The Impact of Bariatric-Surgery-Induced Weight Loss on Patients Undergoing Liver Transplant: A Focus on Metabolism, Pathophysiological Changes, and Outcome in Obese Patients Suffering NAFLD-Related Cirrhosis
Abstract
:1. Introduction
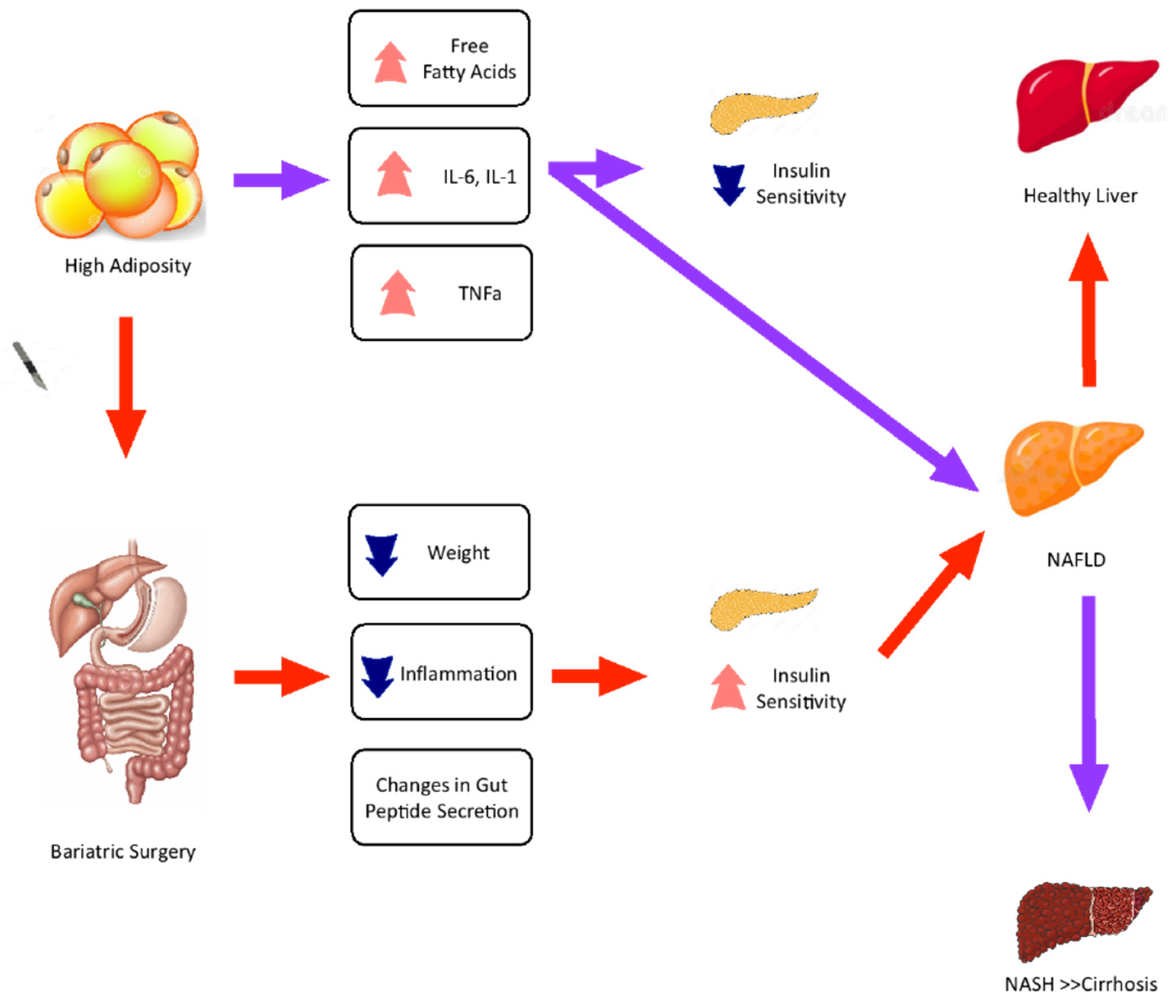
2. Metabolic Changes and Liver Pathophysiology Related to Bariatric Surgery
3. Impact of Bariatric Surgery on Absorption and Effectiveness of Immunosuppressants
4. Outcomes and Complications of Bariatric Surgery on Liver Transplantation
5. Future Perspectives
6. Cost-Effectiveness of Bariatric Surgery in Liver Transplantation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Schiavo, L.; Busetto, L.; Cesaretti, M.; Zelber-Sagi, S.; Deutsch, L.; Iannelli, A. Nutritional issues in patients with obesity and cirrhosis. World J. Gastroenterol. 2018, 24, 3330–3346. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, A.; Fontas, E.; Grec, L.; Nocca, D.; Robert, M.; Schiavo, L.; Schneck, A.S. Four-week omega-3 polyunsaturated fatty acids supplementation for liver left lateral section volume reduction in individuals with morbid obesity undergoing bariatric surgery: A double blind, multicenter, randomized placebo-controlled trial. Int. J. Surg. 2022, 101, 106614. [Google Scholar] [CrossRef]
- Addeo, P.; Cesaretti, M.; Anty, R.; Iannelli, A. Liver transplantation for bariatric surgery-related liver failure: A systematic review of a rare condition. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2019, 15, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Rinella, M.E. Nonalcoholic Fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin. Liver Dis. 2015, 19, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Brethauer, S.A.; Navaneethan, S.D.; Aminian, A.; Pothier, C.E.; Kim, E.S.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—3-Year Outcomes. N. Engl. J. Med. 2014, 370, 2002–2013. [Google Scholar] [CrossRef] [PubMed]
- Lassailly, G.; Caiazzo, R.; Buob, D.; Pigeyre, M.; Verkindt, H.; Labreuche, J.; Raverdy, V.; Leteurtre, E.; Dharancy, S.; Louvet, A.; et al. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology 2015, 149, 379–388; quiz e15–e16. [Google Scholar] [CrossRef]
- Leoni, S.; Tovoli, F.; Napoli, L.; Serio, I.; Ferri, S.; Bolondi, L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J. Gastroenterol. 2018, 24, 3361–3373. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Francque, S.M.; Marchesini, G.; Kautz, A.; Walmsley, M.; Dorner, R.; Lazarus, J.V.; Zelber-Sagi, S.; Hallsworth, K.; Busetto, L.; Frühbeck, G.; et al. Non-alcoholic fatty liver disease: A patient guideline. JHEP Rep. Innov. Hepatol. 2021, 3, 100322. [Google Scholar] [CrossRef]
- Colles, S.L.; Dixon, J.B.; Marks, P.; Strauss, B.J.; O’Brien, P.E. Preoperative weight loss with a very-low-energy diet: Quantitation of changes in liver and abdominal fat by serial imaging. Am. J. Clin. Nutr. 2006, 84, 304–311. [Google Scholar] [CrossRef]
