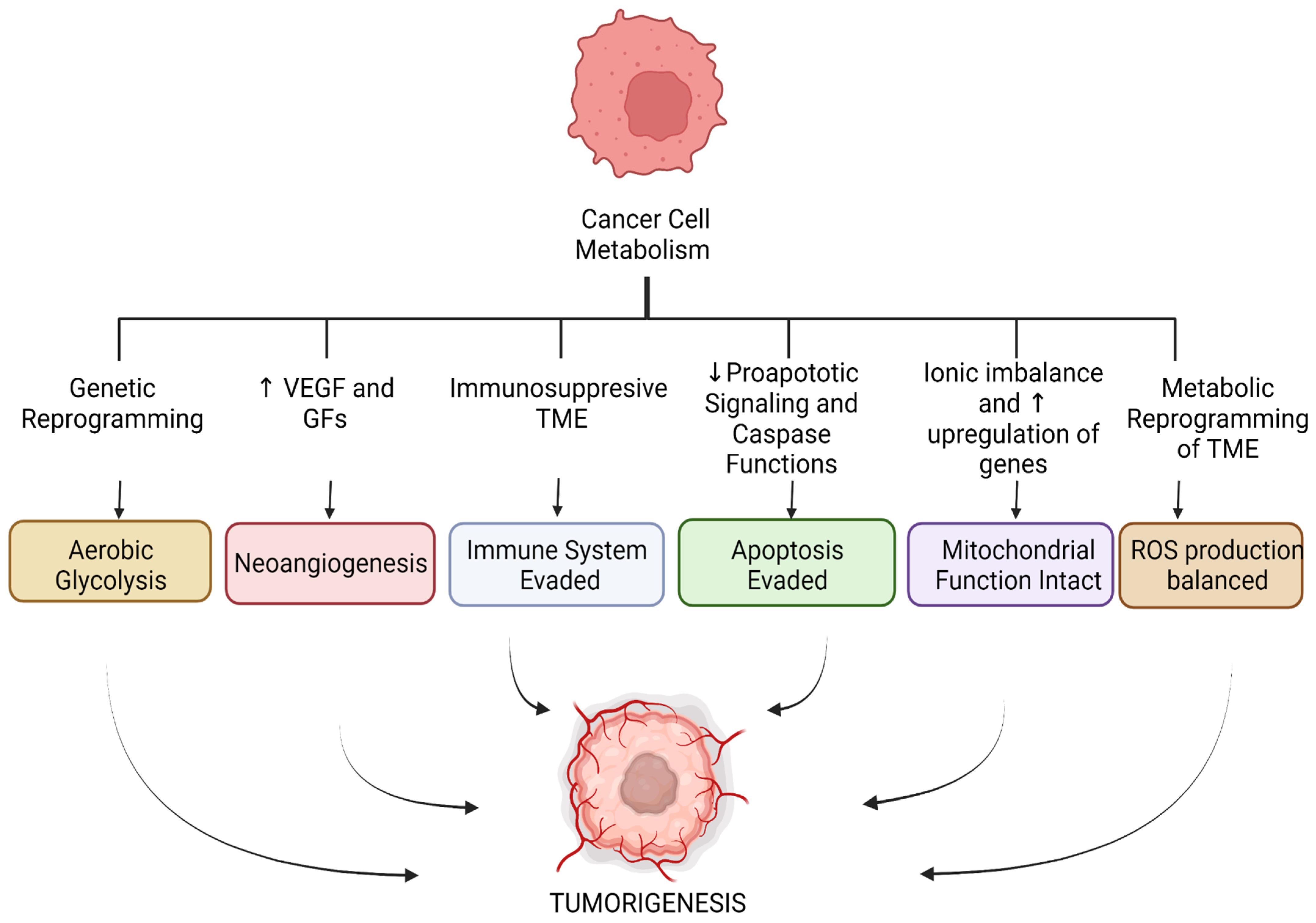
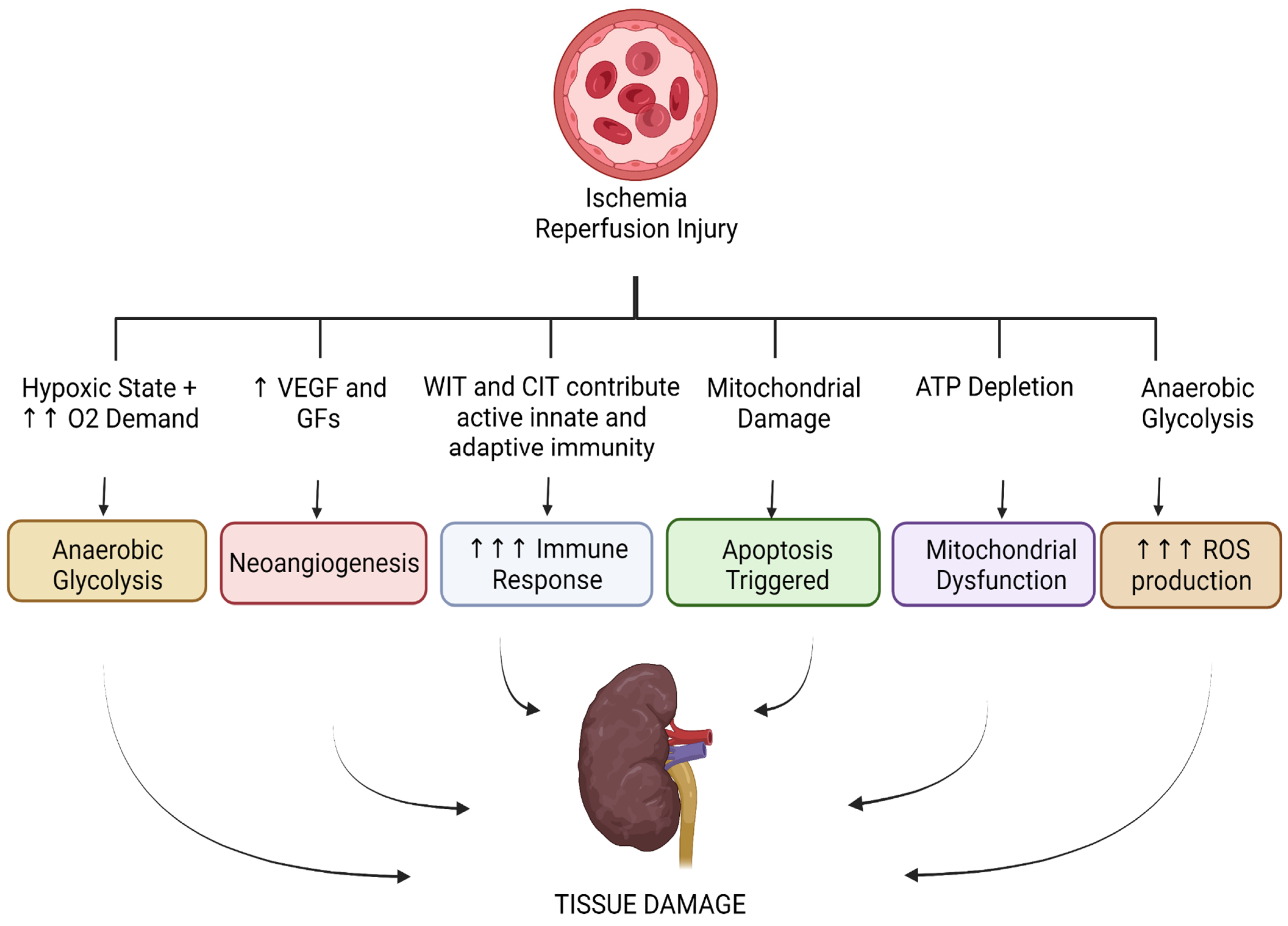
Cancer Metabolism and Ischemia-Reperfusion Injury: Two Sides of the Same Coin
Abstract
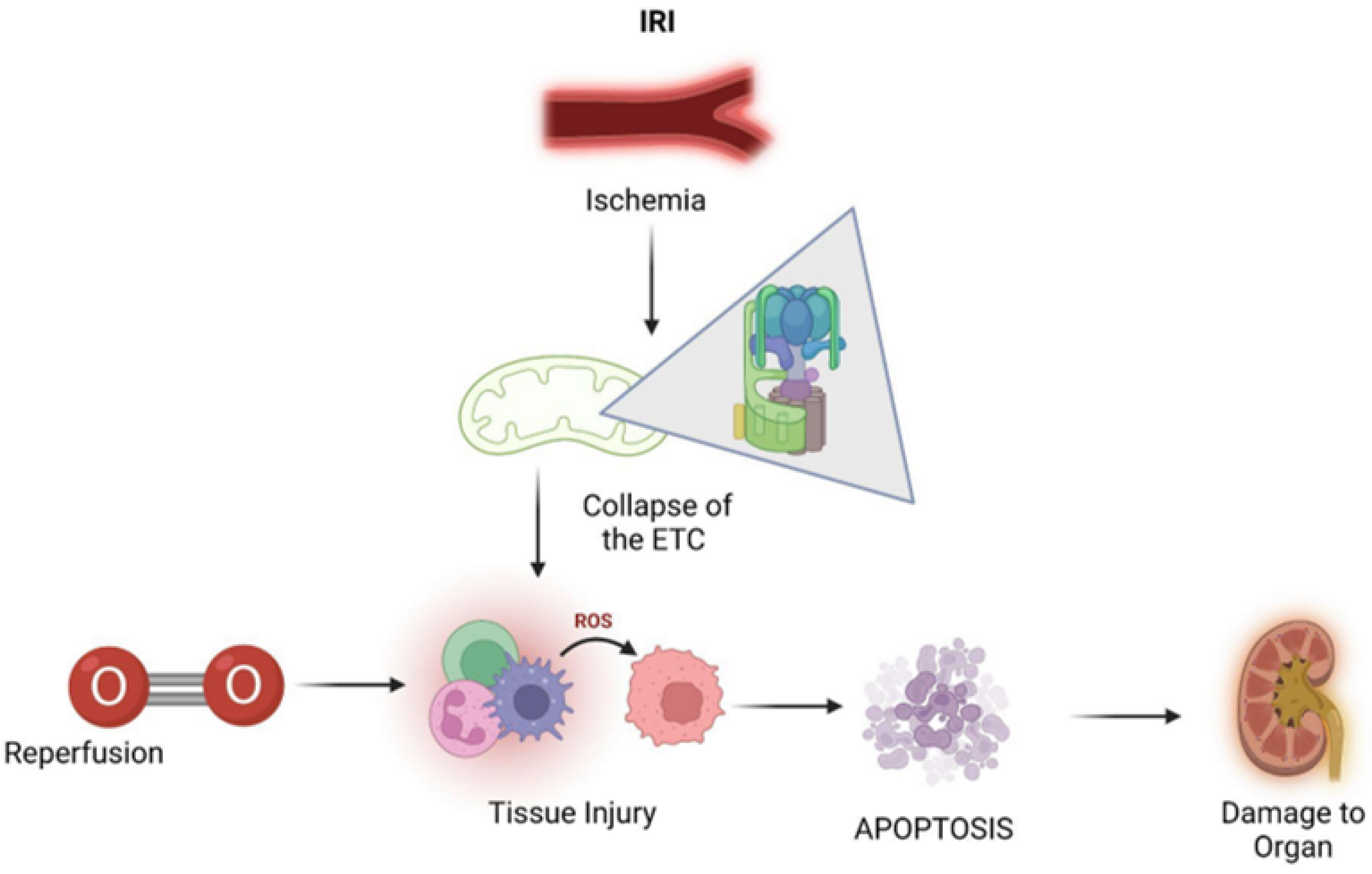
:1. Introduction
2. Literature Search
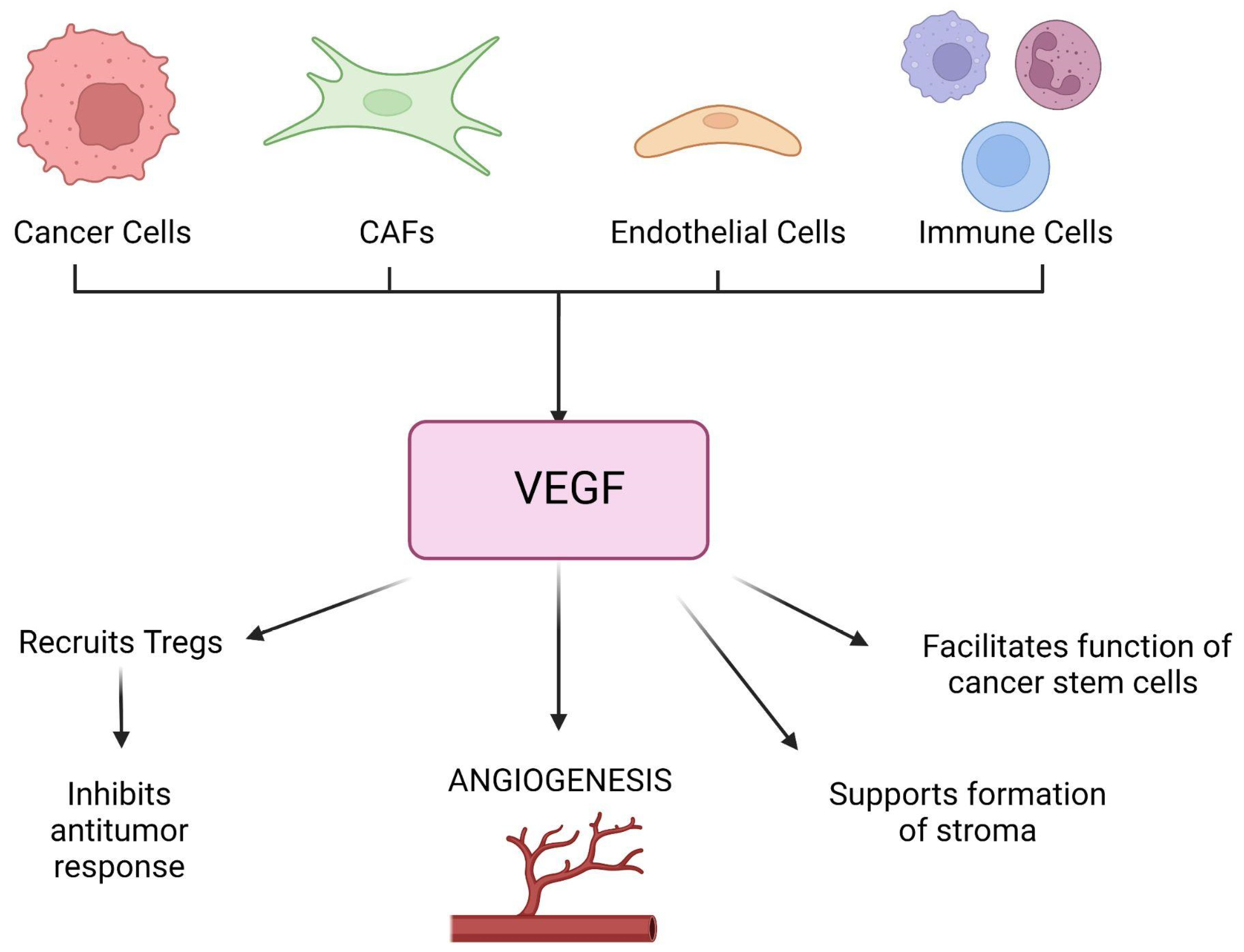
2.1. Tumor Microenvironment and the Significance of Stroma in Cancer and IRI
2.2. Glycolytic Pathways in Cancer and IRI
2.3. Neoangiogenesis in Cancer and IRI
2.4. The Immune System in Cancer and IRI
2.5. Apoptosis in Cancer and IRI
2.6. Mitochondrial Function in Cancer and IRI
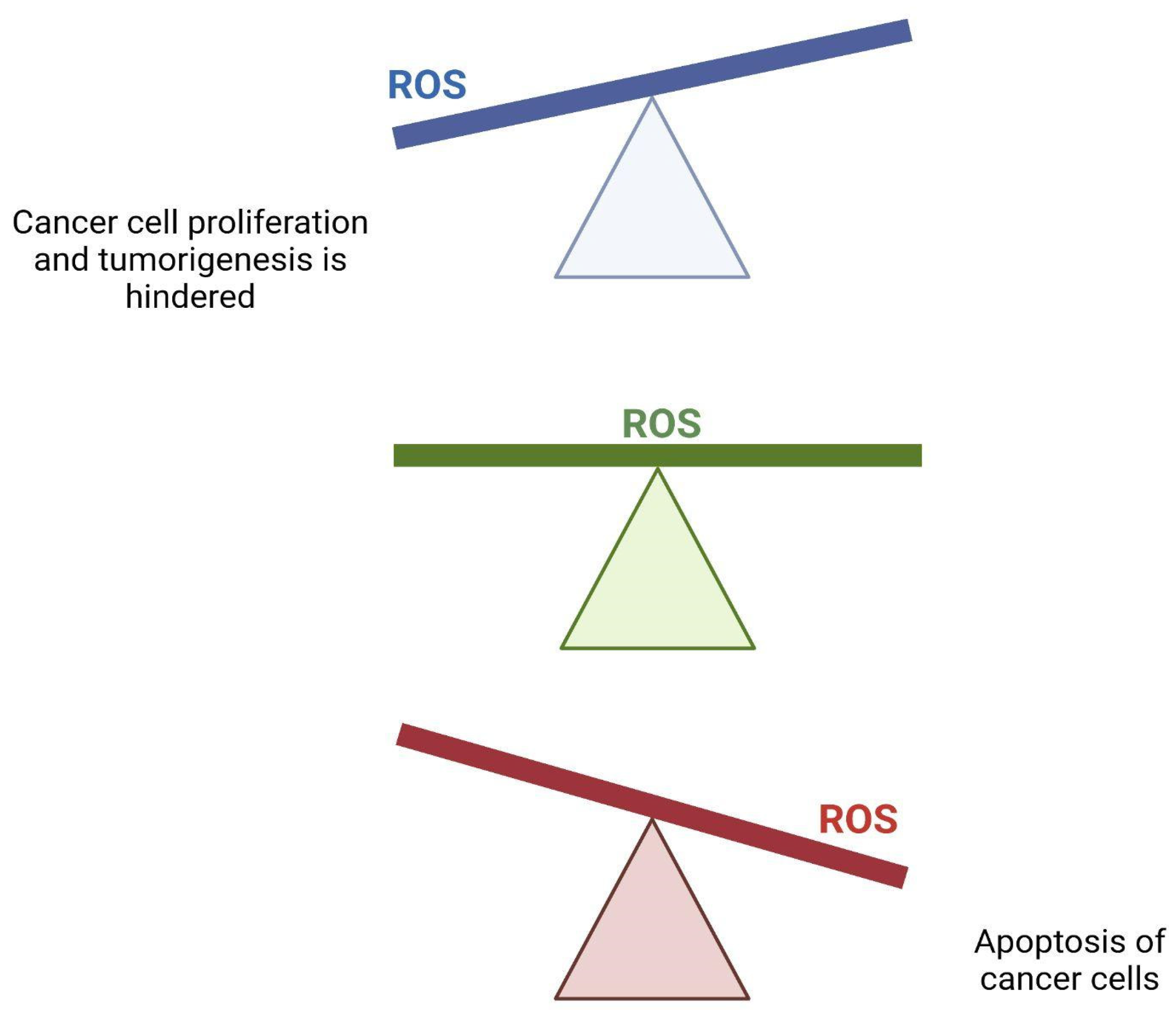
2.7. Production of Reactive Oxygen Species (ROS) in Cancer and IRI
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cell |
| AKT | Serine/threonine kinase 1 |
| ATP | Adenosine triphosphate |
| CAFs | Cancer-associated fibroblasts |
| CIT | Cold ischemia time |
| DBD | Donation after brain death |
| DCD | Donation after circulatory death |
| DGF | Delayed graft function |
| ECD | Extended criteria donor |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EVNP | Ex vivo normothermic perfusion |
| FADH2 | Flavin adenine dinucleotide |
| FasL | Fas ligand |
| FGF | Fibroblast growth factor |
| GLUT-1 | Glucose transporter 1 |
| GF | Growth factors |
| HIF | Hypoxia-inducible factor |
| IRI | Ischemia-reperfusion injury |
| MCT | Monocarboxylate transporter |
| mtDNA | Mitochondrial DNA |
| MHC | Major histocompatibility complex |
| mTOR | Mammalian target of rapamycin |
| NADH | Nicotinamide adenine dinucleotide hydride |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| OPS | Organ preservation solutions |
| PDGF | Platelet-derived growth factor |
| PI3K | Phosphatidylinositol-3-Kinase |
| PTEN | Phosphatase and tensin homolog |
| ROS | Reactive oxygen species |
| siRNA | Small interfering RNA |
| TAMs | Tumor-associated macrophages |
| TGF-β | Transforming growth factor-beta |
| TME | Tumor micro-environment |
| TNF | Tumor necrosis factor |
| TSC | Tuberous sclerosis complex |
| TSP-1 | Thrombospondin-1 |
| VEGF | Vascular endothelial growth factor |
| VHL | Von Hippel-Lindau |
| WIT | Warm ischemia time |
References
- Duan, Q.; Yang, T. Ischaemia reperfusion may be a new approach in cancer interventional therapy. J. Med. Hypotheses Ideas 2012, 6, 50–52. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Li, X.F. Hypoxia and the tumor microenvironment. Technol. Cancer Res. Treat. 2021, 20, 15330338211036304. [Google Scholar] [CrossRef] [PubMed]
