Use of Melatonin and/on Ramelteon for the Treatment of Insomnia in Older Adults: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Search Strategy
2.3. Data Extraction
2.4. Outcomes
2.5. Data Analysis
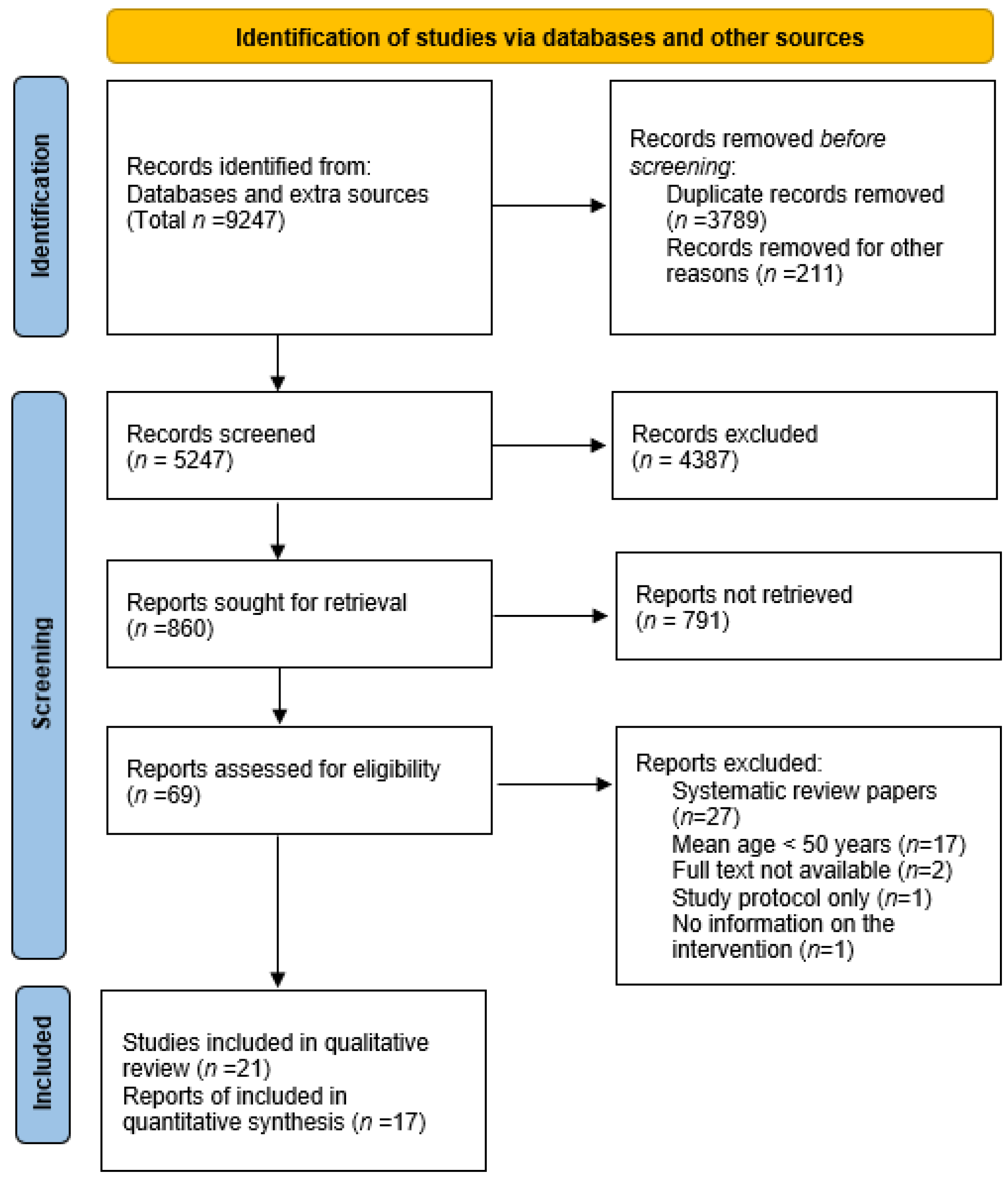
3. Results
3.1. Characteristics of the Included Studies
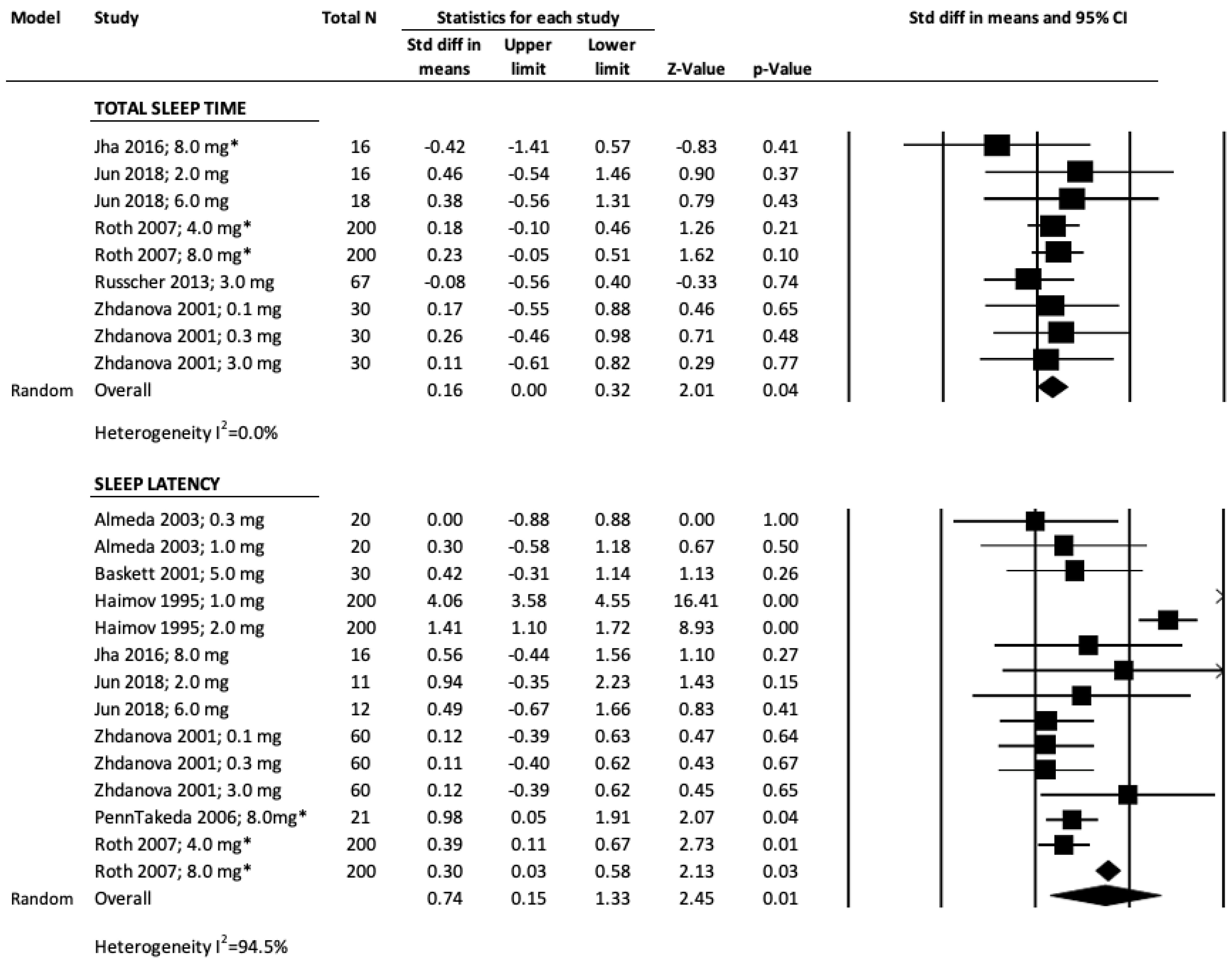