- Iannelli, A.; Petrucciani, N.; Schiavo, L.; Anty, R. Severe Protein Malnutrition After Bariatric Surgery and Liver Failure: A Dangerous Sequence. Obes. Surg. 2021, 31, 3860–3861. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.R.; Burns, J.M.; Pedersen, R.A.; Watt, K.D.; Heimbach, J.K.; Dierkhising, R.A. Frequency and Outcomes of Liver Transplantation for Nonalcoholic Steatohepatitis in the United States. Gastroenterology 2011, 141, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Ney, M.; Irwin, I.; Ma, M.M.; Gramlich, L.; Bain, V.G.; Esfandiari, N.; Baracos, V.; Montano-Loza, A.J.; Myers, R.P. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2012, 18, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Spengler, E.K.; O’Leary, J.G.; Te, H.S.; Rogal, S.; Pillai, A.A.; Al-Osaimi, A.; Desai, A.; Fleming, J.; Ganger, D.; Seetharam, A.; et al. Liver Transplantation in the Obese Cirrhotic Patient. Transplantation 2017, 101, 2288–2296. [Google Scholar] [CrossRef]
- Weinheimer, E.M.; Sands, L.P.; Campbell, W.W. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: Implications for sarcopenic obesity. Nutr. Rev. 2010, 68, 375–388. [Google Scholar] [CrossRef]
- Iannelli, A.; Schiavo, L. Very Low-Calorie Diet, the Morbidly Obese With Liver Cirrhosis and Bariatric Surgery. Transplantation 2018, 102, e188–e189. [Google Scholar] [CrossRef]
- Dasarathy, S.; Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Barbarisi, A.; Cesaretti, M.; Iannelli, A. A 4-Week Preoperative Ketogenic Micronutrient-Enriched Diet Is Effective in Reducing Body Weight, Left Hepatic Lobe Volume, and Micronutrient Deficiencies in Patients Undergoing Bariatric Surgery: A Prospective Pilot Study. Obes. Surg. 2018, 28, 2215–2224. [Google Scholar] [CrossRef]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2017, 13, 727–741. [Google Scholar] [CrossRef]
- Allied Health Sciences Section Ad Hoc Nutrition Committee; Aills, L.; Blankenship, J.; Buffington, C.; Furtado, M.; Parrott, J. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2008, 4 (Suppl. 5), S73–S108. [Google Scholar] [CrossRef]
- Sinclair, P.; Brennan, D.J.; Le Roux, C.W. Gut adaptation after metabolic surgery and its influences on the brain, liver and cancer. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 606–624. [Google Scholar] [CrossRef] [PubMed]
- Sayiner, M.; Koenig, A.; Henry, L.; Younossi, Z.M. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin. Liver Dis. 2015, 20, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, V.W.; Stram, D.O.; Porcel, J.; Lu, S.C.; Le Marchand, L.; Noureddin, M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: The multiethnic cohort. Hepatology 2016, 64, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Garcêz, L.S.; Avelar, C.R.; Fonseca, N.S.S.; Costa, P.R.F.; Lyra, A.C.; Cunha, C.M.; Jesus, R.P.; Oliveira, L.P.M. Effect of dietary carbohydrate and lipid modification on clinical and anthropometric parameters in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 1321–1337. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Ness, E.; Kowdley, K.V. Nutritional Approaches to Achieve Weight Loss in Nonalcoholic Fatty Liver Disease. Adv. Nutr. 2017, 8, 253–265. [Google Scholar] [CrossRef]
- Ahn, J.; Jun, D.W.; Lee, H.Y.; Moon, J.H. Critical appraisal for low-carbohydrate diet in nonalcoholic fatty liver disease: Review and meta-analyses. Clin. Nutr. 2019, 38, 2023–2030. [Google Scholar] [CrossRef]
