Prevalence, Characteristics, Management and Outcomes of Patients with Heart Failure with Preserved, Mildly Reduced, and Reduced Ejection Fraction in Spain
Abstract
:1. Introduction
2. Methods
Statistical Analysis
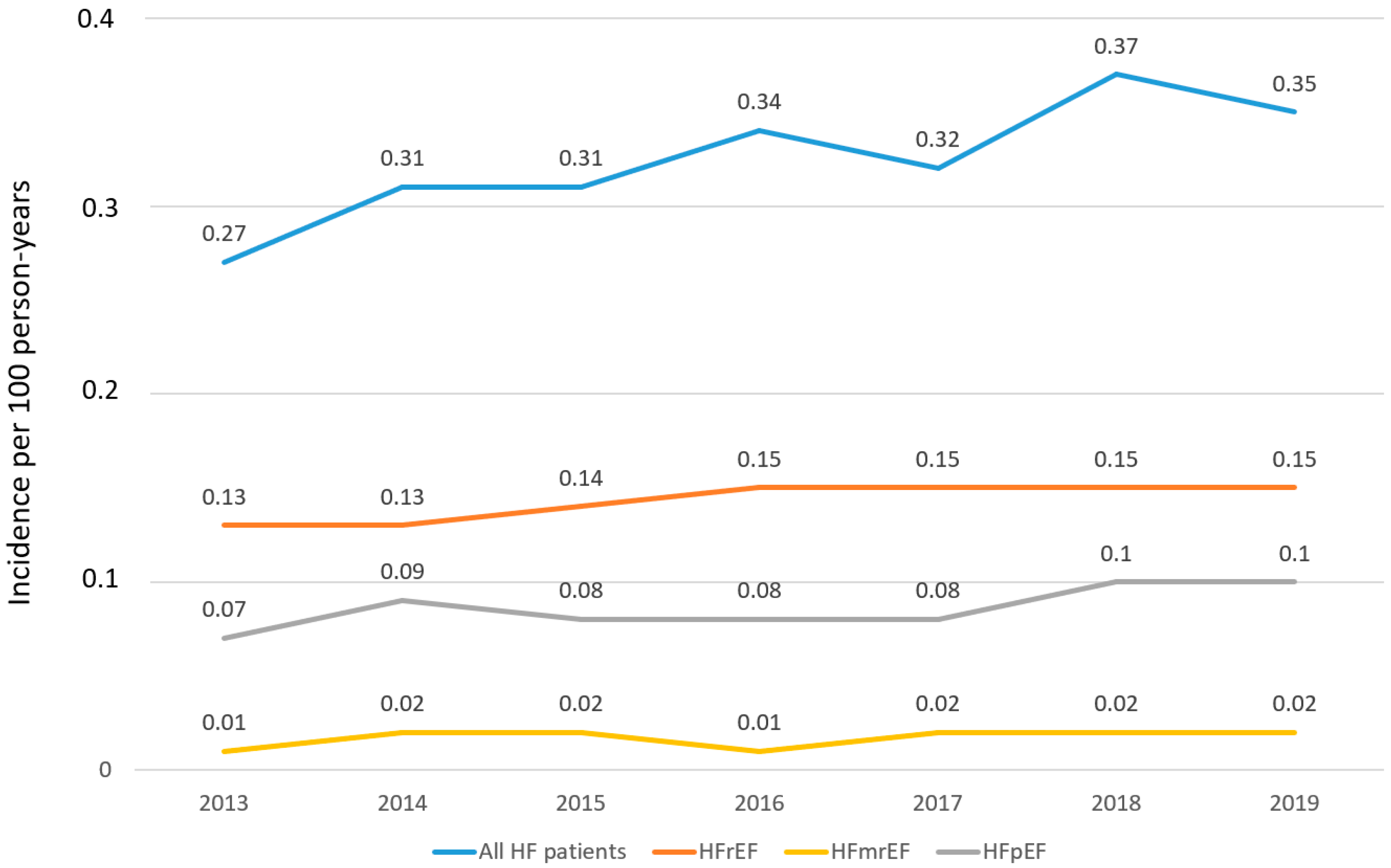
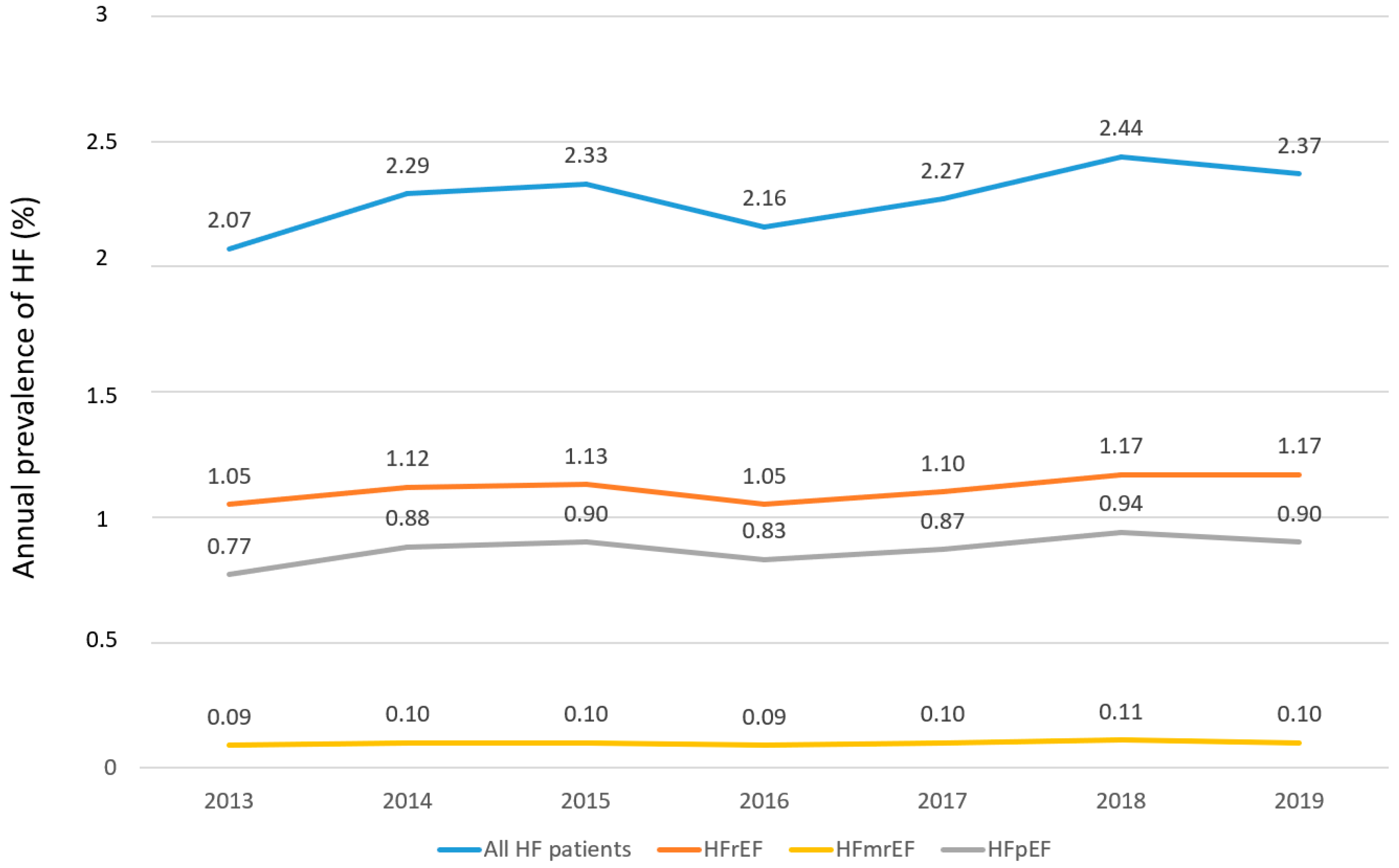
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart. J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton., A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart. Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Cordero, A.; Rodríguez-Mañero, M.; Bertomeu-González, V.; García-Acuña, J.M.; Baluja, A.; Agra-Bermejo, R.; Álvarez-Álvarez, B.; Cid, B.; Zuazola, P.; González-Juanatey, J.R. New-onset heart failure after acute coronary syndrome in patients without heart failure or left ventricular dysfunction. Rev. Esp. Cardiol. 2021, 74, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Van Riet, E.E.; Hoes, A.W.; Wagenaar, K.P.; Limburg, A.; Landman, M.A.; Rutten, F.H. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart. Fail. 2016, 18, 242–252. [Google Scholar] [CrossRef]
- Fonseca, C.; Brás, D.; Araújo, I.; Ceia, F. Heart failure in numbers: Estimates for the 21st century in Portugal. Rev. Port. Cardiol. 2018, 37, 97–104. [Google Scholar] [CrossRef]
- Sicras-Mainar, A.; Sicras-Navarro, A.; Palacios, B.; Varela, L.; Delgado, J.F. Epidemiology and treatment of heart failure in Spain: The HF-PATHWAYS study. Rev. Esp. Cardiol. 2022, 75, 31–38. [Google Scholar] [CrossRef]
- Escobar, C.; Varela, L.; Palacios, B.; Capel, M.; Sicras-Mainar, A.; Sicras-Navarro, A.; Hormigo, A.; Alcázar, R.; Manito, N.; Botana, M. Clinical characteristics, management, and one-year risk of complications among patients with heart failure with and without type 2 diabetes in Spain. Rev. Clin. Esp. 2022, 222, 195–204. [Google Scholar] [CrossRef]
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015, 175, 996–1004. [Google Scholar] [CrossRef]
- Shafie, A.A.; Tan, Y.P.; Ng, C.H. Systematic review of economic burden of heart failure. Heart. Fail. Rev. 2018, 23, 131–145. [Google Scholar] [CrossRef]
- Savarese, G.; Lund, L.H. Global public health burden of heart failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Anguita, M.; Bonilla, J.L.; García, M.; Bernal, J.L.; Elola, F.J.; Marín, F. Temporal trends in hospitalization and in-hospital mortality rates due to heart failure by age and sex in Spain (2003–2018). Rev. Esp. Cardiol. 2021, 74, 993–996. [Google Scholar]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Farré, N.; Lupon, J.; Roig, E.; Gonzalez-Costello, J.; Vila, J.; Perez, S.; de Antonio, M.; Solé-González, E.; Sánchez-Enrique, C.; Moliner, P.; et al. Clinical characteristics, one-year change in ejection fraction and long-term outcomes in patients with heart failure with mid-range ejection fraction: A multicentre prospective observational study in Catalonia (Spain). BMJ Open 2017, 7, e018719. [Google Scholar] [CrossRef]