3.2. Total Sleep Time, TST
3.3. Sleep Latency, SL
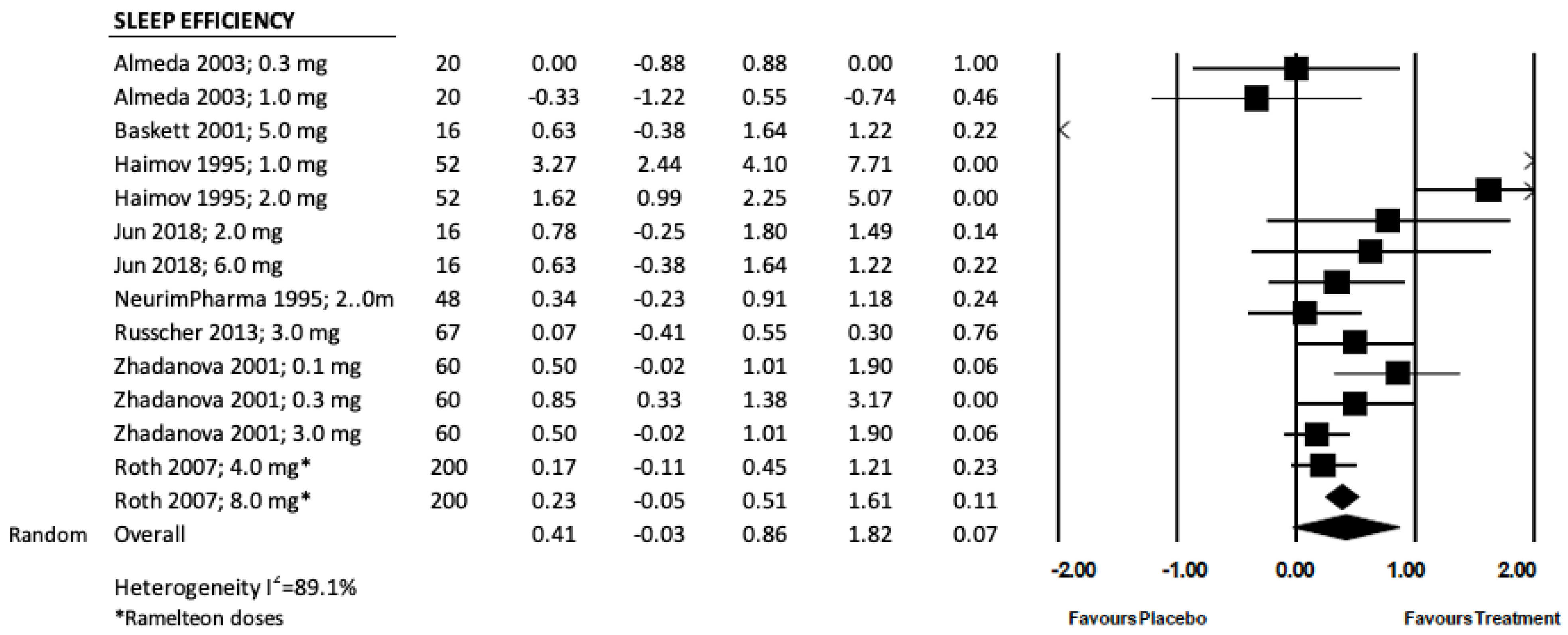
3.4. Sleep Efficiency, SE
3.5. Sleep Quality
3.6. Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maternal and Child Health Bureau; Women’s Health USA: Avon, CT, USA, 2011. Available online: https://mchb.hrsa.gov/whusa11/hstat/hshi/downloads/pdf/224sd.pdf (accessed on 8 February 2020).
- Aasm.org. 2017. Available online: https://aasm.org/resources/clinicalguidelines/040515.pdf (accessed on 2 March 2020).
- What is Insomnia? | National Sleep Foundation. Sleepfoundation.org. 2020. Available online: https://www.sleepfoundation.org/insomnia/what-insomnia (accessed on 9 March 2020).