- Dehkordy, S.F.; Fowler, K.J.; Mamidipalli, A.; Wolfson, T.; Hong, C.W.; Covarrubias, Y.; Hooker, J.C.; Sy, E.Z.; Schlein, A.N.; Cui, J.Y.; et al. Hepatic steatosis and reduction in steatosis following bariatric weight loss surgery differs between segments and lobes. Eur. Radiol. 2019, 29, 2474–2480. [Google Scholar] [CrossRef]
- Panunzi, S.; Maltese, S.; Verrastro, O.; Labbate, L.; De Gaetano, A.; Pompili, M.; Capristo, E.; Bornstein, S.R.; Mingrone, G. Pioglitazone and bariatric surgery are the most effective treatments for non-alcoholic steatohepatitis: A hierarchical network meta-analysis. Diabetes Obes. Metab. 2021, 23, 980–990. [Google Scholar] [CrossRef]
- Liu, S.Y.-W.; Wong, V.W.-S.; Wong, S.K.-H.; Wong, G.L.-H.; Lai, C.M.-S.; Lam, C.C.-H.; Shu, S.S.-T.; Chan, H.L.-Y.; Ng, E.K.-W. A prospective 5-year study on the use of transient elastography to monitor the improvement of non-alcoholic fatty liver disease following bariatric surgery. Sci. Rep. 2021, 11, 5416. [Google Scholar] [CrossRef]
- Nickel, F.; Tapking, C.; Benner, L.; Sollors, J.; Billeter, A.T.; Kenngott, H.G.; Bokhary, L.; Schmid, M.; von Frankenberg, M.; Fischer, L.; et al. Bariatric Surgery as an Efficient Treatment for Non-Alcoholic Fatty Liver Disease in a Prospective Study with 1-Year Follow-up: BariScan Study. Obes. Surg. 2018, 28, 1342–1350. [Google Scholar] [CrossRef]
- Von Schönfels, W.; Beckmann, J.H.; Ahrens, M.; Hendricks, A.; Röcken, C.; Szymczak, S.; Hampe, J.; Schafmayer, C. Histologic improvement of NAFLD in patients with obesity after bariatric surgery based on standardized NAS (NAFLD activity score). Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2018, 14, 1607–1616. [Google Scholar] [CrossRef]
- Haddad, A.; Bashir, A. The Hardship of Recovering a Patient from Liver Failure after One Anastomosis Gastric Bypass. Obes. Surg. 2021, 31, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, Y.P.; Becchetti, C.; Watt, K.D.; Berzigotti, A. Risks and Rewards of Bariatric Surgery in Advanced Chronic Liver Diseases. Semin. Liver Dis. 2021, 41, 448–460. [Google Scholar] [CrossRef]
- Patton, H.; Heimbach, J.; McCullough, A. AGA Clinical Practice Update on Bariatric Surgery in Cirrhosis: Expert Review. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2021, 19, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Tapia, N.C.; Tellez-Avila, F.I.; Barrientos-Gutierrez, T.; Mendez-Sanchez, N.; Lizardi-Cervera, J.; Uribe, M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst. Rev. 2010, 2010, CD007340. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Khalouf, A.; Acosta, A. Management of Obesity and Nonalcoholic Fatty Liver Disease: A Literature Review. Semin. Liver Dis. 2021, 41, 435–447. [Google Scholar] [CrossRef] [PubMed]
- De Barros, F.; Cardoso Faleiro Uba, P.H. Liver transplantation and bariatric surgery: A new surgical reality: A systematic review of the best time for bariatric surgery. Updates Surg. 2021, 73, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, L.A.; Chaar, B.B.; Um, I.S. Pharmacokinetic changes post-bariatric surgery: A scoping review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2020, 21, e12988. [Google Scholar] [CrossRef]