- Gómez-Otero, I.; Ferrero-Gregori, A.; Román, A.V.; Seijas, J.; Pascual-Figal, D.A.; Delgado, J.; Álvarez-García, J.; Fernández-Avilés, F.; Worner, F.; Alonso-Pulpón, L.; et al. Mid-range ejection fraction does not permit risk stratification among patients hospitalized for heart failure. Rev. Esp. Cardiol. 2017, 70, 338–346. [Google Scholar]
- Guo, L.; Guo, X.; Chang, Y.; Yang, J.; Zhang, L.; Li, T.; Sun, Y. Prevalence and Risk Factors of Heart Failure with Preserved Ejection Fraction: A Population-Based Study in Northeast China. Int. J. Environ. Res. Public Health 2016, 13, 770. [Google Scholar] [CrossRef] [PubMed]
- Kraigher-Krainer, E.; Lyass, A.; Massaro, J.M.; Lee, D.S.; Ho, J.E.; Levy, D.; Kannel, W.B.; Vasan, R.S. Association of physical activity and heart failure with preserved vs. reduced ejection fraction in the elderly: The Framingham Heart Study. Eur. J. Heart Fail. 2013, 15, 742–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiller, D.; Russ, M.; Greiser, K.H.; Nuding, S.; Ebelt, H.; Kluttig, A.; Kors, J.A.; Thiery, J.; Bruegel, M.; Haerting, J.; et al. Prevalence of symptomatic heart failure with reduced and with normal ejection fraction in an elderly general population-the CARLA study. PLoS ONE 2013, 8, e59225. [Google Scholar]
- Hamada, T.; Kubo, T.; Kawai, K.; Nakaoka, Y.; Yabe, T.; Furuno, T.; Yamada, E.; Kitaoka, H. Clinical characteristics and frailty status in heart failure with preserved vs. reduced ejection fraction. ESC Heart Fail. 2022, 9, 1853–1863. [Google Scholar] [CrossRef]
- Rywik, T.M.; Doryńska, A.; Wiśniewska, A.; Cegłowska, U.; Drohomirecka, A.; Topór-Mądry, R.; Łazarczyk, H.; Połaska, P.; Leszek, P.; Zieliński, T. Epidemiology and clinical characteristics of hospitalized heart failure patients with a reduced, mildly reduced and preserved ejection fraction. Pol. Arch. Intern. Med. 2022, 132, 16227. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; O’Connor, C.M.; Chiswell, K.; Anstrom, K.J.; Newby, L.K.; Mentz, R.J. Survival in Patients with Nonischemic Cardiomyopathy With Preserved vs Reduced Ejection Fraction. CJC Open 2021, 3, 1333–1340. [Google Scholar] [CrossRef]
- De Boer, A.R.; Vaartjes, I.; Gohar, A.; Valk, M.J.M.; Brugts, J.J.; Boonman-de Winter, L.J.M.; van Riet, E.E.; van Mourik, Y.; Brunner-La Rocca, H.P.; Linssen, G.C.M.; et al. Heart failure with preserved, mid-range, and reduced ejection fraction across health care settings: An observational study. ESC Heart Fail. 2022, 9, 363–372. [Google Scholar] [CrossRef]
- Escobar, C.; Varela, L.; Palacios, B.; Capel, M.; Sicras, A.; Sicras, A.; Hormigo, A.; Alcázar, R.; Manito, N.; Botana, M. Costs and healthcare utilisation of patients with heart failure in Spain. BMC Health Serv. Res. 2020, 20, 964. [Google Scholar] [CrossRef]
- KDIGO. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef]
- Quan, H.L.B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD): World Health Organization. Available online: https://www.who.int/classifications-/atcddd/en/ (accessed on 20 April 2022).
