Transcatheter Aortic Valve Implantation for Pure Native Aortic Regurgitation: The Last Frontier
Abstract
:1. Introduction
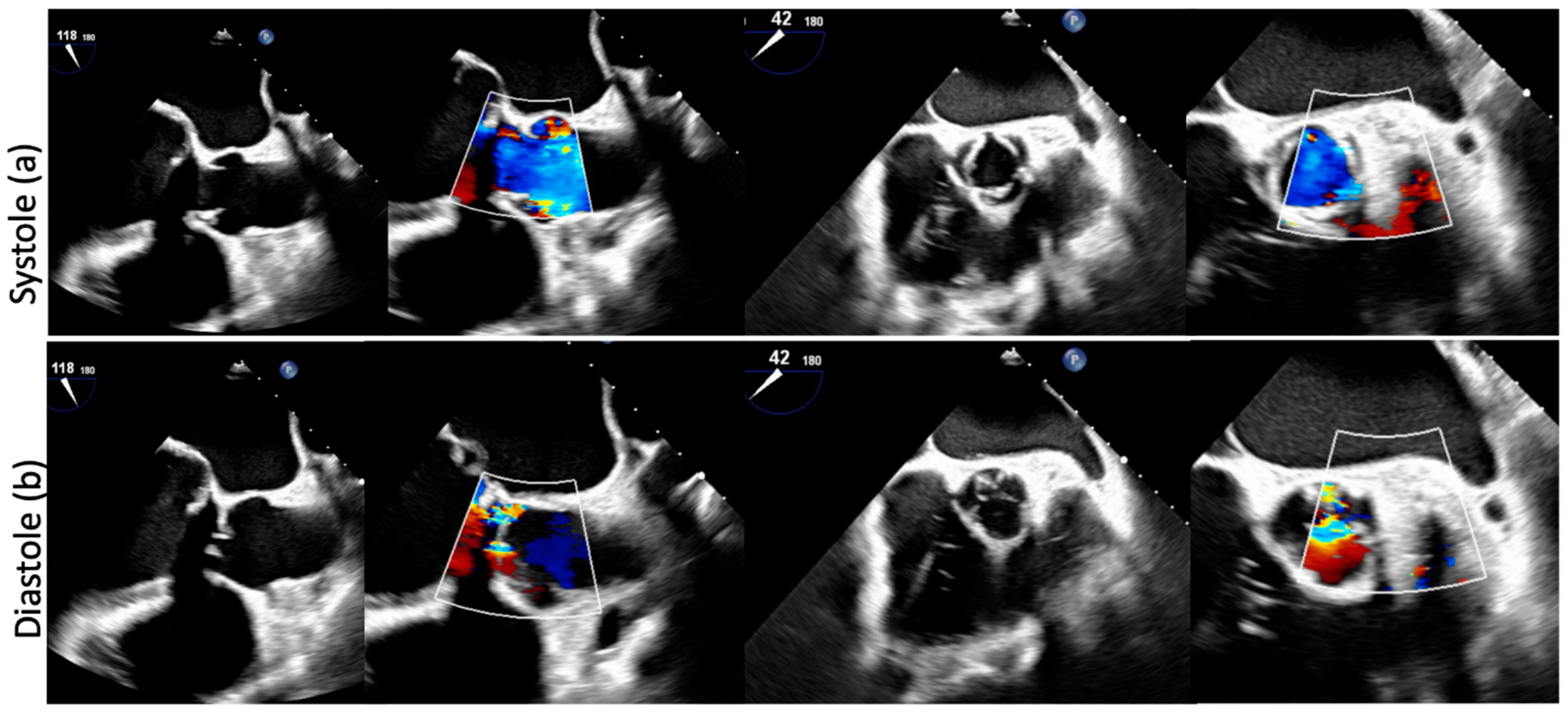
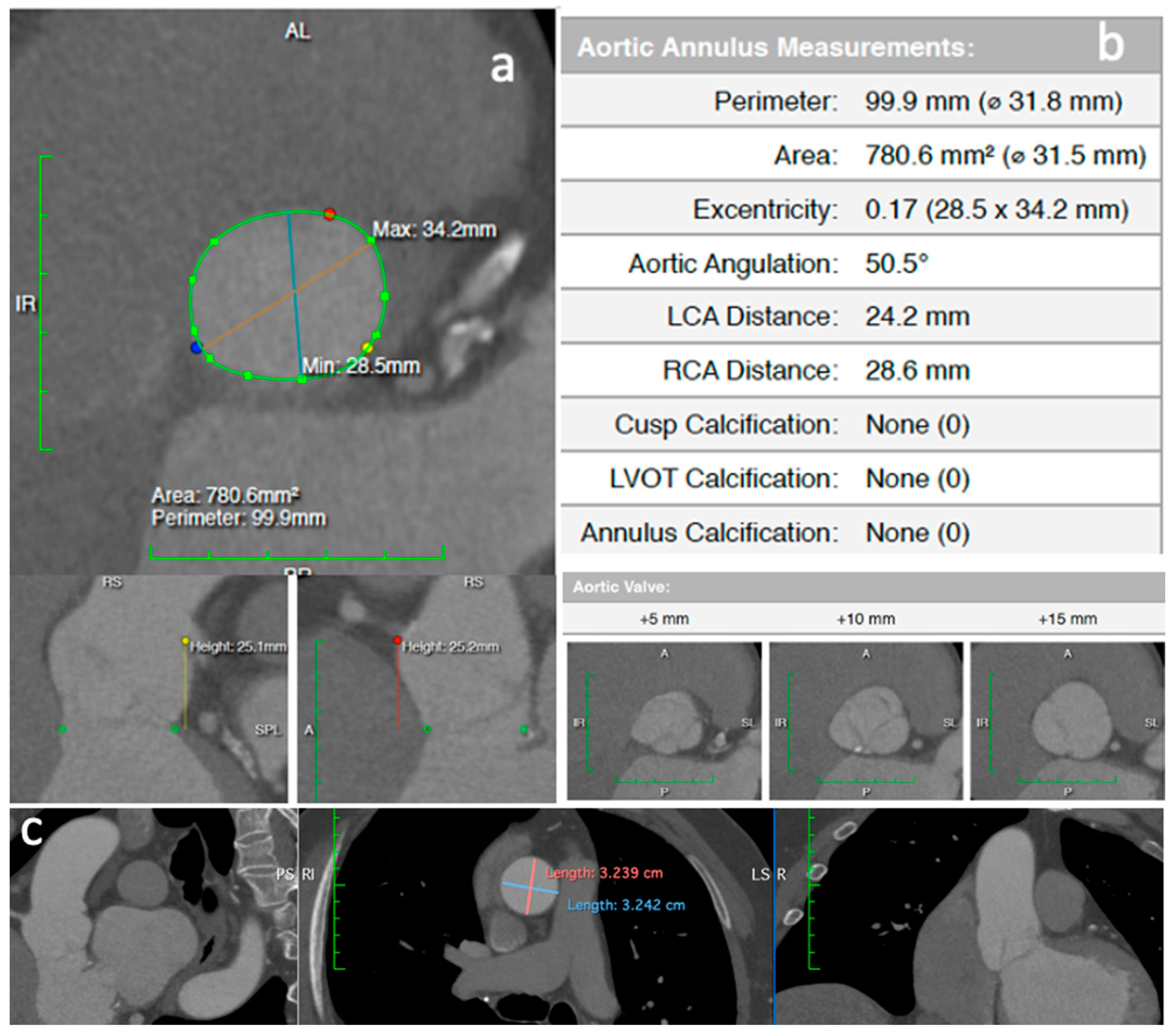
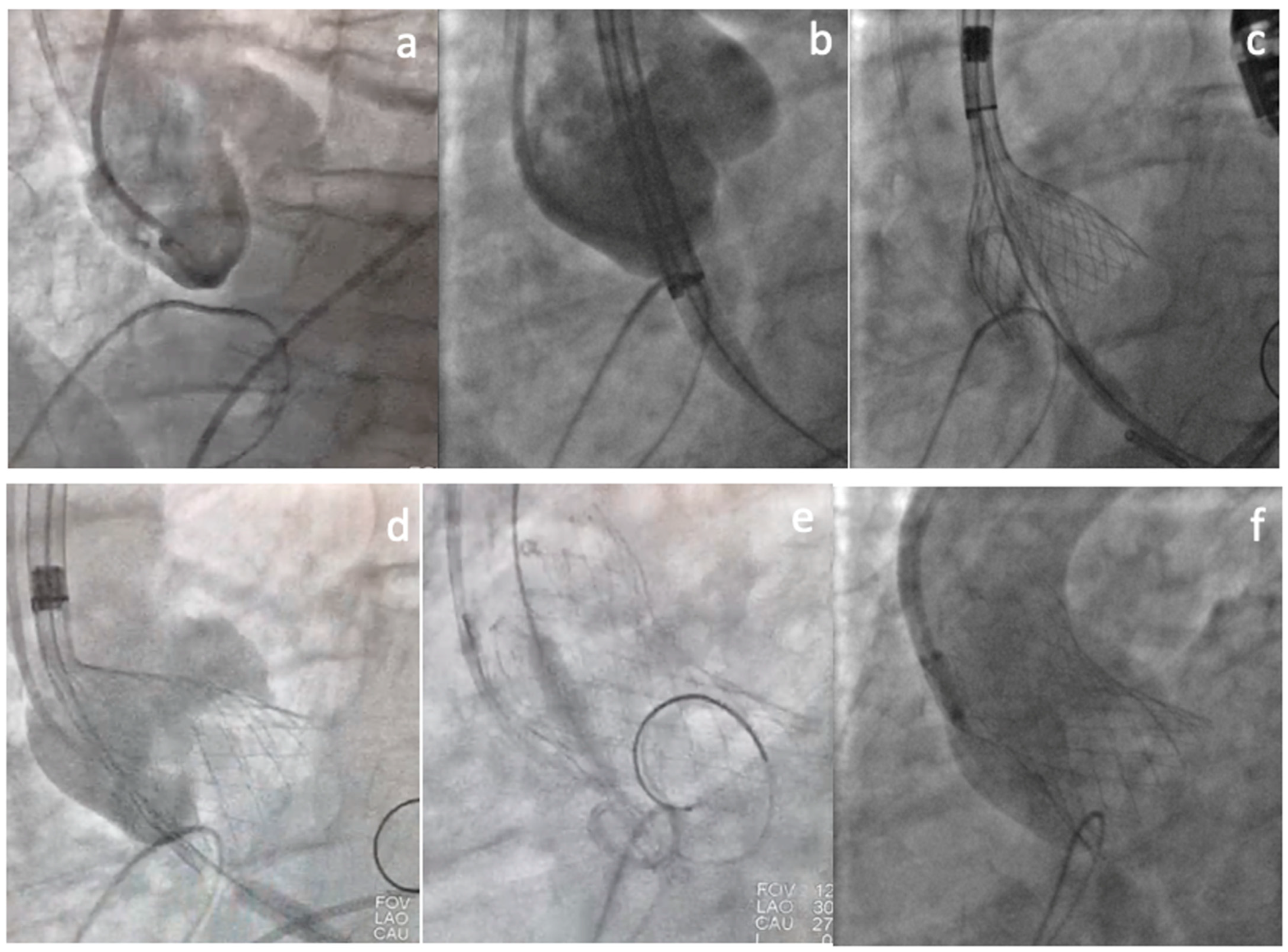
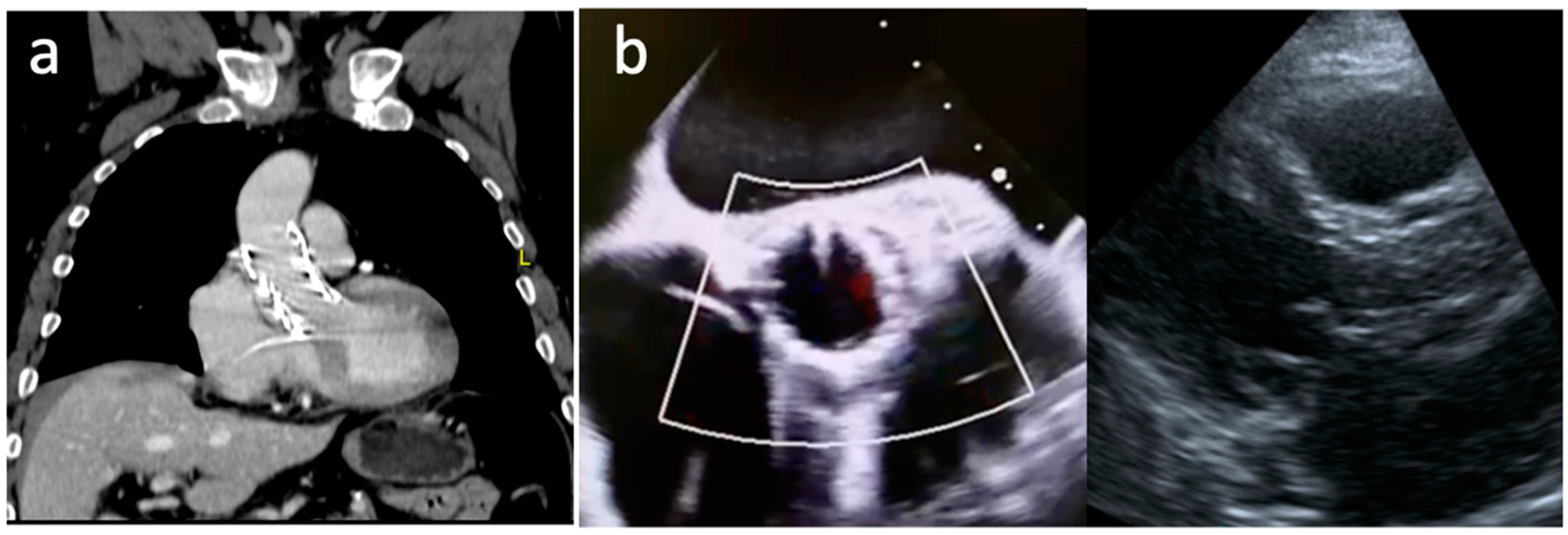
2. Clinical Vignette
3. Discussion
3.1. Aortic Regurgitation Relevance
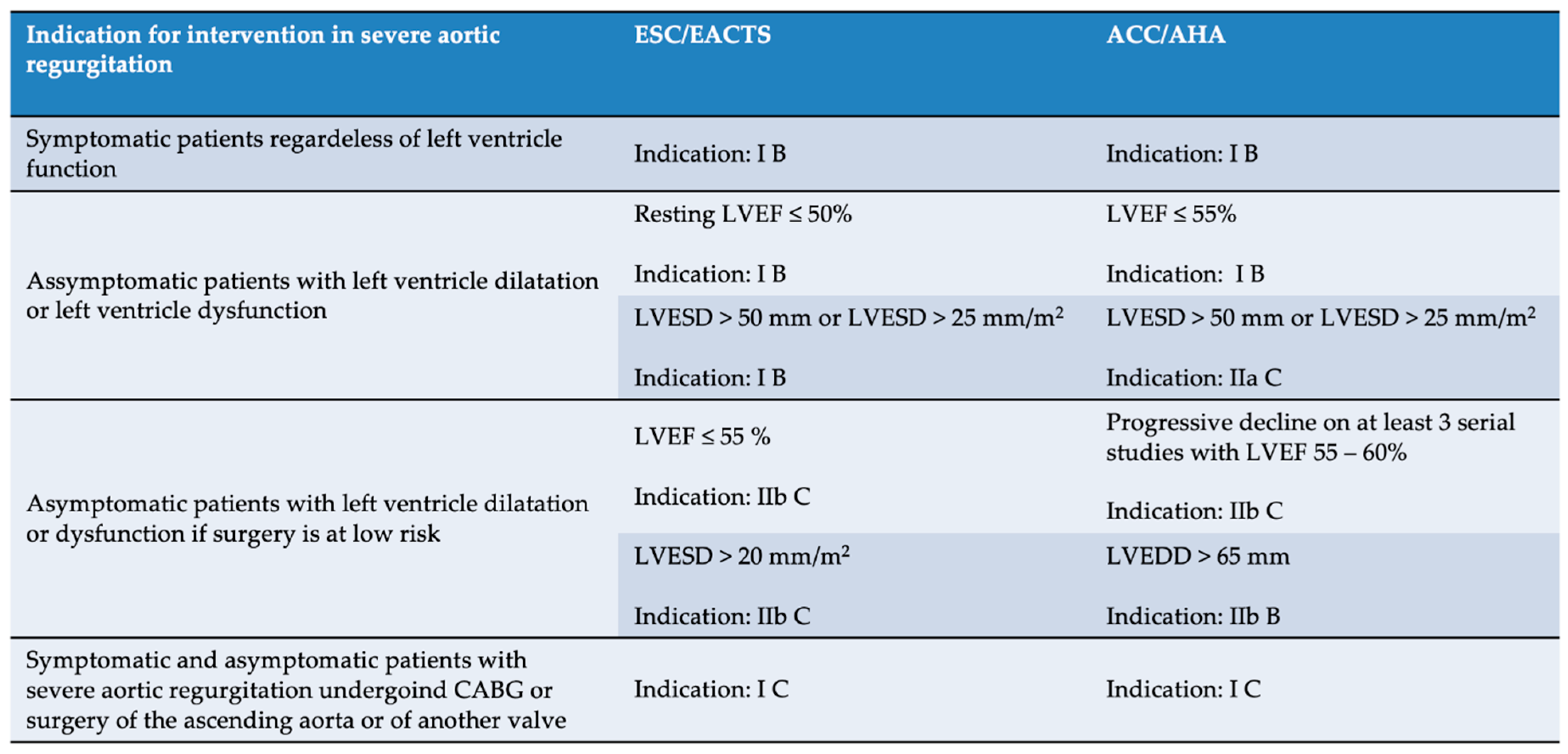
3.2. Intervention in Aortic Regurgitation
3.3. Aortic Regurgitation Particularities
3.4. TAVI in Aortic Regurgitation
3.5. Results of TAVI for PNAR Comparing First- Versus Newer-Generation Devices
3.6. Device selection and Procedural Considerations
3.7. Dedicated Devices
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J., Jr.; Kleiman, N.S.; et al. U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. PARTNER 2 Investigators. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. SURTAVI Investigators. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Evolut Low Risk Trial Investigators. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- D’Ancona, G.; Pasic, M.; Buz, S.; Drews, T.; Dreysse, S.; Hetzer, R.; Unbehaun, A. TAVI for pure aortic valve insufficiency in a patient with a left ventricular assist device. Ann. Thorac. Surg. 2012, 93, e89–e91. [Google Scholar] [CrossRef]
- Roy, D.A.; Schaefer, U.; Guetta, V.; Hildick-Smith, D.; Möllmann, H.; Dumonteil, N.; Modine, T.; Bosmans, J.; Petronio, A.S.; Moat, N.; et al. Transcatheter aortic valve implantation for pure severe native aortic valve regurgitation. J. Am. Coll. Cardiol. 2013, 61, 1577–1584. [Google Scholar] [CrossRef]