- Schiavo, L.; Giosuè, A.; Izzo, V.; Piaz, F.D.; Filippelli, A.; Pilone, V. Liquid levothyroxine sodium therapy improves pharmacologic thyroid-stimulating hormone homeostasis in patients with reduced efficacy for tablet levothyroxine sodium after sleeve gastrectomy. A case report. Obes. Surg. 2021, 31, 4649–4652. [Google Scholar] [CrossRef]
- Lupoli, R.; Lembo, E.; Giosuè, A.; Schiavo, L.; Capaldo, B. Clinical insights into management options for recurrent type 2 diabetes and cardiovascular risk after metabolic-bariatric surgery. Nutr. Metab. Cardiovasc. Dis. NMCD 2022, 32, 1335–1342. [Google Scholar] [CrossRef]
- Miedziaszczyk, M.; Ciabach, P.; Szałek, E. The Effects of Bariatric Surgery and Gastrectomy on the Absorption of Drugs, Vitamins, and Mineral Elements. Pharmaceutics 2021, 13, 2111. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lin, Y.; Luo, P.; Yang, N.; Yang, G.; Zhu, L.; Pei, Q. Effect of laparoscopic sleeve gastrectomy on drug pharmacokinetics. Expert Rev. Clin. Pharmacol. 2021, 14, 1481–1495. [Google Scholar] [CrossRef] [PubMed]
- Kermansaravi, M.; Davarpanah Jazi, A.H.; Talebian, P.; Rokhgireh, S.; Kabir, A.; Pazouki, A. Bariatric surgery in transplant recipients: A narrative review. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2021, 26, 44. [Google Scholar]
- Chenhsu, R.-Y.; Wu, Y.; Katz, D.; Rayhill, S. Dose-Adjusted Cyclosporine C2 in a Patient with Jejunoileal Bypass as Compared to Seven Other Liver Transplant Recipients. Ther. Drug Monit. 2003, 25, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.C.; Alloway, R.R.; Alexander, J.W.; Cardi, M.; Trofe, J.; Vinks, A.A. Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: A pilot study. Clin. Transplant. 2007, 22, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Diwan, T.S.; Lee, T.C.; Nagai, S.; Benedetti, E.; Posselt, A.; Bumgardner, G.; Noria, S.; Whitson, B.A.; Ratner, L.; Mason, D.; et al. Obesity, transplantation, and bariatric surgery: An evolving solution for a growing epidemic. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant. Surg. 2020, 20, 2143–2155. [Google Scholar] [CrossRef]
- Moctezuma-Velazquez, C.; Márquez-Guillén, E.; Torre, A. Obesity in the Liver Transplant Setting. Nutrients 2019, 11, 2552. [Google Scholar] [CrossRef]
- Chandan, S.; Mohan, B.P.; Khan, S.R.; Facciorusso, A.; Ramai, D.; Kassab, L.L.; Bhogal, N.; Asokkumar, R.; Lopez-Nava, G.; McDonough, S.; et al. Efficacy and Safety of Intragastric Balloon (IGB) in Non-alcoholic Fatty Liver Disease (NAFLD): A Comprehensive Review and Meta-analysis. Obes. Surg. 2021, 31, 1271–1279. [Google Scholar] [CrossRef]
- Schiavo, L.; De Stefano, G.; Persico, F.; Gargiulo, S.; Di Spirito, F.; Griguolo, G.; Petrucciani, N.; Fontas, E.; Iannelli, A.; Pilone, V. A Randomized, Controlled Trial Comparing the Impact of a Low-Calorie Ketogenic vs a Standard Low-Calorie Diet on Fat-Free Mass in Patients Receiving an Elipse™ Intragastric Balloon Treatment. Obes. Surg. 2020, 31, 1514–1523. [Google Scholar] [CrossRef]
- Brandman, D. Obesity Management of Liver Transplant Waitlist Candidates and Recipients. Clin. Liver Dis. 2020, 25, 1–18. [Google Scholar] [CrossRef]
