A Comparison of Different Types of Esophageal Reconstructions: A Systematic Review and Network Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
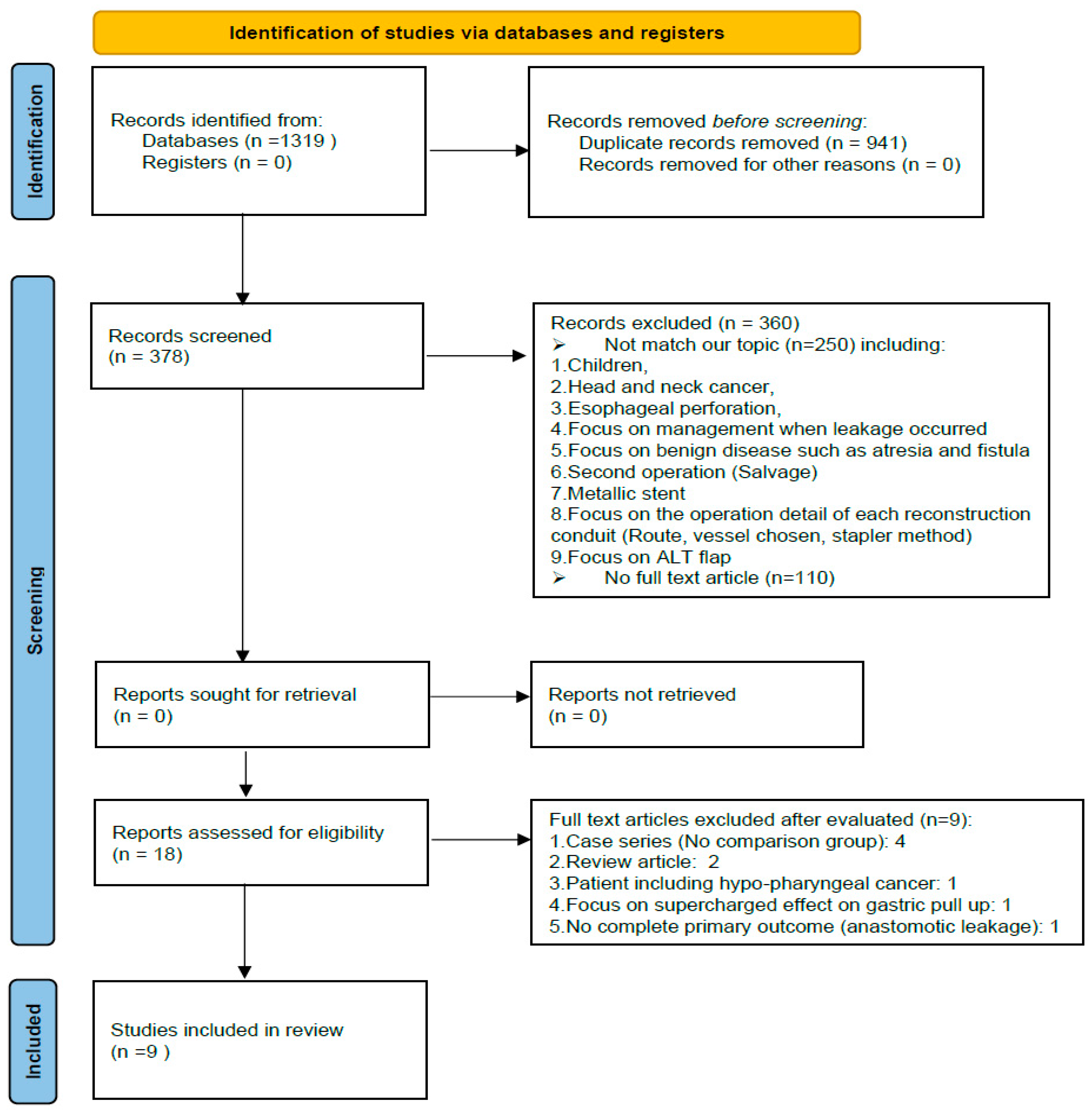
2.1. Literature Search
- Esophageal cancer;
- Esophagectomy;
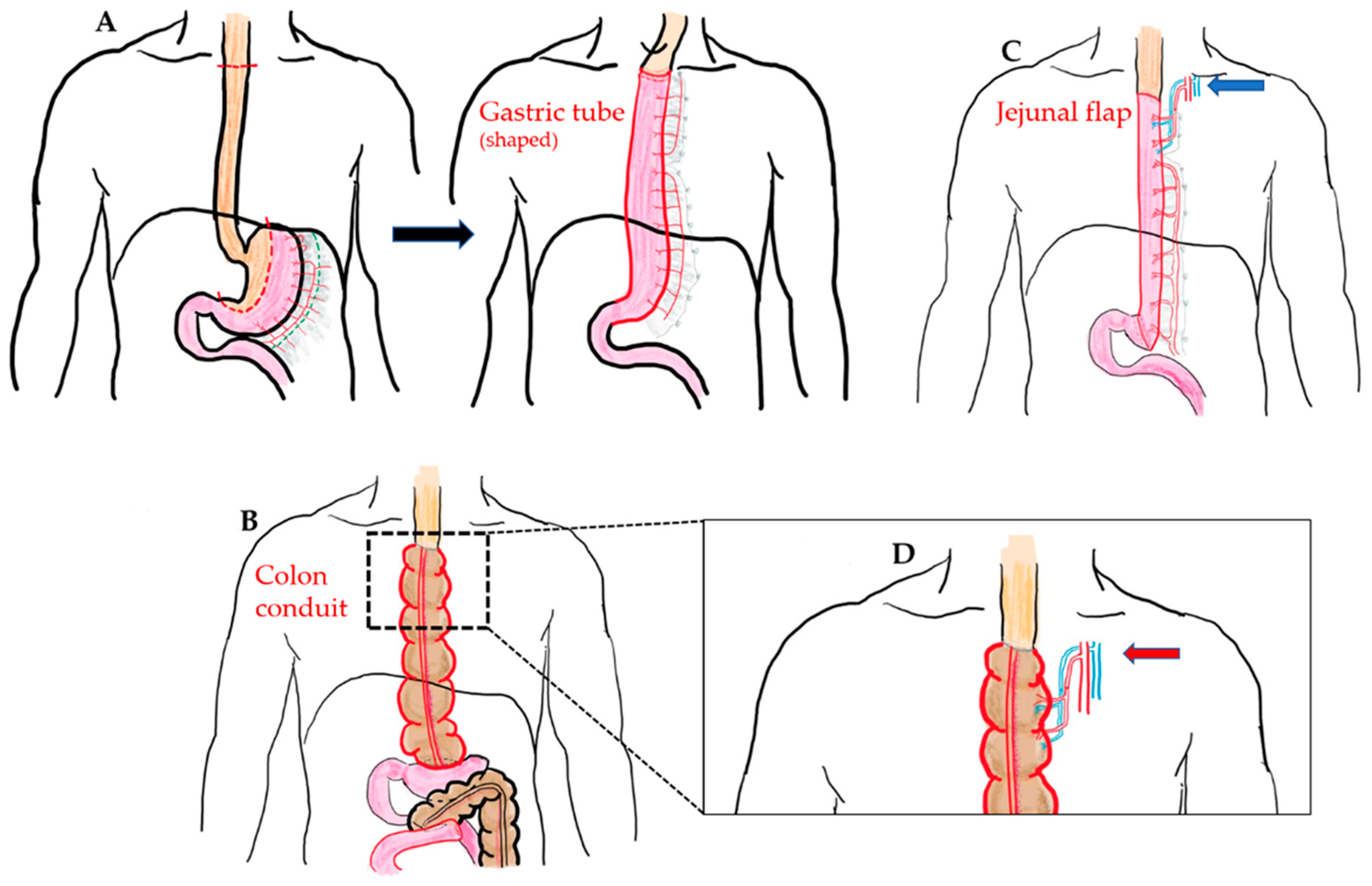
- Gastric pull-up;
- Colon interposition;
- Jejunal flap;
- Anastomotic leakage.
2.2. Article Eligibility
- Patients with esophageal cancer who underwent open or minimally invasive esophagectomy and reconstruction surgery;
- Gastric pull-up, free jejunal flap, or colon interposition reconstruction, with or without the supercharged procedure;
- One of the following data should exist: conduit leaking rate, necrotic conduit rate, or reoperation rate to evaluate whether the conduit functions well;
- Full text article;
- Article in English;
- Published up to 2021 (1991–2021);
- Adult population;
- Cohort study, case-control study, or clinical trial.