- Hildebrandt, H.A.; Erbel, R.; Kahlert, P. Compassionate use of the self-expandable medtronic CoreValve prosthesis for the treatment of pure aortic regurgitation in a patient at prohibitive risk for surgical valve replacement. Catheter. Cardiovasc. Interv. 2013, 82, E939–E943. [Google Scholar] [CrossRef]
- Seiffert, M.; Diemert, P.; Koschyk, D.; Schirmer, J.; Conradi, L.; Schnabel, R.; Blankenberg, S.; Reichenspurner, H.; Baldus, S.; Treede, H. Transapical implantation of a second-generation transcatheter heart valve in patients with noncalcified aortic regurgitation. JACC Cardiovasc. Interv. 2013, 6, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, M.; Bader, R.; Kappert, U.; Rastan, A.; Krapf, S.; Bleiziffer, S.; Hofmann, S.; Arnold, M.; Kallenbach, K.; Conradi, L.; et al. Initial German experience with transapical implantation of a second-generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc. Interv. 2014, 7, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, H.; Zhu, L.; Yang, Y.; Zheng, J.; Guo, K.; Luo, H.; Zhao, W.; Yang, X.; Maimaiti, A.; et al. A New Transcatheter Aortic Valve Replacement System for Predominant Aortic Regurgitation Implantation of the J-Valve and Early Outcome. JACC Cardiovasc. Interv. 2015, 8, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef]
- Brzezinski, A.; Koprivanac, M.; Gillinov, A.M.; Mihaljevic, T. Pathophysiology of aortic valve disease. In Cardiac Surgery in the Adult; Cohn, L.H., Adams, D.H., Eds.; McGraw-Hill Education/Medical: New York, NY, USA, 2018. [Google Scholar]
