Are Lung Ultrasound Features More Severe in Children Diagnosed with Bronchiolitis after the COVID-19 Lockdown Period?
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Collection
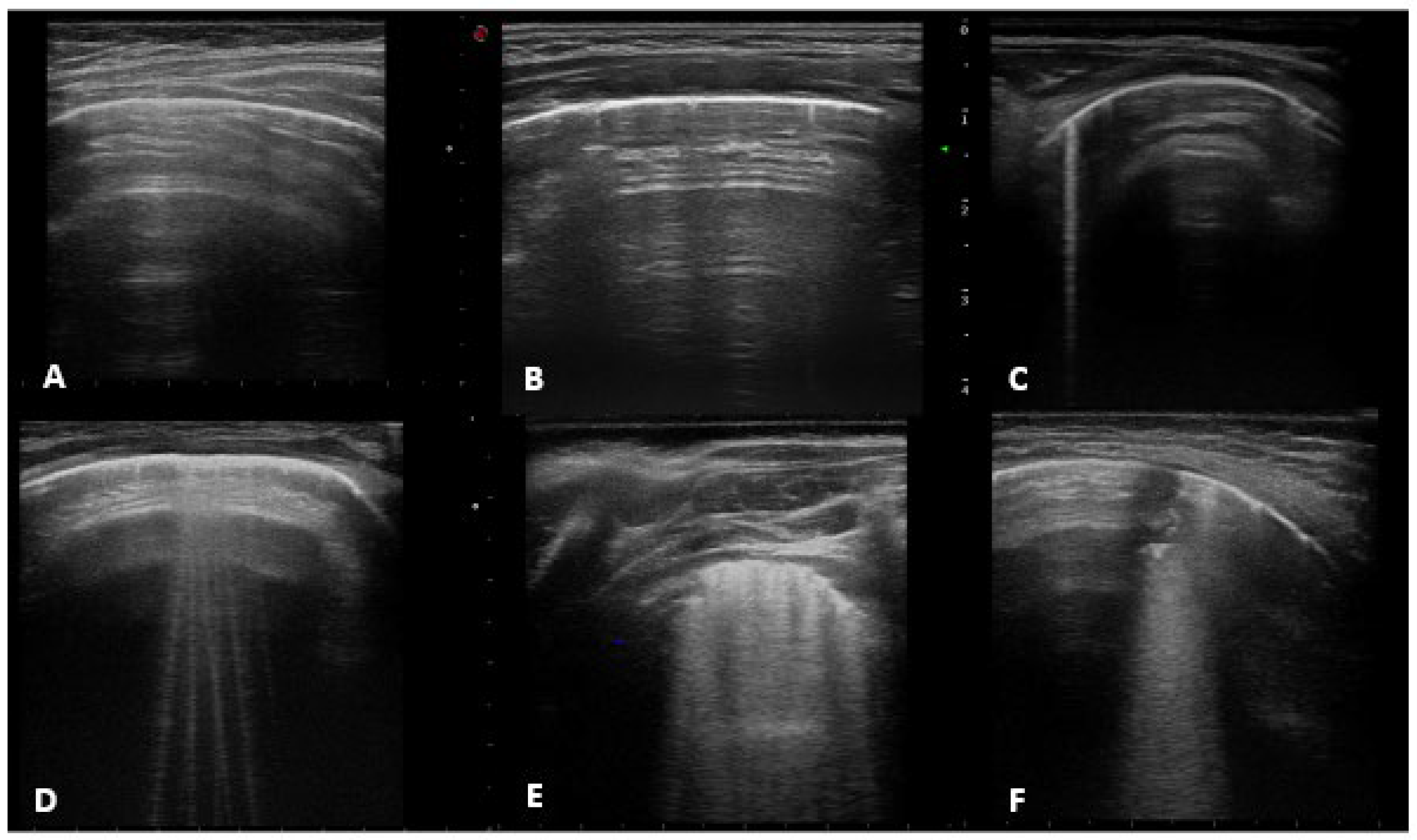
2.3. Chest Ultrasound Examination
- -
- Horizontal artifacts (the summation of the reverberation effects, due to the pleural-line and myofascial acoustic interfaces of the chest wall, and the mirror effects variable in its expression in relation to the thickness of the chest wall-reproducing beyond the pleural line, in a specular way, the myofascial planes of the chest wall) [25].