- Van Riet, E.E.; Hoes, A.W.; Limburg, A.; Landman, M.A.; van der Hoeven, H.; Rutten, F.H. Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur. J. Heart. Fail. 2014, 16, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Escobar, C.; De La Sierra, A.; Llisterri, J.L.; González-Segura, D. Detection of unrecognized clinical heart failure in elderly hypertensive women attended in primary care setting. Blood. Press. 2010, 19, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Anguita, M.; Bayés-Genís, A.; Cepeda, J.M.; Cinza, S.; Cosín, J.; Crespo, M.; Egocheaga, I.; Escobar, C.; Faraudo, M.; García-Pinilla, J.M.; et al. Expert consensus statement on heart failure with reduced ejection fraction: Beyond the guidelines. Rev. Esp. Cardiol. Supl. 2020, 20, 1–46. [Google Scholar]
- Gevaert, A.B.; Kataria, R.; Zannad, F.; Sauer, A.J.; Damman, K.; Sharma, K.; Shah, S.J.; Van Spall, H.G.C. Heart failure with preserved ejection fraction: Recent concepts in diagnosis, mechanisms and management. Heart 2022, 108, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Dinh, D.; Brennan, A.; Hare, D.L.; Kaye, D.; Lefkovits, J.; Lockwood, S.; Neil, C.; Prior, D.; Nasis, A.; et al. Characteristics and Clinical Outcomes in Patients with Heart Failure With Preserved Ejection Fraction Compared to Heart Failure With Reduced Ejection Fraction: Insights From the VCOR Heart Failure Snapshot. Heart. Lung. Circ. 2022, 31, 623–628. [Google Scholar] [CrossRef]
- Cerqueiro-González, J.M.; González-Franco, Á.; Carrascosa-García, S.; Soler-Rangel, L.; Ruiz-Laiglesia, F.J.; Epelde-Gonzalo, F.; Dávila-Ramos, M.F.; Casado-Cerrada, J.; Casariego-Vales, E.; Manzano, L. Benefits of a comprehensive care model in patients with heart failure and preserved ejection fraction: The UMIPIC program. Rev. Clin. Esp. 2022, 222, 339–347. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef]
- Li, P.; Zhao, H.; Zhang, J.; Ning, Y.; Tu, Y.; Xu, D.; Zeng, Q. Similarities and Differences Between HFmrEF and HFpEF. Front. Cardiovasc. Med. 2021, 8, 614–678. [Google Scholar] [CrossRef]
- Savarese, G.; Stolfo, D.; Sinagra, G.; Lund, L.H. Heart failure with mid-range or mildly reduced ejection fraction. Nat. Rev. Cardiol. 2022, 19, 100–116. [Google Scholar] [CrossRef]
- Girerd, N.; Von Hunolstein, J.J.; Pellicori, P.; Bayés-Genís, A.; Jaarsma, T.; Lund, L.H.; Bilbault, P.; Boivin, J.M.; Chouihed, T.; Costa, J.; et al. Therapeutic inertia in the pharmacological management of heart failure with reduced ejection fraction. ESC. Heart Fail. 2022, 9, 2063–2069. [Google Scholar] [CrossRef]
- Savarese, G.; Vasko, P.; Jonsson, Å.; Edner, M.; Dahlström, U.; Lund, L.H. The Swedish Heart Failure Registry: A living, ongoing quality assurance and research in heart failure. Ups. J. Med. Sci. 2019, 124, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Stolfo, D.; Uijl, A.; Vedin, O.; Strömberg, A.; Faxén, U.L.; Rosano, G.M.C.; Sinagra, G.; Dahlström, U.; Savarese, G. Sex-Based Differences in Heart Failure Across the Ejection Fraction Spectrum: Phenotyping, and Prognostic and Therapeutic Implications. JACC Heart Fail. 2019, 7, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Mamas, M.A.; Sperrin, M.; Watson, M.C.; Coutts, A.; Wilde, K.; Burton, C.; Kadam, U.T.; Kwok, C.S.; Clark, A.B.; Murchie, P.; et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur. J. Heart Fail. 2017, 19, 1095–1104. [Google Scholar] [CrossRef]
- Pandey, A.K.; Dhingra, N.K.; Hibino, M.; Gupta, V.; Verma, S. Sodium-glucose cotransporter 2 inhibitors in heart failure with reduced or preserved ejection fraction: A meta-analysis. ESC Heart Fail. 2022, 9, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, P.; Li, Y.; Han, Y. Sodium-glucose cotransporter-2 inhibitors in heart failure: An updated meta-analysis. ESC Heart Fail. 2022, 9, 1942–1953. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]