- de Meester, C.; Gerber, B.L.; Vancraeynest, D.; Pouleur, A.C.; Noirhomme, P.; Pasquet, A.; de Kerchove, L.; El Khoury, G.; Vanoverschelde, J.L. Do Guideline-Based Indications Result in an Outcome Penalty for Patients With Severe Aortic Regurgitation? JACC Cardiovasc. Imaging 2019, 12 Pt 1, 2126–2138. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- Arias, E.A.; Bhan, A.; Lim, Z.Y.; Mullen, M. TAVI for Pure Native Aortic Regurgitation: Are We There Yet? Interv. Cardiol. 2019, 14, 26–30. [Google Scholar] [CrossRef]
- Schneeberger, Y.; Seiffert, M.; Schaefer, A.; Bhadra, O.D.; Schofer, N.; Pecha, S.; Westermann, D.; Blankenberg, S.; Reichenspurner, H.; Conradi, L. TAVI for Pure Non-calcified Aortic Regurgitation Using a Self-Expandable Transcatheter Heart Valve. Front. Cardiovasc. Med. 2022, 8, 743579. [Google Scholar] [CrossRef]
- Richards, G.; Joshi, N.; Turner, M.; Dorman, S. Transcatheter Aortic Valve Implantation in Severe Native Pure Aortic Regurgitation Following Endocarditis With Large Vegetation. JACC Case Rep. 2021, 3, 859–863. [Google Scholar] [CrossRef]
- Maeno, Y.; Yoon, S.H.; Abramowitz, Y.; Watanabe, Y.; Jilaihawi, H.; Lin, M.S.; Chan, J.; Sharma, R.; Kawashima, H.; Israr, S.; et al. Effect of ascending aortic dimension on acute procedural success following self-expanding transcatheter aortic valve replacement: A multicenter retrospective analysis. Int. J. Cardiol. 2017, 244, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.; MacDonald, Z.; Simard, T.; Russo, J.J.; Feder, J.; Froeschl, M.V.; Dick, A.; Glover, C.; Burwash, I.G.; Latib, A.; et al. Transcatheter Aortic Valve Implantation (TAVI) for Native Aortic Valve Regurgitation—A Systematic Review. Circ. J. 2018, 82, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sato, K.; Banerjee, K.; Narayanswami, J.; Betancor, J.; Menon, V.; Mohananey, D.; Anumandla, A.K.; Sawant, A.C.; Krishnaswamy, A.; et al. Hemodynamic durability of transcatheter aortic valves using the updated Valve Academic Research Consortium-2 criteria. Catheter. Cardiovasc. Interv. 2019, 93, 729–738. [Google Scholar] [CrossRef] [PubMed]