- -
- Short vertical artifact (artifact that do not reach the bottom of the screen).
- -
- B-line (hyperechogenic ultrasonographic artefacts, perpendicular to the pleural line, also known as comet-tail artefacts) that can be isolated (not more than 2 B-lines per intercostal space) or multiple (B-lines with a distance between them of less than half a cm to the confluence, remaining identifiable from each other).
- -
- White lung (characterized by a granular and mostly white texture, which starts at the pleura line and ends at the bottom of the screen as reported in previously mentioned [25]).
- -
- Sub-pleural consolidation (Subpleural echo-poor region interrupting the pleural line).
- -
- A-lines, normal ultrasound with score 0.
- -
- Short vertical artifact and Isolated-B lines with score 1 (counted together according to available literature See: https://doi.org/10.3390/app10051570, accessed on 24 July 2022).
- -
- Multiple B-lines with score 2.
- -
- White lung and subpleural consolidation less than 1 cm in size with score 3.
- -
- Sub-pleural consolidation greater than 1 cm in size, score 4.
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. LUS Features
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Florin, T.A.; Plint, A.C.; Zorc, J.J. Viral bronchiolitis. Lancet 2017, 389, 211–224. [Google Scholar] [CrossRef]
- NICE. Bronchiolitis in Children: Diagnosis and Management; NICE Guideline, No. 9; National Institute for Health and Care Excellence (NICE): London, UK, 2021; ISBN 978-1-4731-1162-2. [Google Scholar]
- Ali, S.; Plint, A.C.; Klassen, T.P. Bronchiolitis. In Kendig and Chernick’s Disorders of the Respiratory Tract in Children, 8th ed.; Wilmott, R.W., Kendig, E.L., Boat, T.F., Bush, A., Chernick, V., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2012; pp. 443–452. [Google Scholar]
- Miller, E.K.; Gebretsadik, T.; Carroll, K.N. Viral etiologies of infant bronchiolitis, croup and upper respiratory illness during 4 consecutive years. Pediatr. Infect. Dis. J. 2013, 32, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fernandez, D.; Casellas, A.; Mellado, M.J.; Calvo, C.; Bassat, Q. Acute bronchiolitis and respiratory syncytial virus seasonal transmission during the COVID-19 pandemic in Spain: A national perspective from the pediatric Spanish Society (AEP). J. Clin. Virol. 2021, 145, 105027. [Google Scholar] [CrossRef] [PubMed]
- Van Brusselen, D.; De Troeyer, K.; Ter Haar, E.; Vander Auwera, A.; Poschet, K.; Van Nuijs, S.; Bael, A.; Stobbelaar, K.; Verhulst, S.; Van Herendael, B.; et al. Bronchiolitis in COVID-19 times: A nearly absent disease? Eur. J. Pediatr. 2021, 180, 1969–1973. [Google Scholar] [CrossRef]
- Guedj, R.; Lorrot, M.; Lecarpentier, T.; Leger, P.L.; Corvol, H.; Carbajal, R. Infant bronchiolitis dramatically reduced during the second French COVID-19 outbreak. Acta Paediatr. 2021, 110, 1297–1299. [Google Scholar] [CrossRef]
- Takia, L.; Awasthi, P.; Angurana, S.K. Impact of COVID-19 on Acute Viral Bronchiolitis Hospitalization among Infants in North India. Indian J. Pediatr. 2021, 88, 1154. [Google Scholar] [CrossRef]
