Risk Factors for Progressive Spinal Sagittal Imbalance in the Short-Term Course after Total Hip Arthroplasty: A 3 Year Follow-Up Study of Female Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Evaluation of the Demographic and Radiographic Parameters of Participants
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillemin, F.; Rat, A.C.; Mazieres, B.; Pouchot, J.; Fautrel, B.; Euller-Ziegler, L.; Fardellone, P.; Morvan, J.; Roux, C.H.; Verrouil, E.; et al. Prevalence of symptomatic hip and knee osteoarthritis: A two-phase population-based survey. Osteoarthr. Cartilage 2011, 19, 1314–1322. [Google Scholar] [CrossRef]
- Ochi, H.; Homma, Y.; Baba, T.; Nojiri, H.; Matsumoto, M.; Kaneko, K. Sagittal spinopelvic alignment predicts hip function after total hip arthroplasty. Gait Posture 2017, 52, 293–300. [Google Scholar] [CrossRef]
- Buckland, A.J.; Steinmetz, L.; Zhou, P.; Vasquez-Montes, D.; Kingery, M.; Stekas, N.D.; Ayres, E.W.; Varlotta, C.G.; Lafage, V.; Lafage, R.; et al. Spinopelvic Compensatory Mechanisms for Reduced Hip Motion (ROM) in the Setting of Hip Osteoarthritis. Spine Deform. 2019, 7, 923–928. [Google Scholar] [CrossRef]
- Day, L.M.; DelSole, E.M.; Beaubrum, B.M.; Zhou, P.L.; Moon, J.Y.; Tishelman, J.C.; Vigdorchik, J.M.; Schwarzkopf, R.; Lafage, R.; Lafage, V.; et al. Radiological severity of hip osteoarthritis in patients with adult spinal deformity: The effect on spinopelvic and lower extremity compensatory mechanisms. Eur. Spine J. 2018, 27, 2294–2302. [Google Scholar] [CrossRef]
- Innmann, M.M.; Merle, C.; Phan, P.; Beaulé, P.E.; Grammatopoulos, G. Differences in Spinopelvic Characteristics Between Hip Osteoarthritis Patients and Controls. J. Arthroplast. 2021, 36, 2808–2816. [Google Scholar] [CrossRef]
- Sculco, P.K.; Windsor, E.N.; Jerabek, S.A.; Mayman, D.J.; Elbuluk, A.; Buckland, A.J.; Vigdorchik, J.M. Preoperative spinopelvic hypermobility resolves following total hip arthroplasty. Bone Jt. J. 2021, 103-B, 1766–1773. [Google Scholar] [CrossRef]
- Weng, W.J.; Wang, W.J.; Wu, M.D.; Xu, Z.H.; Xu, L.L.; Qiu, Y. Characteristics of sagittal spine-pelvis-leg alignment in patients with severe hip osteoarthritis. Eur. Spine J. 2015, 24, 1228–1236. [Google Scholar] [CrossRef]