- Lejay, A.; Fang, F.; John, R.; Van, J.A.; Barr, M.; Thaveau, F.; Chakfe, N.; Geny, B.; Scholey, J.W. Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J. Mol. Cell Cardiol. 2016, 91, 11–22. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [PubMed]
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? small molecules and hypoxia. J. Cell Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Tumor microenvironment. Medicina 2020, 56, 15. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K. Normalization in tumor ecosystem: Opportunities and challenges. Cell Biol. Int. 2021, 45, 2017–2030. [Google Scholar] [CrossRef] [PubMed]
- Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer. Oncology 2002, 16, 217–226. [Google Scholar] [PubMed]
- Werb, Z.; Lu, P. The role of stroma in tumor development. Cancer J. 2015, 21, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer. 2021, 20, 131. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Ding, W.; Duan, Y.; Qu, Z.; Feng, J.; Zhang, R.; Li, X.; Sun, D.; Zhang, X.; Lu, Y. Acidic microenvironment aggravates the severity of hepatic ischemia/reperfusion injury by modulating M1-polarization through regulating PPAR-γ signal. Front. Immunol. 2021, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Qiu, X.; Wang, K.; Shao, C.; Jin, W.; Zhang, Z.; Xu, X. Targeting the hepatic microenvironment to improve ischemia/reperfusion injury: New insights into the immune and metabolic compartments. Aging Dis. 2022, 13, 1196–1214. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.I.; Nozdrin, M.; Yiu, J.; Papalois, V. Machine perfusion for abdominal organ preservation: A systematic review of kidney and liver human grafts. J. Clin. Med. 2019, 8, 1221. [Google Scholar] [CrossRef] [Green Version]
- Petrenko, A.; Carnevale, M.; Somov, A. Organ preservation into the 2020s: The era of dynamic intervention. Transfus. Med. Hemother. 2019, 46, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Fard, A.; Pearson, R.; Lathan, R.; Mark, P.B.; Clancy, M.J. Perfusate composition and duration of ex-vivo normothermic perfusion in kidney transplantation: A systematic review. Transpl. Int. 2022, 35, 10236. [Google Scholar] [CrossRef]