| HF Prevalent Cohort (n = 21,297; 100%) | HFrEF (n = 10,323; 48.5%) | HFmrEF (n = 903; 4.2%) | HFpEF (n = 8225; 38.6%) | HFpEF (50 to <60) (n = 2995; 14.1%) | HFpEF (≥60) (n = 5230; 24.6%) | HFuEF (n = 1846; 8.7%) | p-Value (HFmrEF vs. HFrEF) | p-Value (HFpEF vs. HFrEF) | |
|---|---|---|---|---|---|---|---|---|---|
| Biodemographic Data | |||||||||
| Age (years) at index date Mean (SD) Median (25th75th percentile) Range (min–max) | 78.8 (11.8) 79.1 (71.9–87.6) (18–102.8) | 73.6 (9.7) 73.8 (67.5–79.8) (18–96.36) | 81.7 (9.9) 80.6 (75.6–89.4) (21.6–98.8) | 84.0 (11.4) 85.6 (78.2–91.9) (18.5–102.8) | 84.1 (11.3) 85.7 (78.4–89.4) (18.6–98.8) | 84.0 (11.4) 85.5 (78.2–91.9) (18.5–100.5) | 83.3 (11.9) 85.5 (78.0–91.9) (18.9–100.5) | <0.001 | <0.001 |
| Age groupsn (%) <45 years 45–64 years 65–74 years 75–84 years ≥85 years | 251 (1.2) 2007 (9.4) 5124 (24.1) 6981 (32.8) 6934 (32.6) | 122 (1.2) 1479 (14.3) 4087 (39.6) 3314 (32.1) 1321 (12.8) | 2 (0.2) 34 (3.8) 168 (18.6) 346 (38.3) 353 (39.1) | 103 (1.3) 376 (4.6) 695 (8.5) 2754 (33.5) 4297 (52.2) | 37 (1.2) 134 (4.5) 244 (8.2) 1000 (33.4) 1580 (52.8) | 66 (1.3) 242 (4.6) 451 (8.6) 1754 (33.5) 2717 (52.0) | 24 (1.3) 118 (6.4) 174 (9.4) 567 (30.7) 963 (52.2) | ||
| Gendermalen (%) | 11,278 (53.0) | 6782 (65.7) | 440 (48.7) | 3068 (37.3) | 1102 (36.8) | 1966 (37.6) | 988 (53.5) | <0.001 | <0.001 |
| NYHA at index daten (%) Class I Class II Class III Class IV Unknown | 2137 (10.0) 8949 (42.0) 8728 (41.0) 1013 (4.8) 470 (2.2) | 1030 (10.0) 3689 (35.7) 4750 (46.0) 612 (5.9) 242 (2.3) | 91 (10.1) 332 (36.8) 411 (45.5) 56 (6.2) 13 (1.4) | 817 (9.9) 4176 (50.8) 2783 (33.8) 280 (3.4) 169 (2.1) | 322 (10.8) 1504 (50.2) 1016 (33.9) 92 (3.1) 61 (2.0) | 495 (9.5) 2672 (51.1) 1767 (33.8) 188 (3.6) 108 (2.1) | 199 (10.8) 752 (40.7) 784 (42.5) 65 (3.5) 46 (2.5) | 0.494 | <0.001 |
| Cardiovascular risk factorsn (%) | |||||||||
| Hypertension | 14,379 (67.5) | 6885 (66.7) | 662 (73.3) | 5550 (67.5) | 2021 (67.5) | 3529 (67.5) | 1282 (69.5) | <0.001 | 0.261 |
| Dyslipidemia | 10,457 (49.1) | 4681 (45.4) | 457 (50.6) | 4384 (53.3) | 1601 (53.5) | 2783 (53.2) | 935 (50.7) | 0.002 | <0.001 |
| Diabetes type 1 | 844 (4.0) | 499 (4.8) | 32 (3.5) | 258 (3.1) | 100 (3.3) | 158 (3.0) | 55 (3.0) | 0.080 | <0.001 |
| Diabetes type 2 | 6772 (31.8) | 3331 (32.3) | 236 (26.1) | 2630 (32.0) | 1127 (37.6) | 1503 (28.7) | 575 (31.2) | <0.001 | 0.672 |
| Vascular diseasen (%) | |||||||||
| Coronary artery disease | 8124 (38.2) | 4520 (43.8) | 288 (31.9) | 2653 (32.3) | 971 (32.4) | 1682 (32.2) | 663 (35.9) | <0.001 | <0.001 |
| Chronic kidney disease Stage Unknown Stage I Stage II Stage III Stage IV Stage V | 6452 (30.3) 2706 (12.7) 179 (0.8) 644 (3.0) 2225 (10.5) 524 (2.5) 174 (1.0) | 3411 (33.0) 1451 (14.1) 86 (0.8) 327 (3.2) 1179 (11.4) 281 (2.7) 87 (1.1) | 272 (30.1) 106 (11.7) 6 (0.7) 19 (2.1) 106 (11.7) 26 (2.9) 9 (1.1) | 2286 (27.8) 953 (11.6) 73 (0.9) 250 (3.0) 789 (9.6) 153 (1.9) 68 (0.9) | 849 (28.4) 358 (12.0) 26 (0.9) 85 (2.8) 296 (9.9) 63 (2.1) 21 (0.9) | 1437 (27.5) 595 (11.4) 47 (0.9) 165 (3.2) 493 (9.4) 90 (1.7) 47 (0.9) | 483 (26.2) 196 (10.6) 14 (0.8) 48 (2.6) 151 (8.2) 64 (3.5) 10 (0.6) | 0.073 | <0.001 |
| Myocardial Infarction | 3174 (14.9) | 1645 (15.9) | 103 (11.4) | 1110 (13.5) | 384 (12.8) | 726 (13.9) | 316 (17.1) | <0.001 | <0.001 |
| Stroke | 2254 (10.6) | 1327 (12.9) | 107 (11.9) | 617 (7.5) | 297 (9.9) | 320 (6.1) | 203 (11.0) | 0.385 | <0.001 |
| Peripheral arterial disease | 1074 (5.0) | 616 (6.0) | 24 (2.7) | 337 (4.1) | 146 (4.9) | 191 (3.7) | 97 (5.3) | <0.001 | <0.001 |
| Other comorbiditiesn (%) | |||||||||