- De Backer, O.; Pilgrim, T.; Simonato, M.; Mackensen, G.B.; Fiorina, C.; Veulemanns, V.; Cerillo, A.; Schofer, J.; Amabile, N.; Achkouty, G.; et al. Usefulness of Transcatheter Aortic Valve Implantation for Treatment of Pure Native Aortic Valve Regurgitation. Am. J. Cardiol. 2018, 122, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Schmidt, T.; Bleiziffer, S.; Schofer, N.; Fiorina, C.; Munoz-Garcia, A.J.; Yzeiraj, E.; Amat-Santos, I.J.; Tchetche, D.; Jung, C.; et al. Transcatheter Aortic Valve Replacement in Pure Native Aortic Valve Regurgitation. J. Am. Coll. Cardiol. 2017, 70, 2752–2763. [Google Scholar] [CrossRef]
- Sawaya, F.J.; Deutsch, M.A.; Seiffert, M.; Yoon, S.H.; Codner, P.; Wickramarachchi, U.; Latib, A.; Petronio, A.S.; Rodés-Cabau, J.; Taramasso, M.; et al. Safety and Efficacy of Transcatheter Aortic Valve Replacement in the Treatment of Pure Aortic Regurgitation in Native Valves and Failing Surgical Bioprostheses: Results From an International Registry Study. JACC Cardiovasc. Interv. 2017, 10, 1048–1056. [Google Scholar] [CrossRef]
- Anwaruddin, S.; Desai, N.D.; Szeto, W.Y.; Hermiller, J.B., Jr.; Sorajja, P.; Kodali, S.; Popma, J.J.; Giri, J.; Herrmann, H.C.; Tang, G.; et al. Self-Expanding Valve System for Treatment of Native Aortic Regurgitation by Transcatheter Aortic Valve Implantation (from the STS/ACC TVT Registry). Am. J. Cardiol. 2019, 124, 781–788. [Google Scholar] [CrossRef]