- Ohayon, M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef]
- Bain, K. Management of chronic insomnia in elderly persons. Am. J. Geriatr. Pharmacother. 2006, 4, 168–192. [Google Scholar] [CrossRef]
- Gooneratne, N.; Vitiello, M. Sleep in Older Adults. Clin. Geriatr. Med. 2014, 30, 591–627. [Google Scholar] [CrossRef]
- Boselli, M.; Parrino, L.; Smerieri, A.; Terzano, M.G. Effect of Age on EEG Arousals in Normal Sleep. Sleep 1998, 21, 361–367. [Google Scholar] [CrossRef][Green Version]
- Smagula, S.; Stone, K.; Fabio, A.; Cauley, J. Risk factors for sleep disturbances in older adults: Evidence from prospective studies. Sleep Med. Rev. 2016, 25, 21–30. [Google Scholar] [CrossRef] [PubMed]
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2015, 63, 2227–2246. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, K.; Broomfield, N.; Espie, C. A Systematic Review of the Effectiveness of Oral Melatonin for Adults (18 to 65 Years) with Delayed Sleep Phase Syndrome and Adults (18 to 65 Years) with Primary Insomnia. Curr. Psychiatry Rev. 2005, 1, 103–113. [Google Scholar] [CrossRef][Green Version]
- Buscemi, N.; Vandermeer, B.; Hooton, N.; Pandya, R.; Tjosvold, L.; Hartling, L.; Vohra, S.; Klassen, T.P.; Baker, G. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: Meta-analysis. BMJ 2006, 332, 385–393. [Google Scholar] [CrossRef]
- Axelrod, J.; Weissbach, H. Enzymatic O-methylation of Nacetylserotonin to melatonin. Science 1960, 131, 1312. [Google Scholar] [CrossRef]
- Lemoine, P.; Nir, T.; Laudon, M.; Zisapel, N. Prolonged-release melatonin improves sleep quality and morning alertness 512 in insomnia patients aged 55 years and older and has no withdrawal effects. J. Sleep Res. 2007, 16, 372–380, 513. [Google Scholar] [CrossRef]
- Van Geijlswijk, I.; Korzilius, H.; Smits, M. The Use of Exogenous Melatonin in Delayed Sleep Phase Disorder: A Meta-analysis. Sleep 2010, 33, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.; Bate, G.; Kirkpatrick, P. Ramelteon. Nat. Rev. Drug Discov. 2005, 4, 881–882. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Hirai, K.; Nishiyama, K.; Uchikawa, O.; Fukatsu, K.; Ohkawa, S.; Kawamata, Y.; Hinuma, S.; Miyamoto, M. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology 2005, 48, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Sakamoto, S.; Miyata, K. Effect of Ramelteon on Insomnia Severity: Evaluation of Patient Characteristics Affecting Treatment Response. Sleep Biol. Rhythm. 2019, 17, 379–388. [Google Scholar] [CrossRef]
- Kuriyama, A.; Honda, M.; Hayashino, Y. Ramelteon for the treatment of insomnia in adults: A systematic review and meta-analysis. Sleep Med. 2014, 15, 385–392. [Google Scholar] [CrossRef]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M. Meta-Analysis: Melatonin for the Treatment of Primary Sleep Disorders. PLoS ONE 2013, 8, e63773. [Google Scholar] [CrossRef]
- Rosenthal, J.A. Statistics and Data Interpretation for Social Work; Springer: New York, NY, USA, 2011. [Google Scholar]
- Borenstein, M.; Hedges, L.; Rothstein, H. Meta-Analysis Fixed Effect vs. Random Effects. 2007. Available online: https://www.meta-analysis.com/downloads/M-a_f_e_v_r_e_sv.pdf (accessed on 9 March 2020).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ 2019, 366, 4898. [Google Scholar] [CrossRef]
- Almeida Montes, L.G.; Ontiveros Uribe, M.P.; Cortés Sotres, J.; Heinze Martin, G. Treatment of primary insomnia with melatonin: A double-blind, placebo-controlled, crossover study. J. Psychiatry Neurosci. 2003, 28, 191–196. [Google Scholar]
- Andrade, C.; Srihari, B.S.; Reddy, K.P.; Chandramma, L. Melatonin in Medically Ill Patients with Insomnia. J. Clin. Psychiatry 2001, 62, 41–45. [Google Scholar] [CrossRef]
- Baskett, J.J. Does melatonin improve sleep in older people? A randomised crossover trial. Age Ageing 2003, 32, 164–170. [Google Scholar] [CrossRef][Green Version]
- Dobkin, R.D.; Menza, M.; Bienfait, K.L.; Allen, L.A.; Marin, H.; Gara, M.A. Ramelteon for the treatment of insomnia in menopausal women. Menopause Int. 2009, 15, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Fainstein, I.; Bonetto, A.J.; Brusco, L.I.; Cardinali, D.P. Effects of melatonin in elderly patients with sleep disturbance: A pilot study. Curr. Ther. Res. 1997, 58, 990–1000. [Google Scholar] [CrossRef]
- Haimov, I.; Lavie, P.; Laudon, M.; Herer, P.; Vigder, C.; Zisapel, N. Melatonin Replacement Therapy of Elderly Insomniacs. Sleep 1995, 18, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Jha, L.K.; Fass, R.; Gadam, R.; Maradey-Romero, C.; Nasrollah, L.; Hershcovici, T.; Quan, S.F.; Dickman, R. The Effect of Ramelteon on Heartburn Symptoms of Patients with Gastroesophageal Reflux Disease and Chronic Insomnia. J. Clin. Gastroenterol. 2016, 50, e19–e24. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.S.; Kim, R.; Byun, J.I.; Kim, T.J.; Lim, J.-A.; Sunwoo, J.-S.; Lee, S.-T.; Jung, K.-H.; Park, K.-I.; Chu, K.; et al. Prolonged–release melatonin in patients with idiopathic REM sleep behavior disorder. Ann. Clin. Transl. Neurol. 2019, 6, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, P.; Garfinkel, D.; Laudon, M.; Nir, T.; Zisapel, N. Prolonged-release melatonin for insomnia—An open-label long-term study of efficacy, safety, and withdrawal. Ther. Clin. Risk Manag. 2011, 7, 301–311. [Google Scholar] [CrossRef]
- Neurim Pharmaceuticals Ltd. A Randomized Double-Blind, Crossover Study Comparing the Efficacy of Prolonged-Release Melatonin Versus Placebo in a 3 Week Treatment of Diabetic Patients Suffering from Insomnia. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00869128 (accessed on 21 February 2022).