- Pharyngoesophageal cancer;
- Hypopharyngeal cancer;
- Pharyngolaryngectomy;
- Second operation;
- Case report and case series.
2.3. Data Extraction
- Primary Outcome:The primary outcome was anastomosis leakage.
- Secondary Outcome:The secondary outcomes were stricture formation, length of hospital stay, and mortality.
2.4. Statistical Analysis
2.5. Quality Assessment
2.6. Bias Evaluation
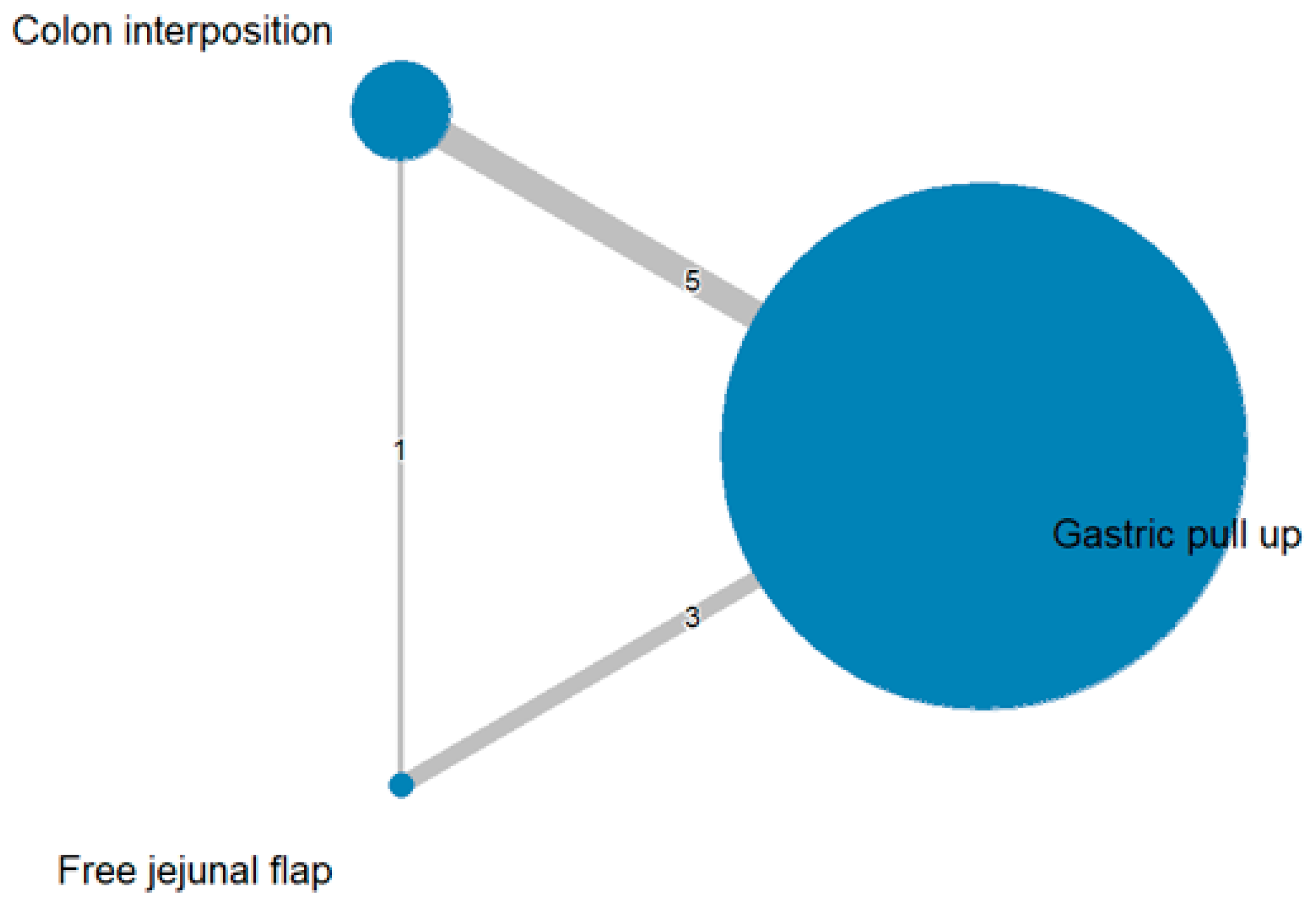
3. Results
| Author (Ref.) | Study Design | Country, Year | Follow-Up Year | Patient Number | Reconstruction Method | Supercharged | NOS | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Method 1 (n) | AL 1 (n,%) | Method 2 (n) | AL 2 (n,%) | |||||||
| Kolh et al. [21] | Retrospective | Belgium, 2000 | 1990–1998 | 130 | GPU (92) | 6, 6.5% | CI (38) | AL (1.2.6) | No | 6 |
| DeMeester et al. [22] * | Questionnaires | USA, 2001 | X | 201 | GPU (116) | 11,9.5% | CI (85) | AL (8.9.4) | No | 1 |
| Huttl et al. [23] | Questionnaires | Germany, 2002 | 1999 | 719 | GPU (653) | 79, 12.1% | CI (66) | 10, 15.1% | No | 4 |
| Davis et al. [24] | Prospective | HK, 2003 | 1982–2000 | 1001 | GPU (959) | 37, 3.9% | CI (42) | 6, 14.3% | No | 8 |
| Briel et al. [11] | Retrospective | USA, 2004 | 1996–2002 | 393 | GPU (230) | 33.14.3% | CI (163) | 10, 6.1% | X (no detail) | 8 |
| Daiko et al. [25] | Retrospective | Japan, 2007 | 1982–2002 | 71 | GPU (21) | 2.9.5% | JF (50) | 2, 4% | X (no detail) | 7 |
| Doki et al. [26] | Retrospective | Japan, 2008 | 1998–2005 | 49 | CI (25) | 13.52% | JF (28) | 6, 21.4% | Yes (both) | 9 |