- Miyagi, M.; Fukushima, K.; Inoue, G.; Nakazawa, T.; Imura, T.; Saito, W.; Takahira, N.; Takaso, M. Hip-spine syndrome: Cross-sectional-study of spinal alignment in patients with coxalgia. Hip Int. 2019, 29, 21–25. [Google Scholar] [CrossRef]
- Sloan, M.; Premkumar, A.; Sheth, N.P. Projected Volume of Primary Total Joint Arthroplasty in the U.S.; 2014 to 2030. J. Bone Jt. Surg. Am. 2018, 100, 1455–1460. [Google Scholar] [CrossRef]
- Kim, Y.; Pour, A.E.; Lazennec, J.Y. How do global sagittal alignment and posture change after total hip arthroplasty? Int. Orthop. 2020, 44, 267–273. [Google Scholar] [CrossRef]
- Jain, D.; Vigdorchik, J.M.; Abotsi, E.; Montes, D.V.; Delsole, E.M.; Lord, E.; Zuckerman, J.D.; Protopsaltis, T.; Passias, P.G.; Buckland, A.J. The Impact of Global Spinal Alignment on Standing Spinopelvic Alignment Change after Total Hip Arthroplasty. Glob. Spine J. 2021, 18, 21925682211026633. [Google Scholar] [CrossRef] [PubMed]
- Piazzolla, A.; Solarino, G.; Bizzoca, D.; Montemurro, V.; Berjano, P.; Lamartina, C.; Martini, C.; Moretti, B. Spinopelvic parameter changes and low back pain improvement due to femoral neck anteversion in patients with severe unilateral primary hip osteoarthritis undergoing total hip replacement. Eur. Spine J. 2018, 27, 125–134. [Google Scholar] [CrossRef]
- Furuhashi, H.; Togawa, D.; Koyama, H.; Hoshino, H.; Yasuda, T.; Matsuyama. Y. Repeated posterior dislocation of total hip arthroplasty after spinal corrective long fusion with pelvic fixation. Eur. Spine J. 2017, 26, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, H.; Yamato, Y.; Hoshino, H.; Shimizu, Y.; Hasegawa, T.; Yoshida, G.; Banno, T.; Arima, H.; Oe, S.; Ushirozako, H.; et al. Dislocation rate and its risk factors in total hip arthroplasty with concurrent extensive spinal corrective fusion with pelvic fixation for adult spinal deformity. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 283–290. [Google Scholar] [CrossRef]
- Bedard, N.A.; Martin, C.T.; Slaven, S.E.; Pugely, A.J.; Mendoza-Lattes, S.A.; Callaghan, J.J. Abnormally High Dislocation Rates of Total Hip Arthroplasty After Spinal Deformity Surgery. J. Arthroplast. 2016, 31, 2884–2885. [Google Scholar] [CrossRef]