- Mini, L.J.; Wang-Weigand, S.; Zhang, J. Self-reported efficacy and tolerability of ramelteon 8 mg in older adults experiencing severe sleep-onset difficulty. Am. J. Geriatr. Pharmacother. 2007, 5, 177–184. [Google Scholar] [CrossRef]
- Penn-Treatment of Insomnia in Elderly Sleep Apnea Patients with Ramelteon (TAK 375)—Full Text 467 View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01048242 (accessed on 21 February 2022).
- Richardson, G.S.; Zammit, G.; Wang-Weigand, S.; Zhang, J. Safety and Subjective Sleep Effects of Ramelteon Administration in Adults and Older Adults with Chronic Primary Insomnia. J. Clin. Psychiatry 2009, 70, 467–476. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Monteferrario, F.; Antoniello, N.; Manni, R.; Klersy, C. The effect of melatonin, magnesium, and zinc on primary insomnia in long-term care facility residents in Italy: A double-blind, placebo-controlled clinical trial. J. Am. Geriatr. Soc. 2011, 59, 82–90. [Google Scholar] [CrossRef]
- Roth, T.; Seiden, D.; Sainati, S.; Wang-Weigand, S.; Zhang, J.; Zee, P. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006, 7, 312–318. [Google Scholar] [CrossRef]
- Roth, T.; Seiden, D.; Wang-Weigand, S.; Zhang, J. A 2-night, 3-period, crossover study of ramelteon’s efficacy and safety in older adults with chronic insomnia. Curr. Med. Res. Opin. 2007, 23, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Russcher, M.; Koch, B.C.P.; Nagtegaal, J.E.; van Ittersum, F.J.; Pasker-de Jong, P.C.M.; Hagen, E.C.; van Dorp, W.T.; Gabreëls, B.; Wildbergh, T.X.; van der Westerlaken, M.M.L.; et al. Long-term effects of melatonin on quality of life and sleep in haemodialysis patients (Melody study): A randomized controlled trial. Br. J. Clin. Pharmacol. 2013, 76, 668–679. [Google Scholar] [CrossRef]
- Wade, A.G.; Ford, I.; Crawford, G.; McMahon, A.D.; Nir, T.; Laudon, M.; Zisapel, N. Efficacy of prolonged release melatonin in insomnia patients aged 55–80 years: Quality of sleep and next-day alertness outcomes. Curr. Med. Res. Opin. 2007, 23, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.G.; Ford, I.; Crawford, G.; McConnachie, A.; Nir, T.; Laudon, M.; Zisapel, N. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: A randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 2010, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhdanova, I.V.; Wurtman, R.J.; Regan, M.M.; Taylor, J.A.; Shi, J.P.; Leclair, O.U. Melatonin Treatment for Age-Related Insomnia. J. Clin. Endocrinol. Metab. 2001, 86, 4727–4730. [Google Scholar] [CrossRef]
- Brzezinski, A.; Vangel, M.G.; Wurtman, R.J.; Norrie, G.; Zhdanova, I.; Ben-Shushan, A.; Ford, I. Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Med. Rev. 2005, 9, 41–50. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.-n. Ramelteon in the treatment of chronic insomnia: Systematic review and meta-analysis. Int. J. Clin. Pract. 2012, 66, 867–873. [Google Scholar] [CrossRef]
- Olde Rikkert, M.G.M.; Rigaud, A.-S.P. Melatonin in elderly patients with insomnia. Z. Gerontol. Geriatr. 2001, 34, 491–497. [Google Scholar] [CrossRef]
- Van Den Berg, J.F.; Van Rooij, F.J.A.; Vos, H.; Tulen, J.H.M.; Hofman, A.; Miedema, H.M.E.; Neven, A.K.; Tiemeier, H. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J. Sleep Res. 2008, 17, 295–302. [Google Scholar] [CrossRef]