- Kaths, J.M.; Hamar, M.; Echeverri, J.; Linares, I.; Urbanellis, P.; Cen, J.Y.; Ganesh, S.; Dingwell, L.; Yip, P.; John, R.; et al. Normothermic ex vivo kidney perfusion for graft quality assessment prior to transplantation. Am. J. Transplant. 2018, 18, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Saeb-Parsy, K.; Hamed, M.O.; Nicholson, M.L. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am. J. Transplant. 2016, 16, 3282–3285. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, E.A.; Schury, M.P. Biochemistry, anaerobic glycolysis. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Naifeh, J.; Dimri, M.; Varacallo, M. Biochemistry, Aerobic Glycolysis; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470170/ (accessed on 3 July 2022).
- Cooper, G. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Liberti, M.V.; Locasale, J.W. The warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Revisiting the warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Courtnay, R.; Ngo, D.C.; Malik, N.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Cancer metabolism and the warburg effect: The role of HIF-1 and PI3K. Mol. Biol. Rep. 2015, 42, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Marbaniang, C.; Kma, L. Dysregulation of glucose metabolism by oncogenes and tumor suppressors in cancer cells. Asian Pac. J. Cancer Prev. APJCP. 2018, 19, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metabolism. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Elstrom, R.L.; Bauer, D.E.; Buzzai, M.; Karnauskas, R.; Harris, M.H.; Plas, D.R.; Zhuang, H.; Cinalli, R.M.; Alavi, A.; Rudin, C.M.; et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004, 64, 3892–3899. [Google Scholar] [CrossRef]
- Hsieh, A.C.; Liu, Y.; Edlind, M.P.; Ingolia, N.T.; Janes, M.R.; Sher, A.; Shi, E.Y.; Stumpf, C.R.; Christensen, C.; Bonham, M.J.; et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 2012, 485, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Magaway, C.; Kim, E.; Jacinto, E. Targeting mTOR and metabolism in cancer: Lessons and innovations. Cells 2019, 8, 1584. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.C.; Rathore, A.; Younas, H.; Gilkes, D.; Polotsky, V.Y. Hypoxia-inducible factors and cancer. Curr. Sleep Med. Rep. 2017, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Akakura, N.; Kobayashi, M.; Horiuchi, I.; Suzuki, A.; Wang, J.; Chen, J.; Niizeki, H.; Ki, K.; Hosokawa, M.; Asaka, M. Constitutive expression of hypoxia-inducible factor-1alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res. 2001, 61, 6548–6554. [Google Scholar] [PubMed]