| COPD | 3319 (15.6) | 1716 (16.6) | 121 (13.4) | 1202 (14.6) | 441 (14.7) | 761 (14.6) | 280 (15.2) | 0.012 | <0.001 |
| Atrial fibrillation | 6785 (31.9) | 2538 (24.6) | 283 (31.3) | 3364 (40.9) | 1205 (40.2) | 2159 (41.3) | 600 (32.5) | <0.001 | <0.001 |
| Anemia within 1 year before index date | 6540 (30.7) | 3266 (31.6) | 255 (28.2) | 2503 (30.4) | 910 (30.4) | 1593 (30.5) | 516 (28.0) | 0.035 | 0.078 |
| Cancer before index date | 2776 (13.0) | 1313 (12.72) | 109 (12.1) | 1077 (13.1) | 368 (12.3) | 709 (13.6) | 277 (15.0) | 0.574 | 0.449 |
| Dementia | 1058 (5.0) | 568 (5.5) | 45 (5.0) | 360 (4.4) | 168 (5.6) | 192 (3.7) | 85 (4.6) | 0.510 | <0.001 |
| Charlson Comorbidity Index Mean (SD) Median (25th75th percentile) | 3.0 (1.5) 3 (2–4) | 3.1 (1.5) 3 (2–4) | 3.0 (1.4) 3 (2–4) | 3.0 (1.4) 3 (2–4) | 3.3 (1.4) 3 (2–4) | 2.8 (1.5) 2 (2–4) | 3.0 (1.5) 3 (2–4) | 0.066 | <0.001 |
| Medicationsn (%) | |||||||||
| HF drugs | |||||||||
| Diuretics | 15,780 (74.1) | 7759 (75.2) | 649 (71.9) | 5964 (72.5) | 2174 (72.6) | 3790 (72.5) | 1408 (76.3) | 0.029 | <0.001 |
| ACEi/ARB | 14,335 (67.3) | 7840 (76.0) | 574 (63.6) | 4806 (58.4) | 1734 (57.9) | 3072 (58.7) | 1115 (60.4) | <0.001 | <0.001 |
| Beta-blockers | 13,043 (61.2) | 6631 (64.2) | 602 (66.7) | 4693 (57.1) | 1711 (57.1) | 2982 (57.0) | 1117 (60.5) | 0.143 | <0.001 |
| Aldosterone antagonists | 4973 (23.4) | 2609 (25.3) | 207 (22.9) | 1765 (21.5) | 654 (21.8) | 1111 (21.2) | 392 (21.2) | 0.118 | <0.001 |
| Digoxin | 4311 (20.2) | 2307 (22.4) | 162 (17.9) | 1437 (17.5) | 526 (17.6) | 911 (17.4) | 405 (21.9) | 0.002 | <0.001 |
| Ivabradine | 1502 (7.1) | 873 (8.5) | 38 (4.2) | 449 (5.5) | 181 (6.0) | 268 (5.1) | 142 (7.7) | <0.001 | <0.001 |
| Hydralazine and nitrate | 19 (0.1) | 7 (0.1) | 1 (0.1) | 11 (0.1) | 5 (0.2) | 6 (0.1) | 0 | 0.643 | 0.152 |
| ARNI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Other cardiovascular drugs at baseline | |||||||||
| Lipid-lowering drugs | 11,282 (53.0) | 5888 (57.0) | 534 (59.1) | 3925 (47.7) | 1419 (47.4) | 2506 (47.9) | 935 (50.7) | 0.222 | <0.001 |
| Any antiplatelet drugs ASA P2Y12 inhibitors DAPT (ASA + P2Y12) | 7732 (36.3) 5269 (24.7) 2174 (10.2) 876 (4.1) | 4223 (40.9) 3012 (29.2) 1181 (11.4) 442 (4.3) | 294 (32.6) 200 (22.2) 80 (8.9) 30 (3.3) | 2567 (31.2) 1647 (20.0) 708 (8.6) 346 (4.2) | 921 (30.8) 596 (19.9) 264 (8.8) 144 (4.8) | 1646 (31.5) 1051 (20.1) 444 (8.5) 202 (3.9) | 648 (35.1) 410 (22.2) 205 (11.1) 58 (3.1) | <0.001 <0.001 0.019 0.168 | <0.001 <0.001 <0.001 0.801 |
| Anticoagulants | 6048 (28.4) | 2271 (22.0) | 242 (26.8) | 2987 (36.3) | 1072 (35.8) | 1915 (36.6) | 548 (29.7) | 0.001 | <0.001 |
| Calcium channel blockers | 4461 (21.0) | 751 (7.3) | 154 | 3155 (38.4) | 1153 (38.5) | 2002 (38.3) | <0.001 | (17.1) | <0.001 |
| Nitrates | 2428 (11.4) | 1196 (11.6) | 116 (12.9) | 878 (10.7) | 329 (11.0) | 549 (10.5) | 238 (12.9) | 0.258 | 0.050 |
| Nicorandil | 14 (0.1) | 5 (0.05) | 0 | 7 (0.1) | 3 (0.10) | 4 (0.1) | 2 (0.1) | 0.508 | 0.329 |
| Antidiabetic drugs at baseline | |||||||||
| Metformin | 6414 (30.1) | 3428 (33.2) | 283 (31.3) | 2138 (26.0) | 763 (25.5) | 1375 (26.3) | 565 (30.6) | 0.253 | <0.001 |
| Sulfonylurea | 2626 (12.3) | 1339 (13.0) | 141 (15.6) | 918 (11.2) | 321 (10.7) | 597 (11.4) | 228 (12.4) | 0.024 | <0.001 |
| DPP-4i | 2509 (11.8) | 1466 (14.2) | 67 (7.4) | 763 (9.3) | 277 (9.3) | 486 (9.3) | 213 (11.5) | <0.001 | <0.001 |
| Insulin | 1571 (7.4) | 790 (7.7) | 58 (6.4) | 591 (7.2) | 196 (6.5) | 395 (7.6) | 132 (7.2) | 0.180 | 0.228 |
| SGLT2i | 1115 (5.2) | 704 (6.8) | 34 (3.8) | 267 (3.3) | 89 (3.0) | 178 (3.4) | 110 (6.0) | <0.001 | <0.001 |
| Other glucose-lowering drugs | 822 (3.9) | 476 (4.6) | 38 (4.2) | 230 (2.8) | 86 (2.9) | 144 (2.8) | 78 (4.2) | 0.579 | <0.001 |