- Takagi, H.; Hari, Y.; Kawai, N.; Ando, T.; ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. Meta-Analysis and Meta-Regression of Transcatheter Aortic Valve Implantation for Pure Native Aortic Regurgitation. Heart Lung Circ. 2020, 29, 729–741. [Google Scholar] [CrossRef]
- Yin, W.H.; Lee, Y.T.; Tsao, T.P.; Lee, K.C.; Hsiung, M.C.; Wei, J. Outcomes of transcatheter aortic valve replacement for pure native aortic regurgitation with the use of newer- vs. early-generation devices. Ann. Transl. Med. 2022, 10, 24. [Google Scholar] [CrossRef]
- Alkhouli, M.; Sengupta, P.; Badhwar, V. Toward Precision in Balloon-Expandable TAVR: Oversizing Tight Versus Just Right. JACC Cardiovasc. Interv. 2017, 10, 821–823. [Google Scholar] [CrossRef]
- Dvir, D.; Webb, J.G.; Piazza, N.; Blanke, P.; Barbanti, M.; Bleiziffer, S.; Wood, D.A.; Mylotte, D.; Wilson, A.B.; Tan, J.; et al. Multicenter evaluation of transcatheter aortic valve replacement using either SAPIEN XT or CoreValve: Degree of device oversizing by computed-tomography and clinical outcomes. Catheter. Cardiovasc. Interv. 2015, 86, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Pesarini, G.; Lunardi, M.; Piccoli, A.; Gottin, L.; Prati, D.; Ferrero, V.; Scarsini, R.; Milano, A.; Forni, A.; Faggian, G.; et al. Effectiveness and Safety of Transcatheter Aortic Valve Implantation in Patients With Pure Aortic Regurgitation and Advanced Heart Failure. Am. J. Cardiol. 2018, 121, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Sachse, S.; Mehilli, J.; Neumann, F.J.; Frerker, C.; Kurz, T.; El-Mawardy, M.; Richardt, G.; Abdel-Wahab, M. Bioprosthetic Valve Performance After Transcatheter Aortic Valve Replacement With Self-Expanding Versus Balloon-Expandable Valves in Large Versus Small Aortic Valve Annuli: Insights From the CHOICE Trial and the CHOICE-Extend Registry. JACC Cardiovasc. Interv. 2018, 11, 2507–2518. [Google Scholar] [CrossRef]