- Friedrich, F.; Ongaratto, R.; Scotta, M.C.; Veras, T.N.; Stein, R.T.; Lumertz, M.S.; Jones, M.H.; Comaru, T.; Pinto, L.A. Early Impact of Social Distancing in Response to Coronavirus Disease 2019 on Hospitalizations for Acute Bronchiolitis in Infants in Brazil. Clin. Infect. Dis. 2021, 72, 2071–2075. [Google Scholar] [CrossRef]
- Saravanos, G.L.; Hu, N.; Homaira, N.; Muscatello, D.J.; Jaffe, A.; Bartlett, A.W.; Wood, N.J.; Rawlinson, W.; Kesson, A.; Lingam, R.; et al. RSV Epidemiology in Australia Before and during COVID-19. Pediatrics 2022, 149, e2021053537. [Google Scholar] [CrossRef]
- Onay, Z.R.; Mavi, D.; Ayhan, Y.; Can Oksay, S.; Bilgin, G.; Girit, S. Did Hospital Admissions Caused by Respiratory Infections and Asthma Decrease During the COVID-19 Pandemic? Medeni. Med. J. 2022, 37, 92–98. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alnasser, Y.; Alenizi, A.S.; Alanazi, A.S.; Alharbi, A.H.; AlQurashi, F.O.; Nafisah, I.; Yousef, A.A. Did the National Lockdown in Saudi Arabia Reduce Lower Respiratory Illnesses in Children? Front. Pediatr. 2021, 9, 717739. [Google Scholar] [CrossRef]
- Doroshenko, A.; Lee, N.; MacDonald, C.; Zelyas, N.; Asadi, L.; Kanji, J.N. Decline of Influenza and Respiratory Viruses with COVID-19 Public Health Measures: Alberta, Canada. Mayo Clin. Proc. 2021, 96, 3042–3052. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, D.K.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; Blyth, C.C. The Interseasonal Resurgence of Respiratory Syncytial Virus in Australian Children Following the Reduction of Coronavirus Disease 2019-Related Public Health Measures. Clin. Infect. Dis. 2021, 73, e2829–e2830. [Google Scholar] [CrossRef]
- Hatter, L.; Eathorne, A.; Hills, T.; Bruce, P.; Beasley, R. Respiratory syncytial virus: Paying the immunity debt with interest. Lancet Child Adolesc. Health 2021, 5, e44–e45. [Google Scholar] [CrossRef]
- Messacar, K.; Baker, R.E.; Park, S.W.; Nguyen-Tran, H.; Cataldi, J.R.; Grenfell, B. Preparing for uncertainty: Endemic paediatric viral illnesses after COVID-19 pandemic disruption. Lancet 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Camporesi, A.; Morello, R.; Ferro, V.; Pierantoni, L.; Rocca, A.; Lanari, M.; Trobia, G.L.; Sciacca, T.; Bellinvia, A.G.; De Ferrari, A.; et al. Epidemiology, Microbiology and Severity of Bronchiolitis in the First Post-Lockdown Cold Season in Three Different Geographical Areas in Italy: A Prospective, Observational Study. Children 2022, 9, 491. [Google Scholar] [CrossRef]
- Pappa, S.; Haidopoulou, K.; Zarras, C.; Theodorakou, E.; Papadimitriou, E.; Iosifidis, E.; Gkeka, I.; Stoikou, K.; Vagdatli, E.; Skoura, L.; et al. Early initiation of the respiratory syncytial virus season in 202120132022, Greece. J. Med. Virol. 2022, 94, 3453–3456. [Google Scholar] [CrossRef]
- Musolino, A.M.; Tomà, P.; De Rose, C.; Pitaro, E.; Boccuzzi, E.; De Santis, R.; Morello, R.; Supino, M.C.; Villani, A.; Valentini, P.; et al. Ten Years of Pediatric Lung Ultrasound: A Narrative Review. Front. Physiol. 2022, 12, 721951. [Google Scholar] [CrossRef]