- Schiavo, L.; Sans, A.; Scalera, G.; Barbarisi, A.; Iannelli, A. Why Preoperative Weight Loss in Preparation for Bariatric Surgery Is Important. Obes. Surg. 2016, 26, 2790–2792. [Google Scholar] [CrossRef] [PubMed]
- Cazzo, E.; Gestic, M.A.; Utrini, M.P.; Chaim, F.D.M.; Callejas-Neto, F.; Pareja, J.C.; Chaim, E.A. Bariatric surgery in individuals with liver cirrhosis: A narrative review. Rev. Assoc. Med. Bras. 2017, 63, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Feltracco, P.; Barbieri, S.; Cillo, U.; Zanus, G.; Senzolo, M.; Ori, C. Perioperative thrombotic complications in liver transplantation. World J. Gastroenterol. 2015, 21, 8004–8013. [Google Scholar] [CrossRef]
- Burra, P.; Becchetti, C.; Germani, G. NAFLD and liver transplantation: Disease burden, current management and future challenges. JHEP Rep. Innov. Hepatol. 2020, 2, 100192. [Google Scholar] [CrossRef] [PubMed]
- Artzner, T.; Michard, B.; Besch, C.; Levesque, E.; Faitot, F. Liver transplantation for critically ill cirrhotic patients: Overview and pragmatic proposals. World J. Gastroenterol. 2018, 24, 5203–5214. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, M.; Langer, F.B.; Beer, A.; Trauner, M.; Prager, G.; Staufer, K. Significant Liver-Related Morbidity after Bariatric Surgery and Its Reversal—A Case Series. Obes. Surg. 2017, 28, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, A.; Treacy, P.; Sebastianelli, L.; Schiavo, L.; Martini, F. Perioperative complications of sleeve gastrectomy: Review of the literature. J. Minimal Access Surg. 2019, 15, 1–7. [Google Scholar]
- Sharpton, S.R.; Terrault, N.A.; Posselt, A.M. Outcomes of Sleeve Gastrectomy in Obese Liver Transplant Candidates. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2019, 25, 538–544. [Google Scholar] [CrossRef]
- Schiavo, L.; Calabrese, P.; Aliberti, S.M.; Tramontano, S.; Iannelli, A.; Pilone, V. Impact of SARS-CoV-2 Lockdown on the Preoperative Care Program of Patients Scheduled for Bariatric Surgery. Nutrients 2022, 14, 1488. [Google Scholar] [CrossRef]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Romano, M.; Pieretti, G.; Schneck, A.-S.; Iannelli, A. Correcting micronutrient deficiencies before sleeve gastrectomy may be useful in preventing early postoperative micronutrient deficiencies. Int. J. Vitam. Nutr. Res. Int. Z. Fur Vitam.-Und Ernahr. J. Int. De Vitaminol. Et De Nutr. 2019, 89, 22–28. [Google Scholar] [CrossRef]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Iannelli, A. The Role of the Nutritionist in a Multidisciplinary Bariatric Surgery Team. Obes. Surg. 2019, 29, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, L.; Scalera, G.; Pilone, V.; De Sena, G.; Capuozzo, V.; Barbarisi, A. Micronutrient Deficiencies in Patients Candidate for Bariatric Surgery: A Prospective, Preoperative Trial of Screening, Diagnosis, and Treatment. Int. J. Vitam. Nutr. Res. Int. Z. Fur Vitam.-Und Ernahrungsforschung. J. Int. De Vitaminol. Et De Nutr. 2015, 85, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, L.; Scalera, G.; Sergio, R.; De Sena, G.; Pilone, V.; Barbarisi, A. Clinical impact of Mediterrane-an-enriched-protein diet on liver size, visceral fat, fat mass, and fat-free mass in patients undergoing sleeve gastrectomy. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2015, 11, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Jung, A.D.; Kim, Y.; Lee, T.C.; Kaiser, T.E.; Thompson, J.R.; Bari, K.; Shah, S.A.; Cohen, R.M.; Schauer, D.P.; et al. Delayed Sleeve Gastrectomy Following Liver Transplantation: A 5-Year Experience. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2019, 25, 1673–1681. [Google Scholar] [CrossRef]
