Snail Track Lesion with Flat Keratometry in Anterior Segment Dysgenesis Caused by a Novel FOXC1 Variant
Abstract
1. Introduction
2. Materials and Methods
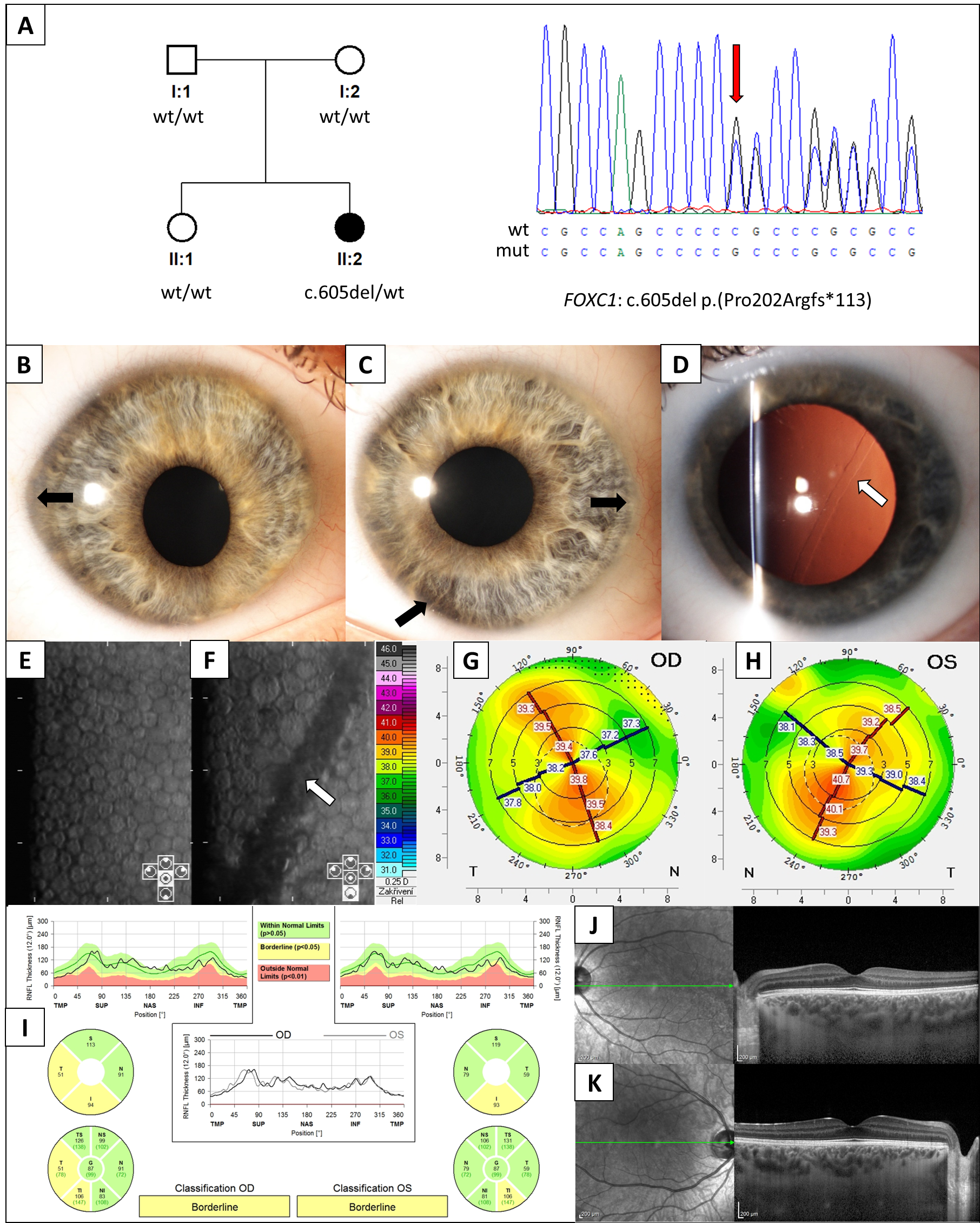
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reis, L.M.; Semina, E.V. Genetics of anterior segment dysgenesis disorders. Curr. Opin. Ophthalmol. 2011, 22, 314–324. [Google Scholar] [CrossRef]
- Ma, A.S.; Grigg, J.R.; Jamieson, R.V. Phenotype-genotype correlations and emerging pathways in ocular anterior segment dysgenesis. Hum. Genet. 2019, 138, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, F.; Gould, D.B.; Heon, E.; Billingsley, G.D.; Cheung, J.C.; Mears, A.J.; Walter, M.A. Axenfeld-Rieger syndrome resulting from mutation of the FKHL7 gene on chromosome 6p25. Eur. J. Hum. Genet. 2000, 8, 71–74. [Google Scholar] [CrossRef]
- Reis, L.M.; Maheshwari, M.; Capasso, J.; Atilla, H.; Dudakova, L.; Thompson, S.; Zitano, L.; Lay-Son, G.; Lowry, R.B.; Black, J.; et al. Axenfeld-Rieger syndrome: More than meets the eye. J. Med. Genet. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Berry, F.B.; Lines, M.A.; Oas, J.M.; Footz, T.; Underhill, D.A.; Gage, P.J.; Walter, M.A. Functional interactions between FOXC1 and PITX2 underlie the sensitivity to FOXC1 gene dose in Axenfeld-Rieger syndrome and anterior segment dysgenesis. Hum. Mol. Genet. 2006, 15, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Kidson, S.H.; Kume, T.; Deng, K.; Winfrey, V.; Hogan, B.L. The forkhead/winged-helix gene, Mf1, is necessary for the normal development of the cornea and formation of the anterior chamber in the mouse eye. Dev. Biol. 1999, 211, 306–322. [Google Scholar] [CrossRef][Green Version]
- Gage, P.J.; Rhoades, W.; Prucka, S.K.; Hjalt, T. Fate maps of neural crest and mesoderm in the mammalian eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4200–4208. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.B.; Mitraud, R.S.; Yamane, R. Anomalia de Axenfeld-Rieger e distrofia corneana endotelial: Uma série de casos. Rev. Bras. Oftalmol. 2008, 67, 303–308. [Google Scholar] [CrossRef]
- Kniestedt, C.; Taralczak, M.; Thiel, M.A.; Stuermer, J.; Baumer, A.; Gloor, B.P. A novel PITX2 mutation and a polymorphism in a 5-generation family with Axenfeld-Rieger anomaly and coexisting Fuchs’ endothelial dystrophy. Ophthalmology 2006, 113, e1791–e1798. [Google Scholar] [CrossRef]