- Suzuki, T. Reliability of measurements of knee extensor muscle strength using a pull-type handheld dynamometer. J. Phys. Ther. Sci. 2015, 27, 967–971. [Google Scholar] [CrossRef]
- Kato, S.; Murakami, H.; Inaki, A.; Mochizuki, T.; Demura, S.; Nakase, J.; Yoshioka, K.; Yokogawa, N.; Igarashi, T.; Takahashi, N.; et al. Innovative exercise device for the abdominal trunk muscles: An early validation study. PLoS ONE 2017, 12, e0172934. [Google Scholar] [CrossRef]
- Kato, S.; Inaki, A.; Murakami, H.; Kurokawa, Y.; Mochizuki, T.; Demura, S.; Yoshioka, K.; Yokogawa, N.; Yonezawa, N.; Shimizu, T.; et al. Reliability of the muscle strength measurement and effects of the strengthening by an innovative exercise device for the abdominal trunk muscles. J. Back Musculoskelet. Rehabil. 2020, 33, 677–684. [Google Scholar] [CrossRef]
- Nakamura, K.; Ogata, T. Locomotive Syndrome: Definition and Management. Clin. Rev. Bone Miner. Metab. 2016, 14, 56–67. [Google Scholar] [CrossRef]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional reach: A new clinical measure of balance. J. Gerontol. 1990, 45, M192–M197. [Google Scholar] [CrossRef]
- Schwab, F.; Patel, A.; Ungar, B.; Farcy, J.P.; Lafage, V. Adult spinal deformity-postoperative standing imbalance: How much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine 2010, 35, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Kanto, M.; Maruo, K.; Tachibana, T.; Fukunishi, S.; Nishio, S.; Takeda, Y.; Arizumi, F.; Kusuyama, K.; Kishima, K.; Yoshiya, S. Influence of Spinopelvic Alignment on Pelvic Tilt after Total Hip Arthroplasty. Orthop. Surg. 2019, 11, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Buckland, A.J.; Fernandez, L.; Shimmim, A.J.; Bare, J.V.; McMahon, S.J.; Vigdorchik, J.M. Effects of Sagittal Spinal Alignment on Postural Pelvic Mobility in Total Hip Arthroplasty Candidates. J. Arthroplast. 2019, 34, 2663–2668. [Google Scholar] [CrossRef]
- Tateiwa, T.; Endo, K.; Matsuoka, Y.; Ishida, T.; Shishido, T.; Yamamoto, K. Pelvic tilt after total hip arthroplasty in patients with osteoarthritis of the hip. J. Orthop. Surg. 2020, 28, 2309499020918317. [Google Scholar] [CrossRef]