- Barbanti, M.; Yang, T.H.; Rodès Cabau, J.; Tamburino, C.; Wood, D.A.; Jilaihawi, H.; Blanke, P.; Makkar, R.R.; Latib, A.; Colombo, A.; et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 2013, 128, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Maeno, Y.; Abramowitz, Y.; Jilaihawi, H.; Israr, S.; Yoon, S.; Sharma, R.P.; Kazuno, Y.; Kawamori, H.; Miyasaka, M.; Rami, T.; et al. Optimal sizing for SAPIEN 3 transcatheter aortic valve replacement in patients with or without left ventricular outflow tract calcification. EuroIntervention 2017, 12, e2177–e2185. [Google Scholar] [CrossRef]
- Le Ruz, R.; Plessis, J.; Guimbretiere, G.; Roussel, J.C.; Piriou, P.G.; Cueff, C.; Maurel, B.; Letocart, V.; Manigold, T. Sapien 3 Embolization From Ventricle to Aorta in the Setting of Noncalcified Aortic Regurgitation. JACC Case Rep. 2021, 3, 64–68. [Google Scholar] [CrossRef]
- Ancona, M.B.; Moroni, F.; Romano, V.; Agricola, E.; Esposito, A.; Ajello, S.; De Bonis, M.; Cappelletti, A.M.; Zangrillo, A.; Scandroglio, A.M.; et al. Transcatheter aortic valve implantation for aortic regurgitation in patients with left ventricular assist device. G Ital. Cardiol. 2021, 22 (Suppl. 1), 39S–42S. [Google Scholar] [CrossRef]
- Silaschi, M.; Treede, H.; Rastan, A.J.; Baumbach, H.; Beyersdorf, F.; Kappert, U.; Eichinger, W.; Rüter, F.; de Kroon, T.L.; Lange, R.; et al. The JUPITER registry: 1-year results of transapical aortic valve implantation using a second-generation transcatheter heart valve in patients with aortic stenosis. Eur. J. CardioThorac. Surg. 2016, 50, 874–881. [Google Scholar] [CrossRef]