- Jun, A.S.; Broman, K.W.; Do, D.V.; Akpek, E.K.; Stark, W.J.; Gottsch, J.D. Endothelial dystrophy, iris hypoplasia, congenital cataract, and stromal thinning (edict) syndrome maps to chromosome 15q22.1-q25.3. Am. J. Ophthalmol. 2002, 134, 172–176. [Google Scholar] [CrossRef]
- Zarouchlioti, C.; Sanchez-Pintado, B.; Hafford Tear, N.J.; Klein, P.; Liskova, P.; Dulla, K.; Semo, M.; Vugler, A.A.; Muthusamy, K.; Dudakova, L.; et al. Antisense Therapy for a Common Corneal Dystrophy Ameliorates TCF4 Repeat Expansion-Mediated Toxicity. Am. J. Hum. Genet. 2018, 102, 528–539. [Google Scholar] [CrossRef]
- Fautsch, M.P.; Wieben, E.D.; Baratz, K.H.; Bhattacharyya, N.; Sadan, A.N.; Hafford-Tear, N.J.; Tuft, S.J.; Davidson, A.E. TCF4-mediated Fuchs endothelial corneal dystrophy: Insights into a common trinucleotide repeat-associated disease. Prog. Retin. Eye Res. 2021, 81, 100883. [Google Scholar] [CrossRef]
- Liskova, P.; Hafford-Tear, N.J.; Skalicka, P.; Malinka, F.; Jedlickova, J.; Dudakova, L.; Pontikos, N.; Davidson, A.E.; Tuft, S. Posterior corneal vesicles are not associated with the genetic variants that cause posterior polymorphous corneal dystrophy. Acta Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Davidson, A.E.; Liskova, P.; Evans, C.J.; Dudakova, L.; Noskova, L.; Pontikos, N.; Hartmannova, H.; Hodanova, K.; Stranecky, V.; Kozmik, Z.; et al. Autosomal-Dominant Corneal Endothelial Dystrophies CHED1 and PPCD1 Are Allelic Disorders Caused by Non-coding Mutations in the Promoter of OVOL2. Am. J. Hum. Genet. 2016, 98, 75–89. [Google Scholar] [CrossRef]
- Liskova, P.; Dudakova, L.; Evans, C.J.; Rojas Lopez, K.E.; Pontikos, N.; Athanasiou, D.; Jama, H.; Sach, J.; Skalicka, P.; Stranecky, V.; et al. Ectopic GRHL2 Expression Due to Non-coding Mutations Promotes Cell State Transition and Causes Posterior Polymorphous Corneal Dystrophy 4. Am. J. Hum. Genet. 2018, 102, 447–459. [Google Scholar] [CrossRef]
- Krafchak, C.M.; Pawar, H.; Moroi, S.E.; Sugar, A.; Lichter, P.R.; Mackey, D.A.; Mian, S.; Nairus, T.; Elner, V.; Schteingart, M.T.; et al. Mutations in TCF8 cause posterior polymorphous corneal dystrophy and ectopic expression of COL4A3 by corneal endothelial cells. Am. J. Hum. Genet. 2005, 77, 694–708. [Google Scholar] [CrossRef]