- Jaworska, J.; Komorowska-Piotrowska, A.; Pomiećko, A.; Wiśniewski, J.; Woźniak, M.; Littwin, B.; Kosiak, W. Consensus on the Application of Lung Ultrasound in Pneumonia and Bronchiolitis in Children. Diagnostics 2020, 10, 935. [Google Scholar] [CrossRef]
- Mongodi, S.; De Luca, D.; Colombo, A.; Stella, A.; Santangelo, E.; Corradi, F.; Gargani, L.; Rovida, S.; Volpicelli, G.; Bouhemad, B.; et al. Quantitative Lung Ultrasound: Technical Aspects and Clinical Applications. Anesthesiology 2021, 134, 949–965. [Google Scholar] [CrossRef]
- Mongodi, S.; Santangelo, E.; De Luca, D.; Rovida, S.; Corradi, F.; Volpicelli, G.; Gargani, L.; Bouhemad, B.; Mojoli, F. Quantitative Lung Ultrasound: Time for a Consensus? Chest 2020, 158, 469–470. [Google Scholar] [CrossRef]
- Baraldi, E.; Lanari, M.; Manzoni, P.; Rossi, G.A.; Vandini, S.; Rimini, A.; Romagnoli, C.; Colonna, P.; Biondi, A.; Biban, P.; et al. Inter-society consensus document on treatment and prevention of bronchiolitis in newborns and infants. Ital. J. Pediatr. 2014, 40, 65. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Proposal for International Standardization of the Use of Lung Ultrasound for Patients With COVID-19: A Simple, Quantitative, Reproducible Method. J. Ultrasound Med. 2020, 39, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Smargiassi, A.; Demi, L.; Inchingolo, R. Artifactual Lung Ultrasonography: It Is a Matter of Traps, Order, and Disorder. Appl. Sci. 2020, 10, 1570. [Google Scholar] [CrossRef]
- Amendolea, A.; Gori, L.; Adamoli, P.; Limoli, G.; Supino, M.C.; Coco, A.D.; Trobia, G.L.; Tursi, F.; Soldati, G.; Buonsenso, D. Gruppo di studio Pediatrico AdET. Pleuropulmonary Ultrasound in Pediatrics: Proposal of a Reporting Model from the Academy of Thoracic Ultrasound. J. Ultrasound Med. 2021. [Google Scholar] [CrossRef]
- Basile, V.; Di Mauro, A.; Scalini, E.; Comes, P.; Lofù, I.; Mostert, M.; Tafuri, S.; Manzionna, M.M. Lung ultrasound: A useful tool in diagnosis and management of bronchiolitis. BMC Pediatr. 2015, 15, 63. [Google Scholar] [CrossRef]
- Caiulo, V.A.; Gargani, L.; Caiulo, S.; Fisicaro, A.; Moramarco, F.; Latini, G.; Picano, E. Lung ultrasound in bronchiolitis: Comparison with chest X-ray. Eur. J. Pediatr. 2011, 170, 1427–1433. [Google Scholar] [CrossRef]
- Kader, M.A.; Samra, M.F.A.; Aal, S.M.A.; Shehata, N.; Khalifa, A. The utility of lung ultrasound in evaluation of infants with suspected bronchitis. Egypt. J. Radiol. Nucl. Med. 2016, 47, 1057–1064. [Google Scholar] [CrossRef][Green Version]
- Cohen, J.S.; Hughes, N.; Tat, S.; Chamberlain, J.M.; Teach, S.J.; Boniface, K. The utility of Bedside lung ultrasound findings in bronchiolitis. Pediatr. Emerg. Care 2017, 33, 97–100. [Google Scholar] [CrossRef]
- Taveira, M.; Yousef, N.; Miatello, J.; Roy, C.; Claude, C.; Boutillier, B.; Dubois, C.; Pierre, A.F.; Tissières, P.; Durand, P. Can a simple lung ultrasound score predict length of ventilation for infants with severe acute viral bronchiolitis? Arch. Pediatr. 2018, 25, 112–117. [Google Scholar] [CrossRef]