- Grigg-Damberger, M.M.; Ianakieva, D. Poor quality control of over-the-counter melatonin: What they say is often not what you get. J. Clin. Sleep Med. 2017, 13, 163–165. [Google Scholar] [CrossRef]
| Study Author, Year | Country | Study Design | Total N | Patient Age, Years Mean ± SD | Male, % | Female, % | Study Settings | Concurrent Disease | Duration of Therapy, Days | Drug, Dose (mg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Andrade et al., 1999 [24] | India | RCT | 33 | 55.6 ± 12.7 | 73 | 27 | Inpatient | None | 8–16 | Melatonin, 5.4 |
| Haimov et al., 1995 [28] | Israel | RCT | 51 | 75.2 ± 6 | 57 | 43 | Inpatient & Outpatient | None | 70 | Melatonin 1.0, 2.0 |
| Jha et al., 2016 [29] | USA | RCT | 16 | 53 ± 4.27 | 88 | 12 | Inpatient | Gastroesophageal Reflux | 28 | Ramelteon, 8.0 |
| Jun et al., 2018 [30] | Korea | RCT | 25 | 66.4 ± 8.64 | 64 | 36 | Inpatient | iRBD | 28 | Melatonin, 2.0, 6.0 |
| Lemoine et al., 2007 [13] | France/Israel | RCT | 170 | 68.5 ± 8.3 | 34 | 66 | Outpatient | Cardiovascular conditions | 21 | Melatonin, 2.0 |
| Mini, et al., 2007 [33] | USA | RCT | 327 | 72.5 ± 5.98 | 39 | 61 | Outpatient | None | 35 | Ramelteon, 8.0 |
| Penn Takeda et al., 2006 [34] | USA | RCT | 27 | 72 ± 5.6 | 70 | 30 | Outpatient | None | 28 | Ramelteon, 8.0 |
| Roth et al., 2006 [37] | USA | RCT | 829 | 72.4 ± 5.95 | 41 | 59 | Outpatient | None | 35 | Ramelteon, 4.0, 8.0 |
| Roth et al., 2007 [38] | USA | RCT | 100 | 70.7 (65–85) | 37 | 63 | Outpatient | None | 63 | Ramelteon, 4.0, 8.0 |
| Russcher et al., 2013 [39] | The Netherlands | RCT | 67 | 65.0 ± 11.9 | 62 | 38 | Long term care | hemodialysis | 365 | Melatonin, 3.0 |
| Wade et al., 2010 [41] | Europe | RCT | 281 | 71.0 ± 4.1 | 35 | 65 | Outpatient | None | 21 | Melatonin, 2.0 |
| Wade et al., 2007 [40] | Europe | RCT | 334 | 65.7 ± 6.4 | 40 | 60 | Outpatient | None | 21 | Melatonin, 2.0 |
| Zhdanova et al., 2001 [42] | USA | RCT | 30 | >50 | N/A | Outpatient | Chronic insomnia | 63 | Melatonin, 0.1, 0.3, 3.0 | |
| Almeida et al., 2003 [23] | Mexico | Crossover | 10 | 50 ± 12.7 | 60 | 40 | Outpatient | None | 7 | Melatonin, 0.3, 1.0 |
| Baskett et al., 2001 [25] | New Zealand | Crossover | 34 | 71.7 ± 4.9 | 32 | 68 | Healthy volunteers | None | 84 | Melatonin, 5 |
| Neurim pharma, 1995 [32] | Israel | Crossover | 36 | 63 ± 8 | 31 | 69 | Outpatient | Diabetes Mellitus, Type 2 | 21 | Melatonin, 2.0 |
| Dobkin et al., 2006 [26] | USA | Open-label study | 20 | 52 ± 4.89 | 0 (only women study) | 100 | Academic medical center | Menopausal women | 42 | Ramelteon, 8.0 |
| Fainstein et al., 1997 [27] | Argentina | Open-label study | 41 | 74 ± 1.2 | 32 | 68 | Inpatient | Depression/Dementia | 21 | Melatonin, 3.0 |
| Lemoine et al., 2011 [31] | France/Israel | Open-Label | 96 | 55.3 ± 13.0 | 31 | 69 | Outpatient | none | 365 | Melatonin, 2.0 |
| Richardson et al., 2009 [35] | USA | Open-label study | 248 | 72.3 ± 5.6 | 47 a | 53 | Outpatient | None | 336 | Ramelteon, 8.0 |
| Rondanelli et al., 2011 [36] | Italy | Open-label study | 43 | 78.3 ± 3.9 | 37 | 63 | Long term care | None | 60 | Melatonin, 5.0 |