| GLP1-RA | 229 (1.1) | 98 (1.0) | 13 (1.4) | 98 (1.2) | 35 (1.2) | 63 (1.2) | 20 (1.1) | 0.153 | 0.109 |
| Other drugs at baseline | |||||||||
| PPIs | 13,942 (65.5) | 7704 (74.6) | 486 (53.8) | 4608 (56.0) | 1655 (55.3) | 2953 (56.5) | 1144 (62.0) | <0.001 | <0.001 |
| NSAIDs | 9978 (46.9) | 5410 (52.4) | 297 (32.9) | 3399 (41.3) | 1215 (40.6) | 2184 (41.8) | 872 (47.2) | <0.001 | <0.001 |
| Number of drugs at index date | |||||||||
| 0 | 5 (0.02) | 1 (0.01) | 0 | 3 (0.04) | 3 (0.1) | 0 | 1 (0.05) | ||
| 1 | 88 (0.4) | 22 (0.2) | 5 (0.6) | 55 (0.7) | 18 (0.6) | 37 (0.7) | 6 (0.3) | ||
| 2 | 460 (2.2) | 127 (1.2) | 38 (4,2) | 261 (3.2) | 97 (3,2) | 164 (3,1) | 34 (1.8) | ||
| 3 | 1489 (7.0) | 508 (4,9) | 86 (9,5) | 751 (9.1) | 258 (8.6) | 493 (9,4) | 144 (7.8) | ||
| 4 | 2884 (13.5) | 1134 (11.0) | 172 (19,1) | 1310 (15.9) | 516 (17.2) | 794 (15,2) | 268 (14.5) | ||
| 5 | 4253 (20.0) | 1865 (18,1) | 185 (20,5) | 1825 (22.2) | 658 (22.0) | 1167 (22,3) | 378 (20.5) | ||
| ≥6 | 12,118 (57.0) | 6666 (64.6) | 417 (46.2) | 4020 (48.9) | 1445 (48.2) | 2575 (49.2) | 1015 (55.0) | ||
| HF Prevalent Cohort (n = 23,806; 100%) | HFrEF (n = 11,746; 49.3%) | HFmrEF (n = 1031; 4.3%) | HFpEF (n = 9062; 38.1%) | HFpEF (50 to <60) (n = 3321; 14.0%) | HFpEF (≥60) (n = 5741; 24.1%) | HFuEF (n = 1967; 8.3%) | p-Value (HFmrEF vs. HFrEF) | p-Value (HFpEF vs. HFrEF) | |
|---|---|---|---|---|---|---|---|---|---|
| Biodemographic data | |||||||||
| Age (years) at index date Mean (SD) Median (25th75th percentile) Range (min–max) | 78.8 (12.4) 79.3 (70.9–88.4) (18.0–99.5) | 73.7 (10.1) 73.2 (68.3–80.2) (18.0–99.4) | 81.0 (11.2) 81.0 (75.9–89.3) (18.1–99.3) | 84.4 (12.1) 86.9 (79.0–93.1) (18.9–99.5) | 84.3 (12.3) 86.9 (79.0–89.3) (19.7–99.3) | 84.5 (12.1) 87.0 (79.1–93.1) (18.9–99.4) | 82.4 (13.8) 86.7 (73.2–93.1) (19.5–99.4) | <0.001 | <0.001 |
| Age groupsn (%) <45 years 45–64 years 65–74 years 75–84 years ≥85 years | 147 (0.6) 1918 (8.1) 5995 (25.2) 8063 (33.9) 7683 (32.3) | 52 (0.4) 1051 (9.0) 5296 (45.1) 4320 (36.8) 1027 (8.7) | 6 (0.6) 57 (5.5) 129 (12.5) 485 (47.0) 354 (34.3) | 64 (0.7) 663 (7.3) 214 (2.4) 2918 (32.2) 5203 (57.4) | 26 (0.9) 242 (7.3) 80 (2.4) 1076 (32.4) 1897 (57.1) | 38 (0.7) 421 (7.3) 134 (2.3) 1842 (32.1) 3306 (57.6) | 25 (1.3) 147 (7.5) 356 (18.1) 340 (17.3) 1099 (55.9) | ||
| Gendermalen (%) | 12,780 (53.7) | 7713 (65.7) | 523 (50.7) | 3528 (38.9) | 1268 (38.2) | 2260 (39.4) | 1016 (51.7) | <0.001 | <0.001 |
| NYHA at index daten (%) Class I Class II Class III Class IV Unknown | 2391 (10.0) 9967 (41.9) 9785 (41.1) 1132 (4.8) 531 (2.2) | 1194 (10.2) 4176 (35.6) 5400 (46.0) 701 (6.0) 275 (2.3) | 99 (9.6) 367 (35.6) 489 (47.4) 58 (5.6) 18 (1.8) | 879 (9.7) 4627 (51.1) 3079 (34.0) 293 (3.2) 184 (2.0) | 312 (9.4) 1669 (50.3) 1153 (34.7) 117 (3.5) 70 (2.1) | 567 (9.9) 2958 (51.5) 1926 (33.6) 176 (3.1) 114 (2.0) | 219 (11.1) 797 (40.5) 817 (41.5) 80 (4.1) 54 (2.8) | 0.665 | <0.001 |
| Cardiovascular risk factorsn (%) | |||||||||
| Hypertension | 16,168 (67.9) | 7897 (67.2) | 775 (75.2) | 6133 (67.7) | 2229 (67.1) | 3904 (68.0) | 1363 (69.3) | <0.001 | 0.495 |
| Dyslipidemia | 11,650 (48.9) | 5373 (45.7) | 492 (47.7) | 4800 (53.0) | 1727 (52.0) | 3073 (53.5) | 985 (50.1) | 0.222 | <0.001 |
| Diabetes type 1 | 960 (4.0) | 565 (4.8) | 41 (4.0) | 279 (3.1) | 128 (3.9) | 151 (2.6) | 75 (3.8) | 0.227 | <0.001 |
| Diabetes type 2 | 7603 (31.9) | 3794 (32.3) | 287 (27.8) | 2899 (32.0) | 1288 (38.8) | 1611 (28.1) | 623 (31.7) | 0.003 | 0.635 |
| Vascular diseasen (%) | |||||||||
| Coronary artery disease | 8986 (37.8) | 5053 (43.0) | 324 (31.4) | 2871 (31.7) | 1071 (32.3) | 1800 (31.4) | 738 (37.5) | <0.001 | <0.001 |