- Garrote, E.Z.; Aparicio, C.G.; Villarroel, C.C.T.; García, A.P.V.; Fontán, M.M.; Erroz, I.O. Utilidad de la ecografia pulmonar precoz en bronquiolitis aguda leve-moderada: Estudio piloto. Ann. Pediatr. 2018, 90, 10–18. [Google Scholar] [CrossRef]
- Biagi, C.; Pierantoni, L.; Baldazzi, M.; Greco, L.; Dormi, A.; Dondi, A.; Faldella, G.; Lanari, M. Lung ultrasuoni for diagnosis of pneumonia in children with acute bronchiolitis. BMC Pulm. Med. 2018, 18, 191. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Supino, M.C.; Giglioni, E.; Battaglia, M.; Mesturino, A.; Scateni, S.; Scialanga, B.; Reale, A.; Musolino, A.M. Point of care diaphragm ultrasound in infants with bronchiolitis: A prospective study. Pediatr. Pulmonol. 2018, 53, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Jaszczołt, S.; Polewczyk, T.; Dołęga-Kozierowska, M.; Wo’zniak, M.; Doniec, Z. Comparison of lung ultrasound and chest x-ray findings in children with bronchiolitis. J. Ultrason. 2018, 18, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Sainz, T.; Alba, M.; Del Rosal, T.; Mendez-Echevarría, A.; Echevarria, R.; Tagarro, A.; Ruperez-Lucas, M.; Herrreros, M.L.; Latorre, L.; et al. Lung ultrasound for prediction of respiratory support in infants with acute bronchiolitis: A cohort study. Pediatr. Pulmonol. 2019, 54, 873–880. [Google Scholar] [CrossRef]
- Özkaya, A.K.; Yilmaz, H.L.; Kendir, Ö.T.; Gökay, S.S.; Eyüboglu, I. Lung ultrasound findings and bronchiolitis ultrasound score for predicting hospital admission in children with acute bronchiolitis. Pediatr. Emerg. Care 2018, 36, e135–e142. [Google Scholar] [CrossRef] [PubMed]
- Supino, M.C.; Buonsenso, D.; Scateni, S.; Scialanga, B.; Mesturino, M.A.; Bock, C.; Chiaretti, A.; Giglioni, E.; Reale, A.; Musolino, A.M. Point-of-care lung ultrasound in infants with bronchiolitis in the pediatric emergency department: A prospective study. Eur. J. Pediatr. 2019, 178, 623–632. [Google Scholar] [CrossRef]
- Ingelse, S.A.; Pisani, L.; Westdorp, M.H.A.; Almakdase, M.; Schultz, M.J.; Woensel, J.B.M.; Bem, R.A. Lung ultrasound scoring in invasive mechanically ventilated children with severe bronchiolitis. Pediatr. Pulmonol. 2020, 55, 2799–2805. [Google Scholar] [CrossRef]
- Musolino, A.M.; Tomà, P.; Supino, M.C.; Scialanga, B.; Mesturino, A.; Scateni, S.; Battaglia, M.; Pirozzi, N.; Bock, C.; Buonsenso, D. Lung ultrasound features of children with complicated and noncomplicated community acquired pneumonia: A prospective study. Pediatr. Pulmonol. 2019, 54, 1479–1486. [Google Scholar] [CrossRef]
- Buonsenso, D.; Musolino, A.; Ferro, V.; De Rose, C.; Morello, R.; Ventola, C.; Liotti, F.M.; De Sanctis, R.; Chiaretti, A.; Biasucci, D.G.; et al. Role of lung ultrasound for the etiological diagnosis of acute lower respiratory tract infection (ALRTI) in children: A prospective study. J. Ultrasound 2022, 25, 185–197. [Google Scholar] [CrossRef]
