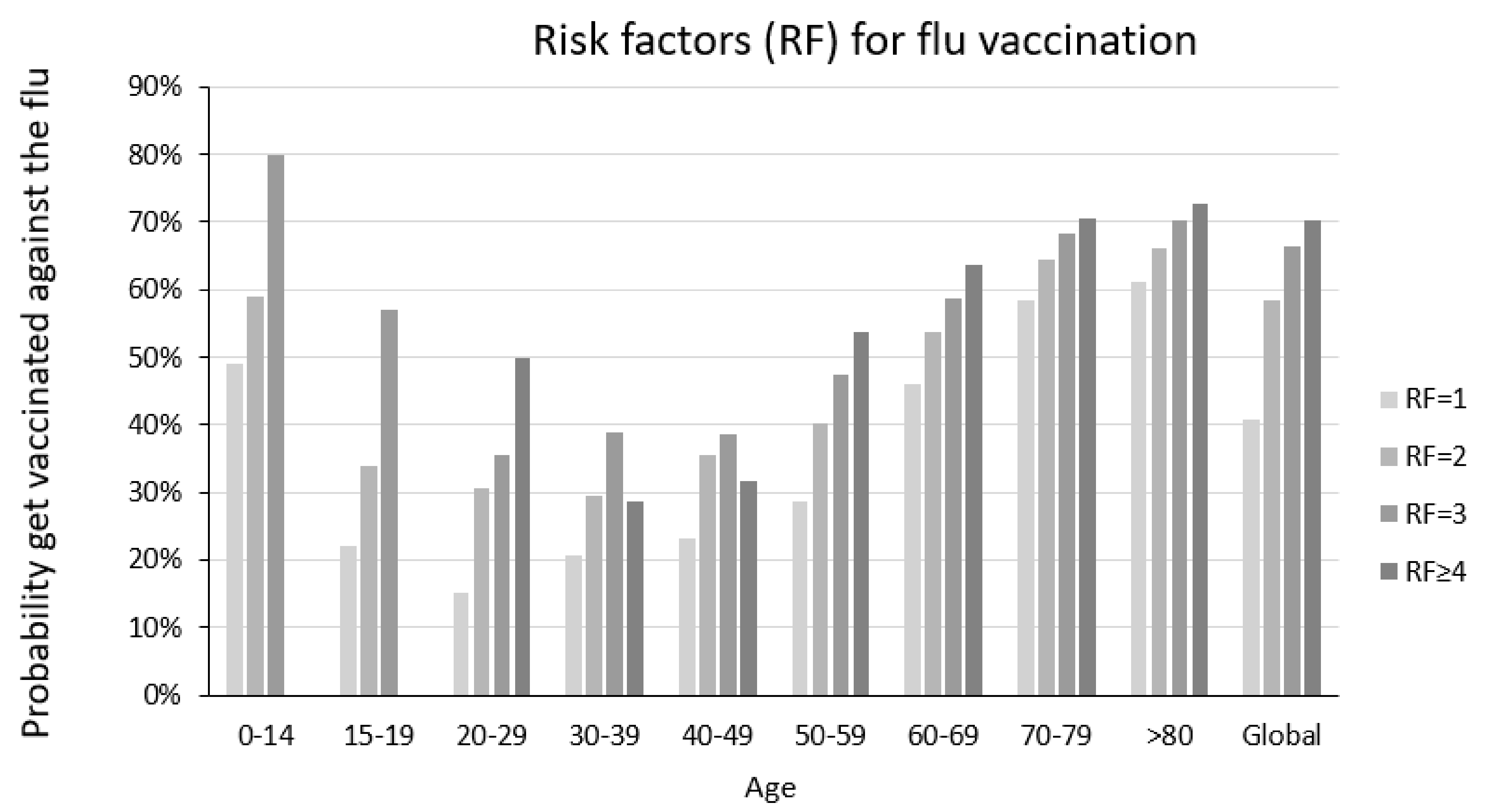
| Number of Risk Factors for Flu Complications | Probability of SARS-CoV-2 Infection | p-Value | |
|---|---|---|---|
| No FVS | Yes FVS | ||
| 0 | 3.3 | 3.4 | 0.3701 |
| 1 | 4.9 | 4.2 | <0.001 |
| 2 | 4.5 | 4.4 | 0.6594 |
| 3 | 5.0 | 5.2 | 0.5417 |
| ≥4 | 7.2 | 6.7 | 0.4702 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alòs, F.; Cánovas Zaldúa, Y.; Feijóo Rodríguez, M.V.; Del Val Garcia, J.L.; Sánchez-Callejas, A.; Colomer, M.À. Does Influenza Vaccination Reduce the Risk of Contracting COVID-19? J. Clin. Med. 2022, 11, 5297. https://doi.org/10.3390/jcm11185297
Alòs F, Cánovas Zaldúa Y, Feijóo Rodríguez MV, Del Val Garcia JL, Sánchez-Callejas A, Colomer MÀ. Does Influenza Vaccination Reduce the Risk of Contracting COVID-19? Journal of Clinical Medicine. 2022; 11(18):5297. https://doi.org/10.3390/jcm11185297
Chicago/Turabian StyleAlòs, Francesc, Yoseba Cánovas Zaldúa, María Victoria Feijóo Rodríguez, Jose Luis Del Val Garcia, Andrea Sánchez-Callejas, and Mª Àngels Colomer. 2022. "Does Influenza Vaccination Reduce the Risk of Contracting COVID-19?" Journal of Clinical Medicine 11, no. 18: 5297. https://doi.org/10.3390/jcm11185297
APA StyleAlòs, F., Cánovas Zaldúa, Y., Feijóo Rodríguez, M. V., Del Val Garcia, J. L., Sánchez-Callejas, A., & Colomer, M. À. (2022). Does Influenza Vaccination Reduce the Risk of Contracting COVID-19? Journal of Clinical Medicine, 11(18), 5297. https://doi.org/10.3390/jcm11185297