| Study Author, Year | Outcome Measure | Total Sleep Time, min Mean ± SD | Sleep Latency, min Mean ± SD | Sleep Efficiency, % Mean ± SD | |||
|---|---|---|---|---|---|---|---|
| Treatment | Placebo | Treatment | Placebo | Treatment | Placebo | ||
| Melatonin | |||||||
| Andrade et al., 1999 [24] | 15-item structure sleep questionnaire | 354 ± 54 | 300 ± 96 | 18 ± 12 | 60 ± 60 | ||
| Haimov et al., 1995 [28] | Actigraphy | 1.0 mg: 14 ± 5.0 2.0 mg: 37 ± 11.0 | 54 ± 13.0 | 1.0 mg: 84.3 ± 2.3 2.0 mg: 80.41 ± 1.8 | 77.4 ± 1.9 | ||
| Jun et al., 2018 [30] | PSG, self-questionnaire | 2.0 mg: 399.4 ± 58.5 6.0 mg: 398.3 ± 73.9 | 374.5 ± 50.4 | 2.0 mg: 20.7 ± 9.5 6.0 mg: 25.9 ± 40.2 | 13.1 ± 7.3 | 2.0 mg: 79.7 ± 10 6.0 mg: 79.6 ± 12.9 | 72.8 ± 7.1 |
| Russcher et al., 2013 [39] | Actigraphy, QoL questionnaire, Melatonin in saliva, Ambulatory blood pressure, echocardiography | 318 ± 29 | 323 ± 82 | Median (IQR) 20.3 (30) | Median (IQR) 25.0 (31) | 66.3 ± 19.7 | 64.9 ± 18.1 |
| Wade et al., 2010 [41] | Sleep diary, PSQI | Change in subjective total sleep time from baseline: 20.4 ± 45 | Change in subjective total sleep time from baseline: 12 ± 47.4 | Change in subjective sleep latency from baseline: −19.1 ± 47.3 | Change in subjective sleep latency from baseline: −1.7 ± 47.8 | ||
| Wade et al., 2007 [40] | PSQI, LSEQ, sleep diary | Subjective sleep latency 40.8 ± 54.5 | Subjective sleep latency 45 ± 59 | ||||
| Zhdanova et al., 2001 [42] | Polysomnography, wrist reports, Actigraphy, Electrocardiography | 0.1 mg: 402 ± 45 0.3 mg: 409 ± 49 3.0 mg: 398 ± 56 | 390 ± 91 | 0.1 mg: 10 ± 6 0.3 mg: 10 ± 8 3.0 mg: 10 ± 7 | 11 ± 10 | 0.1 mg: 84 ± 8 0.3 mg: 88 ± 7 3.0 mg: 84 ± 8 | 78 ± 15 |
| Almeida et al., 2003 [23] | EEG, Sleep logs with analogue visual scale | 0.3 mg: 380 1.0 mg: 375 | 400 | 0.3 mg: 69.2 ± 29.1 1.0 mg: 57.4 ± 47.2 | 69.2 ± 29.1 | 0.3 mg: 84 1.0 mg: 82 | 84 |
| Baskett et al., 2001 [25] | PSQI, Actigraphy | 438 (432, 456) PSQI subjective measures: 340 (290-400) | 444 (420,468) 443 (410,60) | 1.6 (0.6, 2.8) 10 (10-20) | 1.4 (0.4, 2.0) 10 (5-15) | 84.1 (83.8, 86.3) 69 (55-77) | 86.2 (84.9, 87.1) g 91 (86-93) |
| Neurim pharma, 1995 [32] | Wrist actigraphy | 83.1 ± 11.3 | 79.5 ± 9.6 | ||||
| Ramelteon | |||||||
| Jha et al., 2016 [29] | PSQI, sleep diaries, actigraphy | 430.95 ± 95.67 | 466.07 ± 69.49 | 9.64 ± 38.71 | 27.59 ± 23.28 | 87 | 83 |
| Mini, et al., 2007 [33] | Sleep diaries | Change in subjective sleep latency from baseline −37.4 | Change in subjective sleep latency from baseline −17.1 | ||||
| Penn Takeda et al., 2006 [34] | Polysomnography | 9.7 ± 10.3 | 34.4 ± 30.7 | ||||
| Roth et al., 2006 [37] | Sleep diaries | 4.0 mg: 337.5 8.0 mg: 334.4 | 4.0 mg: 330.1 8.0 mg: 330.1 | 4.0 mg: 63.4 8.0 mg: 57.7 | 4.0 mg: 70.6 8.0 mg: 70.6 | ||
| Roth et al., 2007 [38] | Polysomnography; Post Sleep Questionnaire | 4.0 mg: 359.4 (50.6) 8.0 mg: 362.0 (50.3) Subjective scores; 4.0 mg: 337.8 (66.8) 8.0 mg: 337.0 (66.2) | 350.4 (50.4) Subjective score; 333.9 (66.9) | 4.0 mg: 28.7 (24.9) 8.0 mg: 30.8 (25.2) Subjective scores; 4.0 mg: 48.2 (45.3) 8.0 mg: 50.9 (44.6) | 38.4 (24.9) Subjective score; 58.2 (45.3) | 4.0 mg: 74.9 (10.5) 8.0 mg: 75.5 (10.5) | 73.1 (10.5) |