- Silaschi, M.; Conradi, L.; Wendler, O.; Schlingloff, F.; Kappert, U.; Rastan, A.J.; Baumbach, H.; Holzhey, D.; Eichinger, W.; Bader, R.; et al. The JUPITER registry: One-year outcomes of transapical aortic valve implantation using a second generation transcatheter heart valve for aortic regurgitation. Catheter. Cardiovasc. Interv. 2018, 91, 1345–1351. [Google Scholar] [CrossRef]
- Zhu, D.; Hu, J.; Meng, W.; Guo, Y. Successful transcatheter aortic valve implantation for pure aortic regurgitation using a new second generation self-expanding J-Valve(TM) system-the first in-man implantation. Heart Lung Circ. 2015, 24, 411–414. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Wang, W.; Zhu, D.; Wei, L.; Guo, K.; Zhao, W.; Yang, X.; Zhu, L.; Guo, Y.; et al. Transapical transcatheter aortic valve replacement for aortic regurgitation with a second-generation heart valve. J. Thorac. Cardiovasc. Surg. 2018, 156, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, X.; Wang, Y.; Xu, F.; Wang, X.; Li, X.; Wang, W. Structural Valve Deterioration after Transcatheter Aortic Valve Implantation Using J-Valve: A Long-Term Follow-Up. Ann Thorac. Cardiovasc. Surg. 2020, 26, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, U.; Schirmer, J.; Niklas, S.; Harmel, E.; Deuschl, F.; Conradi, L. First-in-human implantation of a novel transfemoral selfexpanding transcatheter heart valve to treat pure aortic regurgitation. EuroIntervention 2017, 13, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. THE ALIGN-AR TRIAL: JenaValve Pericardial TAVR Aortic Regurgitation Study. Available online: https://clinicaltrials.gov/ct2/show/NCT02732704 (accessed on 13 August 2022).