- Parratte, S.; Pagnano, M.W.; Coleman-Wood, K.; Kaufman, K.R.; Berry, D.J. The 2008 Frank Stinchfield award: Variation in postoperative pelvic tilt may confound the accuracy of hip navigation systems. Clin. Orthop. Relat. Res. 2009, 467, 43–49. [Google Scholar] [CrossRef]
- Weng, W.; Wu, H.; Wu, M.; Zhu, Y.; Qiu, Y.; Wang, W. The effect of total hip arthroplasty on sagittal spinal-pelvic-leg alignment and low back pain in patients with severe hip osteoarthritis. Eur. Spine J. 2016, 25, 3608–3614. [Google Scholar] [CrossRef]
- Kim, W.J.; Shin, H.M.; Lee, J.S.; Song, D.G.; Lee, J.W.; Chang, S.H.; Park, K.Y.; Choy, W.S. Sarcopenia and Back Muscle Degeneration as Risk Factors for Degenerative Adult Spinal Deformity with Sagittal Imbalance and Degenerative Spinal Disease: A Comparative Study. World Neurosurg. 2021, 148, e547–e555. [Google Scholar] [CrossRef]
- Jun, H.S.; Kim, J.H.; Ahn, J.H.; Chang, I.B.; Song, J.H.; Kim, T.H.; Park, M.S.; Kim, Y.C.; Kim, S.W.; Oh, J.K.; et al. The Effect of Lumbar Spinal Muscle on Spinal Sagittal Alignment: Evaluating Muscle Quantity and Quality. Neurosurgery 2016, 79, 847–855. [Google Scholar] [CrossRef]
- Imagama, S.; Ito, Z.; Wakao, N.; Seki, T.; Hirano, K.; Muramoto, A.; Sakai, Y.; Matsuyama, Y.; Hamajima, N.; Ishiguro, N.; et al. Influence of spinal sagittal alignment, body balance, muscle strength, and physical ability on falling of middle-aged and elderly males. Eur. Spine J. 2013, 22, 1346–1353. [Google Scholar] [CrossRef]
- Banno, T.; Yamato, Y.; Hasegawa, T.; Kobayashi, S.; Togawa, D.; Oe, S.; Mihara, Y.; Kurosu, K.; Yamamoto, N.; Matsuyama, Y. Assessment of the Cross-Sectional Areas of the Psoas Major and Multifidus Muscles in Patients with Adult Spinal Deformity: A Case-Control Study. Clin. Spine Surg. 2017, 30, E968–E973. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Kato, S.; Demura, S.; Shinmura, K.; Yokogawa, N.; Yonezawa, N.; Shimizu, T.; Kitagawa, R.; Miaki, H.; Tsuchiya, H.; et al. Validation and comparison of trunk muscle activities in male participants during exercise using an innovative device and abdominal bracing maneuvers. J. Back Musculoskelet. Rehabil. 2022, 35, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Akuthota, V.; Ferreiro, A.; Moore, T.; Fredericson, M. Core stability exercise principles. Curr. Sports Med. Rep. 2008, 7, 39–44. [Google Scholar] [CrossRef]