| Dobkin et al., 2006 [26] | Sleep diaries and self-report questionnaires * | 420 ± 38 | 336 ± 62 | 24.0 ± 15.0 | 46.2 ± 19.8 | 91 ± 6 | 80 ± 10 |
| Richardson et al., 2009 [35] | Sleep diaries | 370 | 350 | 42 | 50 | ||
| Study Author, Year | Randomization | Deviations from the Intended Intervention | Missing Outcome Data | Measurement of Outcome | Selection of the Reported Results | Overall |
|---|---|---|---|---|---|---|
| Almeida et al., 2003 [23] |  |  |  |  |  |  |
| Andrade et al., 1999 [24] |  |  |  |  |  | 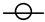 |
| Baskett et al., 2001 [25] |  |  |  |  |  |  |
| Haimov et al., 1995 [28] |  | 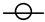 | 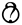 |  |  | 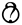 |
| Jha et al., 2016 [29] |  |  |  |  |  |  |
| Jun et al., 2018 [30] |  |  |  |  |  |  |
| Neurim pharma, 1995 [32] |  |  | 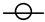 |  |  | 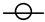 |
| Mini, et al., 2007 [33] |  |  | 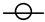 | 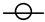 | 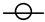 | 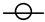 |
| Pen state Takeda et al., 2006 [34] |  |  | 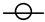 |  |  | 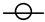 |
| Rondanelli et al., 2011 [36] |  |  |  |  |  |  |
| Roth et al., 2006 [37] |  |  | 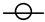 |  |  | 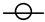 |
| Roth et al., 2007 [38] |  |  |  |  |  |  |
| Russcher et al., 2013 [39] |  |  |  |  |  |  |
| Wade et al., 2010 [41] | 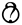 | 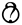 |  |  |  | 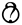 |
| Wade et al., 2007 [40] |  |  | 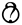 |  |  | 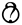 |
| Zhdanova et al., 2001 [42] |  |  | 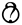 |  |  | 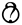 |
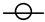 , Low risk of bias =
, Low risk of bias =  , Some concern =
, Some concern = 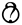 .
.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marupuru, S.; Arku, D.; Campbell, A.M.; Slack, M.K.; Lee, J.K. Use of Melatonin and/on Ramelteon for the Treatment of Insomnia in Older Adults: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5138. https://doi.org/10.3390/jcm11175138
Marupuru S, Arku D, Campbell AM, Slack MK, Lee JK. Use of Melatonin and/on Ramelteon for the Treatment of Insomnia in Older Adults: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(17):5138. https://doi.org/10.3390/jcm11175138
Chicago/Turabian StyleMarupuru, Srujitha, Daniel Arku, Ashley M. Campbell, Marion K. Slack, and Jeannie K. Lee. 2022. "Use of Melatonin and/on Ramelteon for the Treatment of Insomnia in Older Adults: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 17: 5138. https://doi.org/10.3390/jcm11175138
APA StyleMarupuru, S., Arku, D., Campbell, A. M., Slack, M. K., & Lee, J. K. (2022). Use of Melatonin and/on Ramelteon for the Treatment of Insomnia in Older Adults: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(17), 5138. https://doi.org/10.3390/jcm11175138