| Chronic kidney disease Stage Unknown Stage I Stage II Stage III Stage IV Stage V | 7271 (30.5) 2985 (12.5) 190 (0.8) 725 (3.01) 2586 (10.9) 576 (2.4) 209 (1.0) | 3935 (33.5) 1642 (14.0) 99 (0.8) 376 (3.2) 1390 (11.8) 316 (2.7) 112 (1.1) | 328 (31.8) 132 (12.8) 8 (0.8) 34 (3.3) 122 (11.8) 21 (2.0) 11 (1.1) | 2490 (27.5) 1001 (11.1) 72 (0.8) 269 (3.0) 896 (9.9) 183 (2.0) 69 (0.9) | 923 (27.8) 366 (11.0) 19 (0.6) 97 (2.9) 344 (10.4) 75 (2.3) 22 (0.9) | 1567 (27.3) 635 (11.1) 53 (0.9) 172 (3.0) 552 (9.6) 108 (1.9) 47 (0.9) | 518 (26.3) 210 (10.7) 11 (0.6) 46 (2.3) 178 (9.1) 56 (2.9) 17 (0.6) | 0.271 | <0.001 |
| Myocardial Infarction | 3571 (15.0) | 1904 (16.2) | 118 (11.5) | 1239 (13.7) | 458 (13.8) | 781 (13.6) | 310 (15.8) | <0.001 | <0.001 |
| Stroke | 2525 (10.6) | 1497 (12.7) | 122 (11.8) | 692 (7.6) | 320 (9.6) | 372 (6.5) | 214 (10.9) | 0.399 | <0.001 |
| Peripheral arterial disease | 1169 (4.9) | 684 (5.8) | 29 (2.8) | 349 (3.9) | 164 (4.9) | 185 (3.2) | 107 (5.4) | <0.001 | <0.001 |
| Other comorbiditiesn (%) | |||||||||
| COPD | 3658 (15.4) | 1905 (16.2) | 127 (12.3) | 1319 (14.6) | 503 (15.2) | 816 (14.2) | 307 (15.6) | 0.001 | 0.001 |
| Atrial fibrillation | 7596 (31.9) | 2931 (25.0) | 315 (30.6) | 3718 (41.0) | 1353 (40.7) | 2365 (41.2) | 632 (32.1) | <0.001 | <0.001 |
| Anemia within 1 year before index date | 7276 (30.6) | 3721 (31.7) | 290 (28.1) | 2707 (29.9) | 973 (29.3) | 1734 (30.2) | 558 (28.4) | 0.018 | 0.005 |
| Cancer before index date | 3160 (13.3) | 1514 (12.9) | 125 (12.1) | 1230 (13.6) | 435 (13.1) | 795 (13.9) | 291 (14.8) | 0.481 | 0.148 |
| Dementia | 1264 (5.31) | 655 (5.6) | 64 (6.2) | 440 (4.9) | 200 (6.0) | 240 (4.2) | 105 (5.3) | 0.399 | 0.021 |
| Charlson Comorbidity Index Mean (SD) Median (25th75th percentile) | 3.1 (1.4) 3 (2–4) | 3.2 (1.5) 3 (2–4) | 3.1 (1.4) 3 (2–4) | 3.1 (1.4) 3 (2–4) | 3.5 (1.4) 3 (2–4) | 2.8 (1.3) 3 (2–4) | 3.1 (1.3) 3 (2–4) | 0.455 3 (2–4) | <0.001 |
| Medicationsn (%) | |||||||||
| HF drugs | |||||||||
| Diuretics | 16,607 (69.8) | 8262 (70.3) | 689 (66.8) | 6277 (69.3) | 2309 (69.5) | 3968 (69.1) | 1379 (70.1) | 0.018 | 0.095 |
| ACEi/ARB | 16,134 (67.8) | 8957 (76.3) | 631 (61.2) | 5331 (58.8) | 2017 (60.7) | 3314 (57.7) | 1215 (61.8) | <0.001 | <0.001 |
| Beta-blockers | 15,690 (65.9) | 8164 (69.5) | 694 (67.3) | 5537 (61.1) | 1996 (60.1) | 3541 (61.7) | 1295 (65.8) | 0.143 | <0.001 |
| Aldosterone antagonists | 6594 (27.7) | 4324 (36.8) | 185 (17.9) | 1584 (17.5) | 612 (18.4) | 972 (16.9) | 501 (25.5) | 0.126 | 0.020 |
| Digoxin | 4542 (19.1) | 2481 (21.1) | 172 (16.7) | 1500 (16.6) | 571 (17.2) | 929 (16.2) | 389 (19.8) | 0.001 | <0.001 |
| ARNI | 2857 (12.0) | 1468 (12.5) | 112 (10.9) | 1037 (11.4) | 405 (12.2) | 632 (11.0) | 240 (12.2) | <0.001 | <0.001 |
| Ivabradine | 1440 (6.1) | 848 (7.2) | 40 (3.9) | 424 (4.7) | 161 (4.9) | 263 (4.6) | 128 (6.59) | <0.001 | <0.001 |
| Hydralazine and nitrate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Other cardiovascular drugs at baseline | |||||||||
| Lipid-lowering drugs | 12,700 (53.4) | 6703 (57.1) | 606 (58.8) | 4395 (48.5) | 1611 (48.5) | 2784 (48.5) | 996 (50.6) | 0.287 | <0.001 |
| Any antiplatelet drugs ASA P2Y12 inhibitors DAPT (ASA + P2Y12) | 8710 (36.6) 5830 (24.5) 2417 (10.2) 953 (4.0) | 4862 (41.4) 3349 (28.5) 1364 (11.6) 502 (4.3) | 356 (34.5) 232 (22.5) 92 (8.9) 49 (4.8) | 2819 (31.1) 1791 (19.8) 771 (8.5) 335 (3,7) | 1017 (30.6) 643 (19.4) 281 (8.5) 118 (3.6) | 1802 (31.4) 1148 (20.0) 490 (8.5) 217 (3.8) | 673 (34.2) 458 (23.3) 190 (9.7) 67 (3.4) | <0.001 <0.001 0.009 0.468 | <0.001 <0.001 <0.001 0.036 |
| Anticoagulants | 6640 (27.9) | 2580 (22.0) | 269 (26.1) | 3239 (35,7) | 1167 (35.1) | 2072 (36.1) | 552 (28.1) | 0.002 | <0.001 |