- Hensey, M.; Murdoch, D.J.; Sathananthan, J.; Alenezi, A.; Sathananthan, G.; Moss, R.; Blanke, P.; Leipsic, J.; Wood, D.A.; Cheung, A.; et al. First-in-human experience of a new-generation transfemoral transcatheter aortic valve for the treatment of severe aortic regurgitation: The J-Valve transfemoral system. EuroIntervention 2019, 14, e1553–e1555. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. J-Valve Compassionate Use. Available online: https://clinicaltrials.gov/ct2/show/NCT03876964 (accessed on 13 August 2022).
- Tamm, A.R. JenaValve Trilogy for Treatment of Aortic Regurgitation: Worldwide First Results; EuroPCR: Paris, France, 2022. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagliari, A.P.; Petersen Saadi, R.; Keller Saadi, E. Transcatheter Aortic Valve Implantation for Pure Native Aortic Regurgitation: The Last Frontier. J. Clin. Med. 2022, 11, 5181. https://doi.org/10.3390/jcm11175181
Tagliari AP, Petersen Saadi R, Keller Saadi E. Transcatheter Aortic Valve Implantation for Pure Native Aortic Regurgitation: The Last Frontier. Journal of Clinical Medicine. 2022; 11(17):5181. https://doi.org/10.3390/jcm11175181
Chicago/Turabian StyleTagliari, Ana Paula, Rodrigo Petersen Saadi, and Eduardo Keller Saadi. 2022. "Transcatheter Aortic Valve Implantation for Pure Native Aortic Regurgitation: The Last Frontier" Journal of Clinical Medicine 11, no. 17: 5181. https://doi.org/10.3390/jcm11175181
APA StyleTagliari, A. P., Petersen Saadi, R., & Keller Saadi, E. (2022). Transcatheter Aortic Valve Implantation for Pure Native Aortic Regurgitation: The Last Frontier. Journal of Clinical Medicine, 11(17), 5181. https://doi.org/10.3390/jcm11175181