- Baba, Y.; Nosho, K.; Shima, K.; Irahara, N.; Chan, A.T.; Meyerhardt, J.A.; Chung, D.C.; Giovannucci, E.L.; Fuchs, C.S.; Ogino, S. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am. J. Pathol. 2010, 176, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.M.; Calvisi, D.F.; Simile, M.M.; Feo, C.F.; Feo, F. The warburg effect 97 years after its discovery. Cancers 2020, 12, 2819. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, L.; Tan, G.; Ke, L.; Zhang, S.; Chen, H.; Liu, J. A reactive oxygen species activation mechanism contributes to JS-K-induced apoptosis in human bladder cancer cells. Sci. Rep. 2015, 5, 15104. [Google Scholar] [CrossRef]
- Bellini, M.I.; Charalampidis, S.; Herbert, P.E.; Bonatsos, V.; Crane, J.; Muthusamy, A.; Dor, F.J.M.F.; Papalois, V. Cold pulsatile machine perfusion versus static cold storage in kidney transplantation: A single centre experience. Biomed. Res. Int. 2019, 2019, 7435248. [Google Scholar] [CrossRef] [Green Version]
- Husen, P.; Boffa, C.; Jochmans, I.; Krikke, C.; Davies, L.; Mazilescu, L.; Brat, A.; Knight, S.; Wettstein, D.; Cseprekal, O.; et al. Oxygenated end-hypothermic machine perfusion in expanded criteria donor kidney transplant: A randomized clinical trial. JAMA Surg. 2021, 156, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Beltran, C.; Pardo, R.; Bou-Teen, D.; Ruiz-Meana, M.; Villena, J.A.; Ferreira-González, I.; Barba, I. Enhancing glycolysis protects against ischemia-reperfusion injury by reducing ROS production. Metabolites 2020, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Bielenberg, D.R.; Zetter, B.R. The contribution of angiogenesis to the process of metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef]
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the role of angiogenesis in cancer ecosystems. Front. Oncol. 2018, 8, 248. Available online: https://www.frontiersin.org/article/10.3389/fonc.2018.00248 (accessed on 3 July 2022). [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Baeriswyl, V.; Christofori, G. The angiogenic switch in carcinogenesis. Semin Cancer Biol. 2009, 19, 329–337. [Google Scholar] [CrossRef]