- Yemini, R.; Nesher, E.; Braun, M.; Cohen, M.; Carmeli, I.; Mor, E.; Keidar, A. Long-term outcomes of Roux-en-Y gastric bypass or sleeve gastrectomy in patients with cirrhosis; before, during or after liver transplantation: A single center’s experience. Clin. Transplant. 2021, 35, e14374. [Google Scholar] [CrossRef]
- Suraweera, D.; Saab, E.G.; Choi, G.; Saab, S. Bariatric Surgery and Liver Transplantation. Gastroenterol. Hepatol. 2017, 13, 170–175. [Google Scholar] [CrossRef]
- Suaud-Chagny, M.-F.; Buda, M.; Gonon, F.G. Pharmacology of electrically evoked dopamine release studied in the rat olfactory tubercle by in vivo electrochemistry. Eur. J. Pharmacol. 1989, 164, 273–283. [Google Scholar] [CrossRef]
- Klebanoff, M.J.; Corey, K.E.; Chhatwal, J.; Kaplan, L.M.; Chung, R.T.; Hur, C. Bariatric surgery for nonalcoholic steatohepatitis: A clinical and cost-effectiveness analysis. Hepatology 2017, 65, 1156–1164. [Google Scholar] [CrossRef]
- Klebanoff, M.J.; Corey, K.E.; Samur, S.; Choi, J.G.; Kaplan, L.M.; Chhatwal, J.; Hur, C. Cost-effectiveness Analysis of Bariatric Surgery for Patients with Nonalcoholic Steatohepatitis Cirrhosis. JAMA Netw. Open 2019, 2, e190047. [Google Scholar] [CrossRef]
- Iannelli, A.; Bulsei, J.; Debs, T.; Tran, A.; Lazzati, A.; Gugenheim, J.; Anty, R.; Petrucciani, N.; Fontas, E. Clinical and Economic Impact of Previous Bariatric Surgery on Liver Transplantation: A Nationwide, Population-Based Retrospective Study. Obes. Surg. 2021, 32, 55–63. [Google Scholar] [CrossRef]
- Lauren, B.N.; Lim, F.; Krikhely, A.; Taveras, E.M.; Baidal, J.A.W.; Bellows, B.K.; Hur, C. Estimated Cost-effectiveness of Medical Therapy, Sleeve Gastrectomy, and Gastric Bypass in Patients with Severe Obesity and Type 2 Diabetes. JAMA Netw. Open 2022, 5, e2148317. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.; Syn, W.-K. Health and Economic Burden of Nonalcoholic Fatty Liver Disease in the United States and Its Impact on Veterans. Fed. Pract. Health Care Prof. VA DoD PHS 2019, 36, 14–19. [Google Scholar]
- Sayiner, M.; Otgonsuren, M.; Cable, R.; Younossi, I.; Afendy, M.; Golabi, P.; Henry, L.; Younossi, Z.M. Variables Associated with Inpatient and Outpatient Resource Utilization among Medicare Beneficiaries with Nonalcoholic Fatty Liver Disease with or without Cirrhosis. J. Clin. Gastroenterol. 2017, 51, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idriss, R.; Hasse, J.; Wu, T.; Khan, F.; Saracino, G.; McKenna, G.; Testa, G.; Trotter, J.; Klintmalm, G.; Asrani, S.K. Impact of Prior Bariatric Surgery on Perioperative Liver Transplant Outcomes. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2019, 25, 217–227. [Google Scholar] [CrossRef]