- Brooks, A.M.; Grant, G.; Gillies, W.E. Differentiation of posterior polymorphous dystrophy from other posterior corneal opacities by specular microscopy. Ophthalmology 1989, 96, 1639–1645. [Google Scholar] [CrossRef]
- Dudakova, L.; Tuft, S.; Cheong, S.S.; Skalicka, P.; Jedlickova, J.; Fichtl, M.; Hlozanek, M.; Filous, A.; Vaneckova, M.; Vincent, A.L.; et al. Novel disease-causing variants and phenotypic features of X-linked megalocornea. Acta Ophthalmol. 2022, 100, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Dudakova, L.; Vercruyssen, J.H.J.; Balikova, I.; Postolache, L.; Leroy, B.P.; Skalicka, P.; Liskova, P. Analysis of KERA in four families with cornea plana identifies two novel mutations. Acta Ophthalmol. 2018, 96, e87–e91. [Google Scholar] [CrossRef]
- Rodrigues, E.D.S.; Griffith, S.; Martin, R.; Antonescu, C.; Posey, J.E.; Coban-Akdemir, Z.; Jhangiani, S.N.; Doheny, K.F.; Lupski, J.R.; Valle, D.; et al. Variant-level matching for diagnosis and discovery: Challenges and opportunities. Hum. Mutat. 2022, 43, 782–790. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Williams, E.; Foulger, R.E.; Leigh, S.; Daugherty, L.C.; Niblock, O.; Leong, I.U.S.; Smith, K.R.; Gerasimenko, O.; Haraldsdottir, E.; et al. PanelApp crowdsources expert knowledge to establish consensus diagnostic gene panels. Nat. Genet. 2019, 51, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Ensenberger, M.G.; Thompson, J.; Hill, B.; Homick, K.; Kearney, V.; Mayntz-Press, K.A.; Mazur, P.; McGuckian, A.; Myers, J.; Raley, K.; et al. Developmental validation of the PowerPlex 16 HS System: An improved 16-locus fluorescent STR multiplex. Forensic. Sci. Int. Genet. 2010, 4, 257–264. [Google Scholar] [CrossRef]
- Galgauskas, S.; Krasauskaite, D.; Pajaujis, M.; Juodkaite, G.; Asoklis, R.S. Central corneal thickness and corneal endothelial characteristics in healthy, cataract, and glaucoma patients. Clin. Ophthalmol. 2012, 6, 1195–1199. [Google Scholar] [CrossRef][Green Version]
- Gilani, F.; Cortese, M.; Ambrosio, R.R., Jr.; Lopes, B.; Ramos, I.; Harvey, E.M.; Belin, M.W. Comprehensive anterior segment normal values generated by rotating Scheimpflug tomography. J. Cataract Refract. Surg. 2013, 39, 1707–1712. [Google Scholar] [CrossRef]