- Bickel, S.T.; Juliano, J.D.; Nagy, J.D. Evolution of proliferation and the angiogenic switch in tumors with high clonal diversity. PLoS ONE. 2014, 9, e91992. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in signaling and disease: Beyond discovery and development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer. 2013, 13, 871–882. [Google Scholar] [CrossRef]
- Kardas, G.; Daszyńska-Kardas, A.; Marynowski, M.; Brząkalska, O.; Kuna, P.; Panek, M. Role of platelet-derived growth factor (PDGF) in asthma as an immunoregulatory factor mediating airway remodeling and possible pharmacological target. Front. Pharmacol. 2020, 11, 47. [Google Scholar] [CrossRef]
- Pierce, G.F.; Mustoe, T.A.; Altrock, B.W.; Deuel, T.F.; Thomason, A. Role of platelet-derived growth factor in wound healing. J. Cell Biochem. 1991, 45, 319–326. [Google Scholar] [CrossRef]
- Zhao, Y.; Adjei, A.A. Targeting angiogenesis in cancer therapy: Moving beyond vascular endothelial growth factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef]
- Liu, G.; Chen, T.; Ding, Z.; Wang, Y.; Wei, Y.; Wei, X. Inhibition of FGF-FGFR and VEGF-VEGFR signalling in cancer treatment. Cell Prolif. 2021, 54, e13009. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, S.; Zeng, J. TGF-β signaling: A complex role in tumorigenesis (review). Mol. Med. Rep. 2018, 17, 699–704. [Google Scholar] [CrossRef]
- Gutierrez, L.S.; Gutierrez, J. Thrombospondin 1 in metabolic diseases. Front. Endocrinol. 2021, 12, 638536. [Google Scholar] [CrossRef] [PubMed]
- Papetti, M.; Herman, I.M. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol. Cell Physiol. 2002, 282, 947–970. [Google Scholar] [CrossRef]
- Dudley, A.C. Tumor endothelial cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006536. [Google Scholar] [CrossRef] [PubMed]
- Azzi, S.; Hebda, J.K.; Gavard, J. Vascular permeability and drug delivery in cancers. Front. Oncol. 2013, 3, 211. [Google Scholar] [CrossRef]
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. 2016, 36, 451–455. [Google Scholar] [CrossRef]
- Cho, H.-H.; Kim, H.; Nam, S.Y.; Lee, J.E.; Han, B.-K.; Ko, E.Y.; Choi, J.S.; Park, H.; Ko, E.S. Measurement of perfusion heterogeneity within tumor habitats on magnetic resonance imaging and its association with prognosis in breast cancer patients. Cancers 2022, 14, 1858. [Google Scholar] [CrossRef]
- Qin, Z.; Li, X.; Yang, J.; Cao, P.; Qin, C.; Xue, J.; Jia, R. VEGF and ang-1 promotes endothelial progenitor cells homing in the rat model of renal ischemia and reperfusion injury. Int. J. Clin. Exp. Pathol. 2017, 10, 11896–11908. [Google Scholar]