- Buonsenso, D.; Tomà, P.; Scateni, S.; Curatola, A.; Morello, R.; Valentini, P.; Ferro, V.; D’Andrea, M.L.; Pirozzi, N.; Musolino, A.M. Lung ultrasound findings in pediatric community-acquired pneumonia requiring surgical procedures: A two-center prospective study. Pediatr. Radiol. 2020, 50, 1560–1569. [Google Scholar] [CrossRef]
- Buonsenso, D.; Brancato, F.; Valentini, P.; Curatola, A.; Supino, M.; Musolino, A.M. The Use of Lung Ultrasound to Monitor the Antibiotic Response of Community-Acquired Pneumonia in Children: A Preliminary Hypothesis. J. Ultrasound Med. 2020, 39, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Scialanga, B.; Buonsenso, D.; Scateni, S.; Valentini, P.; Schingo, P.M.S.; Boccuzzi, E.; Mesturino, M.A.; Ferro, V.; Chiaretti, A.; Villani, A.; et al. Lung Ultrasound to Detect Pneumothorax in Children Evaluated for Acute Chest Pain in the Emergency Department: An Observational Pilot Study. Front. Pediatr. 2022, 10, 812246. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total Population n = 108 | Pre-COVID-19 Period n = 52 | COVID-19 Period n = 56 | p Value |
|---|---|---|---|---|
| Sex, n(%) | ||||
| Male | 65 (60.19) | 36 (69.23) | 29 (51.79) | 0.06 |
| Female | 43 (39.81) | 16 (30.77) | 27 (48.21) | |
| Age (months), median (IQR) | 1.74 (1–3.68) | 1.6 (0.97–3.45) | 2.1 (1.02–4.86) | 0.22 |
| Prematurity, n (%) | 14 (12.96) | 5 (9.62) | 9 (16.07) | 0.32 |
| Onset of symptoms (hours), median (IQR) | 72 (48–96) | 48.5 (48–96) | 72 (36–96) | 0.75 |
| First episode, n (%) | 104 (96.30) | 50 (96.15) | 54 (96.43) | 0.94 |
| Coryza, n (%) | 108 (100%) | 56 (100%) | 52 (100%) | - |
| Difficulty of feeding, n (%) | 75 (69.44) | 39 (75.00) | 36 (64.29) | 0.23 |
| On ongoing therapy, n (%) | 36 (33.33) | 17 (32.69) | 19 (33.93) | 0.89 |
| Crackles on thorax auscultation, n (%) | 104 (96.30) | 48 (92.31) | 56 (100.00) | 0.034 |
| Wheeze on thorax auscultation, n (%) | 32 (29.63) | 28 (53.85) | 4 (7.14) | <0.001 |
| Chest retractions, n (%) | 103 (95.37) | 48 (92.31) | 55 (98.21) | 0.144 |
| Fever, n (%) | 41 (37.96) | 17 (32.69) | 24 (42.86) | 0.277 |
| SaO2 < 91%, n (%) | 39 (36.11) | 21 (40.38) | 18 (32.14) | 0.37 |
| RSV detection, n (%) | 76 (70.37) | 31 (59.62) | 45 (80.36) | 0.018 |
| Rhinovirus detection, n (%) | 23 (21.30) | 1 (1.92) | 22 (39.29) | <0.001 |
| Metapneumovirus, n (%) | 4 (3.70) | 1 (1.92) | 3 (5.36) | 0.34 |
| Parainfluenza, n (%) | 2 (1.85) | 0 | 2 (3.57) | 0.27 |
| Adenovirus/Bocavirus, n (%) | 2 (1.85) | 0 | 2 (3.57) | 0.27 |
| LUS*-right anterior side, median, (IQR) | 1 (1–3) | 1 (1–3) | 1.5 (1–3) | 0.94 |
| LUS*-left anterior side, median, (IQR) | 1 (1–2.5) | 1 (1–2) | 1 (1–3) | 0.95 |
| LUS*-right lateral side, median, (IQR) | 1 (1–3) | 1 (1–2.5) | 1 (1–3) | 0.37 |
| LUS*-left lateral side, median, (IQR) | 1 (1–3) | 1 (1–3) | 1 (1–3) | 0.11 |
| LUS*-right posterior side, median, (IQR) | 3 (2–3) | 3 (1–3) | 3 (2–3) | 0.37 |
| LUS*-left posterior side, median, (IQR) | 3 (1–3) | 2 (1–3) | 3 (1–3) | 0.34 |
| LUS*-paravertebral side, median, (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.48 |