- Tsai, C.S.; Ritch, R.; Shin, D.H.; Wan, J.Y.; Chi, T. Age-related decline of disc rim area in visually normal subjects. Ophthalmology 1992, 99, 29–35. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- D’Haene, B.; Meire, F.; Claerhout, I.; Kroes, H.Y.; Plomp, A.; Arens, Y.H.; de Ravel, T.; Casteels, I.; De Jaegere, S.; Hooghe, S.; et al. Expanding the spectrum of FOXC1 and PITX2 mutations and copy number changes in patients with anterior segment malformations. Investig. Ophthalmol. Vis. Sci. 2011, 52, 324–333. [Google Scholar] [CrossRef]
- Jeon, H.S.; Hyon, J.Y. Unilateral Posterior Polymorphous Corneal Dystrophy Presented as Anisometropic Astigmatism: 3 Case Reports. Case Rep. Ophthalmol. 2017, 8, 250–258. [Google Scholar] [CrossRef]
- Dudakova, L.; Palos, M.; Hardcastle, A.J.; Liskova, P. Corneal endothelial findings in a Czech patient with compound heterozygous mutations in KERA. Ophthalmic. Genet. 2014, 35, 252–254. [Google Scholar] [CrossRef]
- Liskova, P.; Evans, C.J.; Davidson, A.E.; Zaliova, M.; Dudakova, L.; Trkova, M.; Stranecky, V.; Carnt, N.; Plagnol, V.; Vincent, A.L.; et al. Heterozygous deletions at the ZEB1 locus verify haploinsufficiency as the mechanism of disease for posterior polymorphous corneal dystrophy type 3. Eur. J. Hum. Genet. 2016, 24, 985–991. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalicka, P.; Jedlickova, J.; Horinek, A.; Trkova, M.; Davidson, A.E.; Tuft, S.J.; Dudakova, L.; Liskova, P. Snail Track Lesion with Flat Keratometry in Anterior Segment Dysgenesis Caused by a Novel FOXC1 Variant. J. Clin. Med. 2022, 11, 5166. https://doi.org/10.3390/jcm11175166
Skalicka P, Jedlickova J, Horinek A, Trkova M, Davidson AE, Tuft SJ, Dudakova L, Liskova P. Snail Track Lesion with Flat Keratometry in Anterior Segment Dysgenesis Caused by a Novel FOXC1 Variant. Journal of Clinical Medicine. 2022; 11(17):5166. https://doi.org/10.3390/jcm11175166
Chicago/Turabian StyleSkalicka, Pavlina, Jana Jedlickova, Ales Horinek, Marie Trkova, Alice E. Davidson, Stephen J. Tuft, Lubica Dudakova, and Petra Liskova. 2022. "Snail Track Lesion with Flat Keratometry in Anterior Segment Dysgenesis Caused by a Novel FOXC1 Variant" Journal of Clinical Medicine 11, no. 17: 5166. https://doi.org/10.3390/jcm11175166
APA StyleSkalicka, P., Jedlickova, J., Horinek, A., Trkova, M., Davidson, A. E., Tuft, S. J., Dudakova, L., & Liskova, P. (2022). Snail Track Lesion with Flat Keratometry in Anterior Segment Dysgenesis Caused by a Novel FOXC1 Variant. Journal of Clinical Medicine, 11(17), 5166. https://doi.org/10.3390/jcm11175166