- Korkmaz, A.; Oyar, E.O.; Yıldırım, Z.; Pampal, A.; Unlu, N.L.; Akbulut, H. Application of vascular endothelial growth factor at different phases of intestinal ischemia/reperfusion: What are its effects on oxidative stress, inflammation and telomerase activity? Adv. Clin. Exp. Med. 2020, 29, 1417–1424. [Google Scholar] [CrossRef]
- Patschan, D.; Krupincza, K.; Patschan, S.; Zhang, Z.; Hamby, C.; Goligorsky, M.S. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: Modulation by ischemic preconditioning. Am. J. Physiol. Renal. Physiol. 2006, 291, 176. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, B.F.; Flyvbjerg, A.; De Vriese, A.S. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004, 65, 2003–2017. [Google Scholar] [CrossRef]
- Gao, L.; Yang, J.; Li, Y.; Liu, K.; Sun, H.; Tang, J.; Xia, Z.; Zhang, L.; Hu, Z. Long noncoding RNA SCIRT promotes HUVEC angiogenesis via stabilizing VEGFA mRNA induced by hypoxia. Oxidative Med. Cell. Longev. 2022, 2022, 9102978. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Lei, Y.; Li, J.K.; Du, W.X.; Li, R.G.; Yang, J.; Li, J.; Li, F.; Tan, H.B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor associated neutrophils. their role in tumorigenesis, metastasis, prognosis and therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Al Tameemi, W.; Dale, T.P.; Al-Jumaily, R.M.K.; Forsyth, N.R. Hypoxia-modified cancer cell metabolism. Front. Cell Dev. Biol. 2019, 7, 4. [Google Scholar] [CrossRef]
- Casey, M.; Nakamura, K. The cancer-immunity cycle in multiple myeloma. Immunotargets Ther. 2021, 10, 247–260. [Google Scholar] [CrossRef]
- Hole, C.; Wager, C.M.L.; Castro-Lopez, N.; Campuzano, A.; Cai, H.; Wozniak, K.L.; Wang, Y.; Jr., F.L.W. Induction of memory-like dendritic cell responses in vivo. Nat. Commun. 2019, 10, 2955. [Google Scholar] [CrossRef]
- Veglia, F.; Gabrilovich, D.I. Dendritic cells in cancer: The role revisited. Curr. Opin. Immunol. 2017, 45, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Siedlecki, A.; Irish, W.; Brennan, D.C. Delayed graft function in the kidney transplant. Am. J. Transplant. 2011, 11, 2279–2296. [Google Scholar] [CrossRef]
- Global Observatory on Donation and Transplantation. Available online: http://www.transplant-observatory.org (accessed on 20 May 2021).