- Roussouly, P.; Pinheiro-Franco, J.L. Biomechanical analysis of the spino-pelvic organization and adaptation in pathology. Eur. Spine J. 2011, 20, 609–618. [Google Scholar] [CrossRef]
- Kato, S.; Demura, S.; Kurokawa, Y.; Takahashi, N.; Shinmura, K.; Yokogawa, N.; Yonezawa, N.; Shimizu, T.; Kitagawa, R.; Tsuchiya, H. Efficacy and Safety of Abdominal Trunk Muscle Strengthening Using an Innovative Device in Elderly Patients with Chronic Low Back Pain: A Pilot Study. Ann. Rehabil. Med. 2020, 44, 246–255. [Google Scholar] [CrossRef]
- Oe, S.; Togawa, D.; Nakai, K.; Yamada, T.; Arima, H.; Banno, T.; Yasuda, T.; Kobayasi, S.; Yamato, Y.; Hasegawa, T.; et al. The Influence of Age and Sex on Cervical Spinal Alignment among Volunteers Aged Over 50. Spine 2015, 40, 1487–1494. [Google Scholar] [CrossRef]



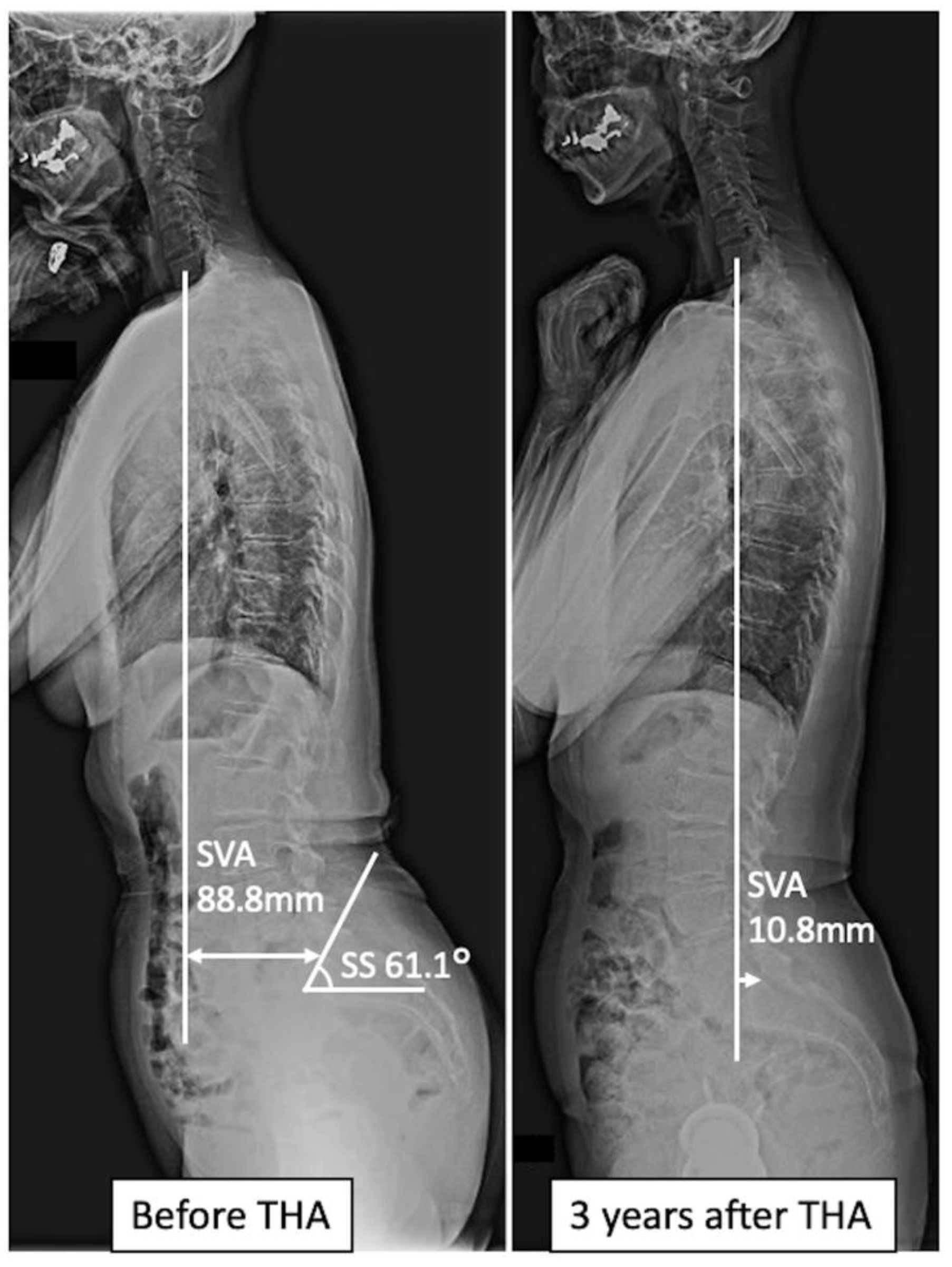
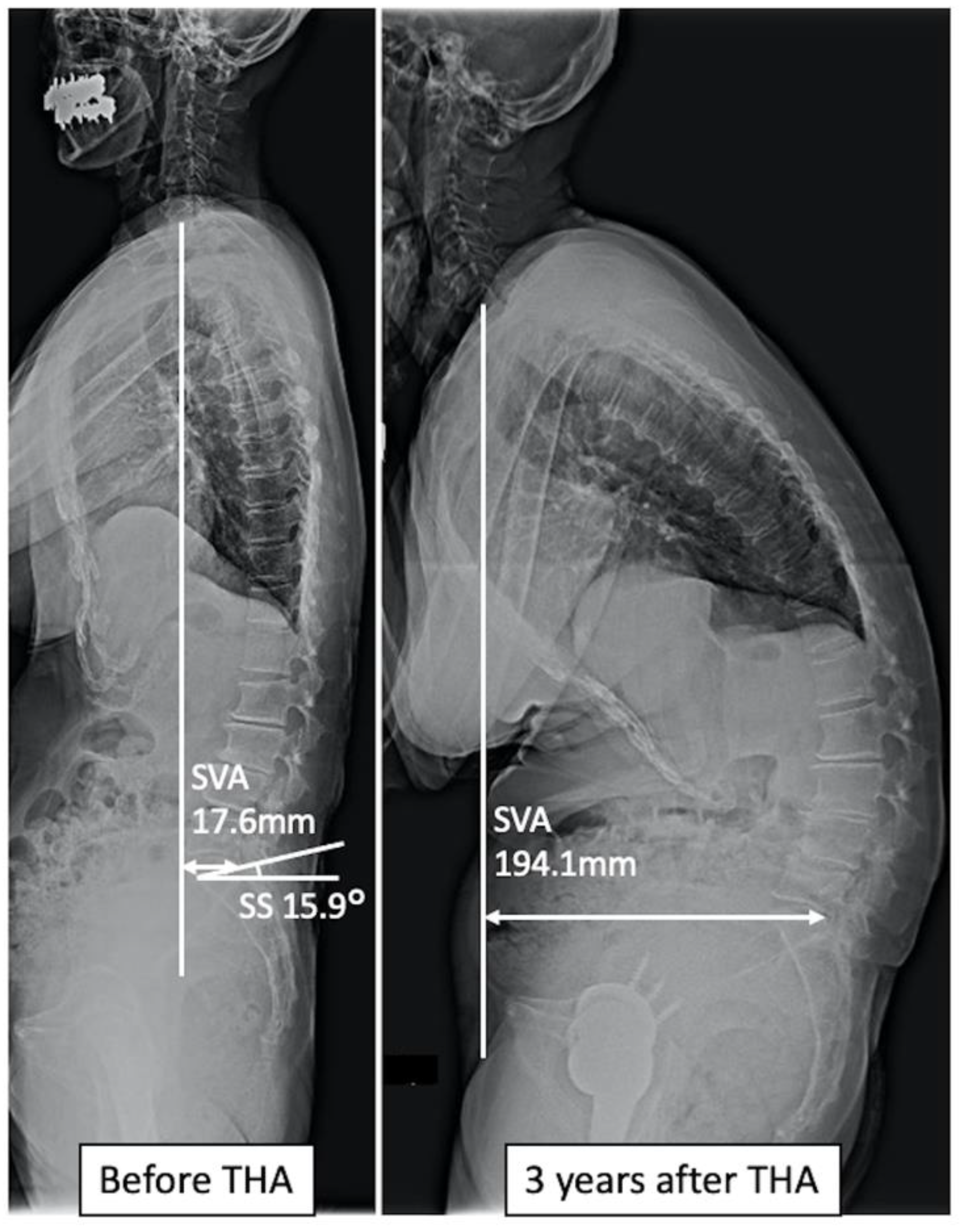
| Imbalance Group | Non-Imbalance Group | p-Value | |
|---|---|---|---|
| No. of subjects | 11 | 92 | |
| Age (years), mean ± SD | 64.7 ± 9.3 | 63.8 ± 8.7 | 0.902 |
| BMI (kg/m2), mean ± SD | 25.0 ± 7.7 | 23.1 ± 3.8 | 0.860 |
| Bilateral simultaneous THA, No. (%) | 2 (18.2) | 18 (19.6) | 0.946 |
| Anterior approach, No. (%) | 9 (81.8) | 67 (72.8) | 0.48 |
| Grip strength (kg), mean ± SD | 20.5 ± 4.9 | 21.1 ± 4.8 | 0.435 |
| KEMS (N/kg), mean ± SD | 2.9 ± 1.4 | 3.9 ± 1.2 | 0.026 |
| ATMS (kPa), mean ± SD | 2.5 ± 1.6 | 5.2 ± 2.9 | 0.013 |
| FRT (cm), mean ± SD | 26.1 ± 6.4 | 30.9 ± 7.1 | 0.019 |
| Walking speed (m/s), mean ± SD | 0.8 ± 0.4 | 0.9 ± 0.2 | 0.359 |
| 1-leg standing time (s), mean ± SD | 25.7 ± 21.4 | 36.7 ± 22.9 | 0.142 |
| 2-step score (point), mean ± SD | 0.8 ± 0.3 | 1.0 ± 0.3 | 0.342 |
| GLFS-25 score (point), mean ± SD | 52.6 ± 18.7 | 43.8 ± 18.5 | 0.173 |
| L-BMD (g/cm2), mean ± SD | 1.2 ± 0.2 | 1.1 ± 0.2 | 0.275 |
| Presence of OVF before THA, No. (%) | 1 (9.0) | 11 (12.0) | 0.624 |