- Orandi, B.J.; Purvis, J.W.; Cannon, R.M.; Smith, A.B.; Lewis, C.E.; Terrault, N.A.; Locke, J.E. Bariatric surgery to achieve transplant in end-stage organ disease patients: A systematic review and meta-analysis. Am. J. Surg. 2020, 220, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Valdes, D.; Watt, K.D.; Kellogg, T.A.; Poterucha, J.J.; Di Cecco, S.R.; Francisco-Ziller, N.M.; Taner, T.; Rosen, C.B.; Heimbach, J.K. Long-term outcomes of patients undergoing simultaneous liver transplantation and sleeve gastrectomy. Hepatology 2018, 68, 485–495. [Google Scholar] [CrossRef] [Green Version]
| Common Complications with NAFLD before Liver Transplantation | Common Complications with NAFLD after Liver Transplantation |
|---|---|
| Diabetes mellitus Cardiovascular disease Kidney dysfunction Altered nutritional status | Diabetes mellitus Obesity Cardiovascular disease Kidney dysfunction Liver failure Graft dysfunction Altered nutritional status |
| Cost Effectiveness of Bariatric Surgery in Liver Transplantation | ||
|---|---|---|
| Before Liver Transplantation | At Liver Transplantation | After Liver Transplantation |
| Meet the right weight-listing requirements Reduce the risk of perioperative transplant morbidity and mortality | Fewer metabolic complications at follow-up More effective and durable weight loss | Improve survival rates Reduce obesity comorbidities Reduce after transplant-related obesity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarno, G.; Schiavo, L.; Calabrese, P.; Álvarez Córdova, L.; Frias-Toral, E.; Cucalón, G.; Garcia-Velasquez, E.; Fuchs-Tarlovsky, V.; Pilone, V. The Impact of Bariatric-Surgery-Induced Weight Loss on Patients Undergoing Liver Transplant: A Focus on Metabolism, Pathophysiological Changes, and Outcome in Obese Patients Suffering NAFLD-Related Cirrhosis. J. Clin. Med. 2022, 11, 5293. https://doi.org/10.3390/jcm11185293
Sarno G, Schiavo L, Calabrese P, Álvarez Córdova L, Frias-Toral E, Cucalón G, Garcia-Velasquez E, Fuchs-Tarlovsky V, Pilone V. The Impact of Bariatric-Surgery-Induced Weight Loss on Patients Undergoing Liver Transplant: A Focus on Metabolism, Pathophysiological Changes, and Outcome in Obese Patients Suffering NAFLD-Related Cirrhosis. Journal of Clinical Medicine. 2022; 11(18):5293. https://doi.org/10.3390/jcm11185293
Chicago/Turabian StyleSarno, Gerardo, Luigi Schiavo, Pietro Calabrese, Ludwig Álvarez Córdova, Evelyn Frias-Toral, Gabriela Cucalón, Eloisa Garcia-Velasquez, Vanessa Fuchs-Tarlovsky, and Vincenzo Pilone. 2022. "The Impact of Bariatric-Surgery-Induced Weight Loss on Patients Undergoing Liver Transplant: A Focus on Metabolism, Pathophysiological Changes, and Outcome in Obese Patients Suffering NAFLD-Related Cirrhosis" Journal of Clinical Medicine 11, no. 18: 5293. https://doi.org/10.3390/jcm11185293
APA StyleSarno, G., Schiavo, L., Calabrese, P., Álvarez Córdova, L., Frias-Toral, E., Cucalón, G., Garcia-Velasquez, E., Fuchs-Tarlovsky, V., & Pilone, V. (2022). The Impact of Bariatric-Surgery-Induced Weight Loss on Patients Undergoing Liver Transplant: A Focus on Metabolism, Pathophysiological Changes, and Outcome in Obese Patients Suffering NAFLD-Related Cirrhosis. Journal of Clinical Medicine, 11(18), 5293. https://doi.org/10.3390/jcm11185293