- Cravedi, P.; Menon, M.; Perico, N.; Remuzzi, G. Chapter 213—Acute renal failure in kidney transplant recipients. In Critical Care Nephrology, 3rd ed.; Ronco, C., Bellomo, R., Kellum, J.A., Ricci, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1279–1285.e3. [Google Scholar]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.M.R.F.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and reperfusion injury in kidney transplantation: Relevant mechanisms in injury and repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef]
- Zhai, Y.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Liver ischemia and reperfusion injury: New insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am. J. Transplant. 2011, 11, 1563–1569. [Google Scholar] [CrossRef]
- Khan, T.F.T.; Ahmad, N.; Serageldeen, A.S.; Fourtounas, K. Implantation warm ischemia time in kidney transplant recipients: Defining its limits and impact on early graft function. Ann. Transplant. 2019, 24, 432–438. [Google Scholar] [CrossRef]
- Denecke, C.; Yuan, X.; Ge, X.; Kim, I.K.; Bedi, D.; Boenisch, O.; Weiland, A.; Jurisch, A.; Kotsch, K.; Pratschke, J.; et al. Synergistic effects of prolonged warm ischemia and donor age on the immune response following donation after cardiac death kidney transplantation. Surgery 2013, 153, 249–261. [Google Scholar] [CrossRef]
- Ferede, A.A.; Walsh, A.L.; Davis, N.F.; Smyth, G.; Mohan, P.; Power, R.; Forde, J.; O’Kelly, P.; Little, D.; Llittle, D. Warm ischemia time at vascular anastomosis is an independent predictor for delayed graft function in kidney transplant recipients. Exp. Clin. Transplant. 2020, 18, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.L.; Palmer, S.M. Danger signals in regulating the immune response to solid organ transplantation. J. Clin. Invest. 2017, 127, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Silvis, M.J.M.; Dengler, S.E.K.G.; Odille, C.A.; Mishra, M.; Van Der Kaaij, N.P.; Doevendans, P.A.; Sluijter, J.P.G.; De Kleijn, D.P.V.; De Jager, S.C.A.; Bosch, L.; et al. Damage-associated molecular patterns in myocardial infarction and heart transplantation: The road to translational success. Front. Immunol. 2020, 11, 599511. [Google Scholar] [CrossRef]
- Braza, F.; Brouard, S.; Chadban, S.; Goldstein, D.R. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat. Rev. Nephrol. 2016, 12, 281–290. [Google Scholar] [CrossRef]
- Kopecky, B.J.; Frye, C.; Terada, Y.; Balsara, K.R.; Kreisel, D.; LaVine, K.J. Role of donor macrophages after heart and lung transplantation. Am. J. Transplant. 2020, 20, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; He, S.; Mao, X.; Zhang, Y.; Cai, Y.; Li, S. Effect of hepatic macrophage polarization and apoptosis on liver ischemia and reperfusion injury during liver transplantation. Front. Immunol. 2020, 11, 1193. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Lu, L.; Zhai, Y. T cells in organ ischemia reperfusion injury. Curr. Opin. Organ. Transplant. 2014, 19, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Loverre, A.; Divella, C.; Castellano, G.; Tataranni, T.; Zaza, G.; Rossini, M.; Ditonno, P.; Battaglia, M.; Palazzo, S.; Gigante, M.; et al. T helper 1, 2 and 17 cell subsets in renal transplant patients with delayed graft function. Transpl. Int. 2011, 24, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Kamo, N.; Shen, X.-D.; Ke, B.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Sotrastaurin, a protein kinase C inhibitor, ameliorates ischemia and reperfusion injury in rat orthotopic liver transplantation. Am. J. Transplant. 2011, 11, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Gandolfo, M.T.; Jang, H.R.; Bagnasco, S.M.; Ko, G.-J.; Agreda, P.; Soloski, M.J.; Crow, M.T.; Rabb, H. Mycophenolate mofetil modifies kidney tubular injury and Foxp3+ regulatory T cell trafficking during recovery from experimental ischemia-reperfusion. Transpl. Immunol. 2010, 23, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef]
- Graham, N.A.; Tahmasian, M.; Kohli, B.; Komisopoulou, E.; Zhu, M.; Vivanco, I.; A Teitell, M.; Wu, H.; Ribas, A.; Lo, R.S.; et al. Glucose deprivation activates a metabolic and signaling amplification loop leading to cell death. Mol. Syst Biol. 2012, 8, 589. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer. 2020, 122, 4–22. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of amino acids in cancer. Front. Cell Dev. Biol. 2021, 8, 603837. [Google Scholar] [CrossRef]