| Total LUS*, median, (IQR) | 12 (9.16) | 11 (9–15) | 13 (8.5–17) | 0.1 |
| Need for oxygen support, n (%) | 90 (83.33) | 39 (75.00) | 51 (91.07) | 0.025 |
| Need for HFNC, n (%) | 48 (44.44) | 17 (32.69) | 31 (55.36) | 0.018 |
| Need d for N-CPAP, n (%) | 6 (5.56) | 0 | 6 (10.71) | 0.015 |
| Need for mechanical ventilation, n (%) | 0 | 0 | 0 | 0 |
| Need for antibiotic therapy, n (%) | 39 (36.11) | 24 (46.15) | 15 (26.79) | 0.036 |
| Trial of bronchodilator, n (%) | 25 (23.15) | 16 (30.77) | 9 (16.7) | 0.07 |
| Need for corticosteroid treatment, n (%) | 35 (32.41) | 24 (46.15) | 11 (19.64) | 0.003 |
| COVID-19 Period vs. Pre COVID-19 Period | Coeff. | OR | Std. Err. | z | p > |z| | 95% CI | |
|---|---|---|---|---|---|---|---|
| Age (months) | 0.33 | 1.4 | 0.15 | 2.23 | 0.026 | 0.04 | 0.6 |
| Sex male vs. female | −0.62 | 0.54 | 0.69 | −0.9 | 0.369 | −2.0 | 0.7 |
| Crackles on thorax auscultation | 2.11 | 8.27 | 2.16 | 0.98 | 0.327 | −2.1 | 6.3 |
| Wheeze on thorax auscultation | −4.26 | 0.01 | 1.48 | −2.88 | 0.004 | −7.2 | −1.4 |
| RSV detection | 0.90 | 2.46 | 1.06 | 0.85 | 0.39 | −1.2 | 3.0 |
| Rhinovirus detection | 3.16 | 23.5 | 1.62 | 1.95 | 0.05 | −0.02 | 6.3 |
| Need for antibiotic therapy | −0.82 | 0.44 | 0.83 | −0.99 | 0.32 | −2.4 | 0.8 |
| Need for corticosteroid treatment | −1.03 | 0.35 | 0.75 | −1.37 | 0.17 | −2.5 | 0.4 |
| Need for oxygen support | −0.75 | 0.47 | 1.33 | −0.56 | 0.57 | −3.4 | 1.9 |
| Need for HFNC | 0.59 | 1.8 | 0.74 | 0.80 | 0.42 | −0.86 | 2.04 |
| Need for hospitalization | 3.41 | 30.4 | 2.41 | 1.42 | 0.16 | −1.31 | 8.13 |
| Need for Intensive Care Unit | 2.66 | 14.3 | 2.28 | 1.17 | 0.24 | −1.80 | 7.12 |
| Constant | −5.19 | 0.005 | 2.80 | −1.86 | 0.06 | −10.67 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonsenso, D.; Morello, R.; Ferro, V.; Musolino, A.M.; De Rose, C.; Inchingolo, R.; Valentini, P. Are Lung Ultrasound Features More Severe in Children Diagnosed with Bronchiolitis after the COVID-19 Lockdown Period? J. Clin. Med. 2022, 11, 5294. https://doi.org/10.3390/jcm11185294
Buonsenso D, Morello R, Ferro V, Musolino AM, De Rose C, Inchingolo R, Valentini P. Are Lung Ultrasound Features More Severe in Children Diagnosed with Bronchiolitis after the COVID-19 Lockdown Period? Journal of Clinical Medicine. 2022; 11(18):5294. https://doi.org/10.3390/jcm11185294
Chicago/Turabian StyleBuonsenso, Danilo, Rosa Morello, Valentina Ferro, Anna Maria Musolino, Cristina De Rose, Riccardo Inchingolo, and Piero Valentini. 2022. "Are Lung Ultrasound Features More Severe in Children Diagnosed with Bronchiolitis after the COVID-19 Lockdown Period?" Journal of Clinical Medicine 11, no. 18: 5294. https://doi.org/10.3390/jcm11185294
APA StyleBuonsenso, D., Morello, R., Ferro, V., Musolino, A. M., De Rose, C., Inchingolo, R., & Valentini, P. (2022). Are Lung Ultrasound Features More Severe in Children Diagnosed with Bronchiolitis after the COVID-19 Lockdown Period? Journal of Clinical Medicine, 11(18), 5294. https://doi.org/10.3390/jcm11185294