| Occurrence of OVF after THA, No. (%) | 0 (0.0) | 2 (2.1) | 0.797 |
| SVA (mm), mean ± SD | 47.9 ± 26.1 | 45.3 ± 47.2 | 0.665 |
| LL (degrees), mean ± SD | 40.1 ± 18.7 | 53.3 ± 17.9 | 0.019 |
| PI (degrees), mean ± SD | 51.0 ± 12.6 | 57.2 ± 11.6 | 0.075 |
| SS (degrees), mean ± SD | 32.7 ± 10.2 | 42.7 ± 12.4 | 0.006 |
| PT (degrees), mean ± SD | 18.3 ± 8.9 | 14.4 ± 11.4 | 0.175 |
| PI-LL (degrees), mean ± SD | 10.9 ± 15.2 | 3.9 ± 16.9 | 0.172 |
| OR | p-Value | 95% CI | |
|---|---|---|---|
| ATMS (kPa) | 0.504 | 0.007 | 0.307–0.827 |
| SS (degrees) | 0.924 | 0.005 | 0.875–0.976 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagatani, S.; Demura, S.; Kato, S.; Kabata, T.; Kajino, Y.; Yokogawa, N.; Inoue, D.; Kurokawa, Y.; Kobayashi, M.; Yamada, Y.; et al. Risk Factors for Progressive Spinal Sagittal Imbalance in the Short-Term Course after Total Hip Arthroplasty: A 3 Year Follow-Up Study of Female Patients. J. Clin. Med. 2022, 11, 5179. https://doi.org/10.3390/jcm11175179
Nagatani S, Demura S, Kato S, Kabata T, Kajino Y, Yokogawa N, Inoue D, Kurokawa Y, Kobayashi M, Yamada Y, et al. Risk Factors for Progressive Spinal Sagittal Imbalance in the Short-Term Course after Total Hip Arthroplasty: A 3 Year Follow-Up Study of Female Patients. Journal of Clinical Medicine. 2022; 11(17):5179. https://doi.org/10.3390/jcm11175179
Chicago/Turabian StyleNagatani, Satoshi, Satoru Demura, Satoshi Kato, Tamon Kabata, Yoshitomo Kajino, Noriaki Yokogawa, Daisuke Inoue, Yuki Kurokawa, Motoya Kobayashi, Yohei Yamada, and et al. 2022. "Risk Factors for Progressive Spinal Sagittal Imbalance in the Short-Term Course after Total Hip Arthroplasty: A 3 Year Follow-Up Study of Female Patients" Journal of Clinical Medicine 11, no. 17: 5179. https://doi.org/10.3390/jcm11175179
APA StyleNagatani, S., Demura, S., Kato, S., Kabata, T., Kajino, Y., Yokogawa, N., Inoue, D., Kurokawa, Y., Kobayashi, M., Yamada, Y., Kawai, M., & Tsuchiya, H. (2022). Risk Factors for Progressive Spinal Sagittal Imbalance in the Short-Term Course after Total Hip Arthroplasty: A 3 Year Follow-Up Study of Female Patients. Journal of Clinical Medicine, 11(17), 5179. https://doi.org/10.3390/jcm11175179