- Priante, G.; Gianesello, L.; Ceol, M.; Del Prete, D.; Anglani, F. Cell death in the kidney. Int. J. Mol. Sci. 2019, 20, 3598. [Google Scholar] [CrossRef]
- Bellini, M.; Tortorici, F.; Amabile, M.; D’Andrea, V. Assessing kidney graft viability and its cells metabolism during machine perfusion. Int. J. Mol. Sci. 2021, 22, 1121. [Google Scholar] [CrossRef] [PubMed]
- Eefting, F.; Rensing, B.; Wigman, J.; Pannekoek, W.J.; Liu, W.M.; Cramer, M.J.; Lips, D.J.; Doevendans, P.A. Role of apoptosis in reperfusion injury. Cardiovasc. Res. 2004, 61, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer. 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Dominy, J.E.; Puigserver, P. Mitochondrial biogenesis through activation of nuclear signaling proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a015008. [Google Scholar] [CrossRef] [PubMed]
- Chio, I.I.C.; Tuveson, D.A. ROS in cancer: The burning question. Trends Mol. Med. 2017, 23, 411–429. [Google Scholar] [CrossRef]
- Cameron, E.; Campbell, A. The orthomolecular treatment of cancer. II. clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem. Biol. Interact. 1974, 9, 285–315. [Google Scholar] [CrossRef]
- Fritz, H.; Flower, G.; Weeks, L.; Cooley, K.; Callachan, M.; McGowan, J.; Skidmore, B.; Kirchner, L.; Seely, D. Intravenous vitamin C and cancer: A systematic review. Integr. Cancer Ther. 2014, 13, 280–300. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, N.; Creagan, E.; Witzig, T.; Levine, M. Ascorbic acid in cancer treatment: Let the phoenix fly. Cancer Cell 2018, 34, 700–706. [Google Scholar] [CrossRef]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.S.; Chandel, N.S. Mitochondrial metabolism and ROS generation are essential for kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef]
- Pizzimenti, S.; Toaldo, C.; Pettazzoni, P.; Dianzani, M.U.; Barrera, G. The “two-faced” effects of reactive oxygen species and the lipid peroxidation product 4-hydroxynonenal in the hallmarks of cancer. Cancers 2010, 2, 338–363. [Google Scholar] [CrossRef]
- Zabłocka, A.; Janusz, M. The two faces of reactive oxygen species. Postepy Hig. Med. Dosw. 2008, 62, 118–124. [Google Scholar]
- Wilde, L.; Roche, M.; Domingo-Vidal, M.; Tanson, K.; Philp, N.; Curry, J.; Martinez-Outschoorn, U. Metabolic coupling and the reverse warburg effect in cancer: Implications for novel biomarker and anticancer agent development. Semin Oncol. 2017, 44, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.I.; Yiu, J.; Nozdrin, M.; Papalois, V. The effect of preservation temperature on liver, kidney, and pancreas tissue ATP in animal and preclinical human models. J. Clin. Med. 2019, 8, 1421. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.H.; Jaberi, A.; Gordon, C.E.; Beck, L.H.; Francis, J. The complement system in the modern era of kidney transplantation: Mechanisms of injury and targeted therapies. Semin Nephrol. 2022, 42, 14–28. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef]
- Chen, S.; Meng, X.-F.; Zhang, C. Role of NADPH oxidase-mediated reactive oxygen species in podocyte injury. BioMed Res. Int. 2013, 2013, 839761. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Y.; Chabrashvili, T.; Aslam, S.; Conde, L.J.B.; Umans, J.G.; Wilcox, C.S. Role of oxidative stress in endothelial dysfunction and enhanced responses to angiotensin II of afferent arterioles from rabbits infused with angiotensin II. J. Am. Soc. Nephrol. 2003, 14, 2783–2789. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemeth, D.V.; Baldini, E.; Sorrenti, S.; D’Andrea, V.; Bellini, M.I. Cancer Metabolism and Ischemia-Reperfusion Injury: Two Sides of the Same Coin. J. Clin. Med. 2022, 11, 5096. https://doi.org/10.3390/jcm11175096
Nemeth DV, Baldini E, Sorrenti S, D’Andrea V, Bellini MI. Cancer Metabolism and Ischemia-Reperfusion Injury: Two Sides of the Same Coin. Journal of Clinical Medicine. 2022; 11(17):5096. https://doi.org/10.3390/jcm11175096
Chicago/Turabian StyleNemeth, Denise V., Enke Baldini, Salvatore Sorrenti, Vito D’Andrea, and Maria Irene Bellini. 2022. "Cancer Metabolism and Ischemia-Reperfusion Injury: Two Sides of the Same Coin" Journal of Clinical Medicine 11, no. 17: 5096. https://doi.org/10.3390/jcm11175096
APA StyleNemeth, D. V., Baldini, E., Sorrenti, S., D’Andrea, V., & Bellini, M. I. (2022). Cancer Metabolism and Ischemia-Reperfusion Injury: Two Sides of the Same Coin. Journal of Clinical Medicine, 11(17), 5096. https://doi.org/10.3390/jcm11175096