| Calcium channel blockers | 4815 (20.2) | 833 (7.1) | 157 (15.2) | 3406 (37.6) | 1197 (36.0) | 2209 (38.5) | 419 (21.3) | <0.001 | <0.001 |
| Nitrates | 2710 (11.4) | 1409 (12.0) | 133 (12.9) | 935 (10.3) | 362 (10.9) | 573 (10.0) | 233 (11.9) | 0.393 | <0.001 |
| Nicorandil | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Antidiabetic drugs at baseline | |||||||||
| Metformin | 6936 (29.1) | 3751 (31.9) | 297 (28.8) | 2323 (25.6) | 824 (24.8) | 1499 (26.1) | 565 (28.7) | 0.039 | <0.001 |
| Sulfonylurea | 2874 (12.1) | 1403 (11.9) | 146 (14.2) | 1094 (12.1) | 417 (12.6) | 677 (11.8) | 231 (11.7) | 0.037 | 0.778 |
| DPP-4i | 2785 (11.7) | 1622 (13.8) | 80 (7.8) | 814 (9.0) | 292 (8.8) | 522 (9.1) | 269 (13.7) | <0.001 | <0.001 |
| Insulin | 1764 (7.4) | 930 (7.9) | 66 (6.4) | 626 (6.9) | 223 (6.7) | 403 (7.0) | 142 (7.2) | 0.082 | 0.006 |
| SGLT2i | 1218 (5.1) | 792 (6.7) | 26 (2.5) | 291 (3.2) | 94 (2.8) | 197 (3.4) | 109 (5.5) | <0.001 | <0.001 |
| Other glucose-lowering drugs | 948 (4.0) | 552 (4.7) | 37 (3.6) | 270 (3.0) | 117 (3.5) | 153 (2.7) | 89 (4.5) | 0.103 | <0.001 |
| GLP1-RA | 239 (1.0) | 112 (1.0) | 15 (1.5) | 94 (1.0) | 33 (1.0) | 61 (1.1) | 18 (0.9) | 0.120 | 0.545 |
| Other drugs at baseline | |||||||||
| PPIs | 15,533 (65.3) | 8724 (74.3) | 540 (52.4) | 5052 (55.8) | 1864 (56.1) | 3188 (55.5) | 1217 (61.9) | <0.001 | <0.001 |
| NSAIDs | 11,246 (47.2) | 6217 (52.9) | 344 (33.4) | 3769 (41.6) | 1386 (41.7) | 2383 (41.5) | 916 (46.6) | <0.001 | <0.001 |
| Number of drugs at index date | |||||||||
| 0 | 11 (0.05) | 3 (0.03) | 1 (0.1) | 7 (0.1) | 6 (0.2) | 1 (0.02) | 0 | ||
| 1 | 121 (0.5) | 17 (0.1) | 12 (1.2) | 79 (0.9) | 29 (0.9) | 50 (0.9) | 13 (0.7) | ||
| 2 | 532 (2.2) | 129 (1.1) | 37 (3.6) | 321 (3.5) | 118 (3.6) | 203 (3.5) | 45 (2.3) | ||
| 3 | 1693 (7.1) | 511 (4.4) | 105 (10.2) | 947 (10.5) | 361 (10.9) | 586 (10.2) | 130 (6.6) | ||
| 4 | 3193 (13.4) | 1158 (9.9) | 214 (20.8) | 1548 (17.1) | 586 (17.7) | 962 (16.8) | 273 (13.9) | ||
| 5 | 4720 (19.8) | 2041 (17.4) | 249 (24.2) | 2012 (22.2) | 736 (22.2) | 1276 (22.2) | 418 (21.3) | ||
| ≥6 | 13,536 (56.9) | 7887 (67.1) | 413 (40.1) | 4148 (45.8) | 1485 (44.7) | 2663 (46.4) | 1088 (55.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar, C.; Palacios, B.; Varela, L.; Gutiérrez, M.; Duong, M.; Chen, H.; Justo, N.; Cid-Ruzafa, J.; Hernández, I.; Hunt, P.R.; et al. Prevalence, Characteristics, Management and Outcomes of Patients with Heart Failure with Preserved, Mildly Reduced, and Reduced Ejection Fraction in Spain. J. Clin. Med. 2022, 11, 5199. https://doi.org/10.3390/jcm11175199
Escobar C, Palacios B, Varela L, Gutiérrez M, Duong M, Chen H, Justo N, Cid-Ruzafa J, Hernández I, Hunt PR, et al. Prevalence, Characteristics, Management and Outcomes of Patients with Heart Failure with Preserved, Mildly Reduced, and Reduced Ejection Fraction in Spain. Journal of Clinical Medicine. 2022; 11(17):5199. https://doi.org/10.3390/jcm11175199
Chicago/Turabian StyleEscobar, Carlos, Beatriz Palacios, Luis Varela, Martín Gutiérrez, Mai Duong, Hungta Chen, Nahila Justo, Javier Cid-Ruzafa, Ignacio Hernández, Phillip R. Hunt, and et al. 2022. "Prevalence, Characteristics, Management and Outcomes of Patients with Heart Failure with Preserved, Mildly Reduced, and Reduced Ejection Fraction in Spain" Journal of Clinical Medicine 11, no. 17: 5199. https://doi.org/10.3390/jcm11175199
APA StyleEscobar, C., Palacios, B., Varela, L., Gutiérrez, M., Duong, M., Chen, H., Justo, N., Cid-Ruzafa, J., Hernández, I., Hunt, P. R., & Delgado, J. F. (2022). Prevalence, Characteristics, Management and Outcomes of Patients with Heart Failure with Preserved, Mildly Reduced, and Reduced Ejection Fraction in Spain. Journal of Clinical Medicine, 11(17), 5199. https://doi.org/10.3390/jcm11175199