| Stephens et al. [27] | Questionnaires | USA, 2015 | 2009–2013 | 45 | GPU (31) | 7.22.5% | JF (14) | 4, 28.5% | X(no detail) | 4 |
| Luan et al. [28] | Retrospective | USA, 2018 | 2004–2014 | 100 | GPU(85) | 7.22.5% | CI (15) | 4, 28.5% | Yes (JF) | 6 |
| Author (Ref.) | Mean Age | Pathology (n) | Tumor Location | Pstage | Preoperative Chemoradiotherapy | Colon Conduit Choice | ||||
| Method 1 | Method 2 | Method 1 | Method 2 | |||||||
| Kolh et al. [21] | 63.4 ± 10.2 | 52.3 ± 12.8 | Adeno: 62 SqCC: 28 Cardia: 33 | Upper: 14 Middle: 49 Lower: 33 Cardia: 34 | I: 21 II:51 III:52 IV:6 | X | X | Light side colon isoperistaltic | ||
| DeMeester et al. [22] * | X | X | X | X | X | X | X | X | ||
| Huttl et al. [23] | X | X | SqCC: 706 Barret: 282 | X | X | X | X | Right side colon antiperistaltic | ||
| Davis et al. [24] | 62.8 ± 9.3 | 62 ± 9.7 | Adeno: 107 SqCC: 873 other: 21 | Cervical: 52 Upper: 64 Middle: 503 Lower: 253 Cardia: 104 Double: 25 | 0:37 I: 48 II:249 III:553 IV:113 | 23, 25% | 7, 18% | Right side colon antiperistaltic (mostly) | ||
| Briel et al. [11] | X | X | X | X | X | X | X | X | ||
| Daiko et al. [25] | X | X | SqCC: 74 | Cervical: 74 | I: 6 II:30 III:38 | X | X | X | ||
| Doki et al. [26] | 63.75 ± 7.2 | 66.5 ± 7.8 | X | X | 0:4; I: 7 II:17 III:15 IV:10 | 9, 35% | 8, 35% | Right side colon antiperistaltic | ||
| Stephens et al. [27] | 63 ± 10 | 55 ± 15 | Cancer: 39; benign: 6 | X | X | X | X | X | ||
| Luan et al. [28] | 63.1 ± 13.1 | 60.2 ± 11.2 | X | X | X | 30, 35% | 6, 40% | X | ||
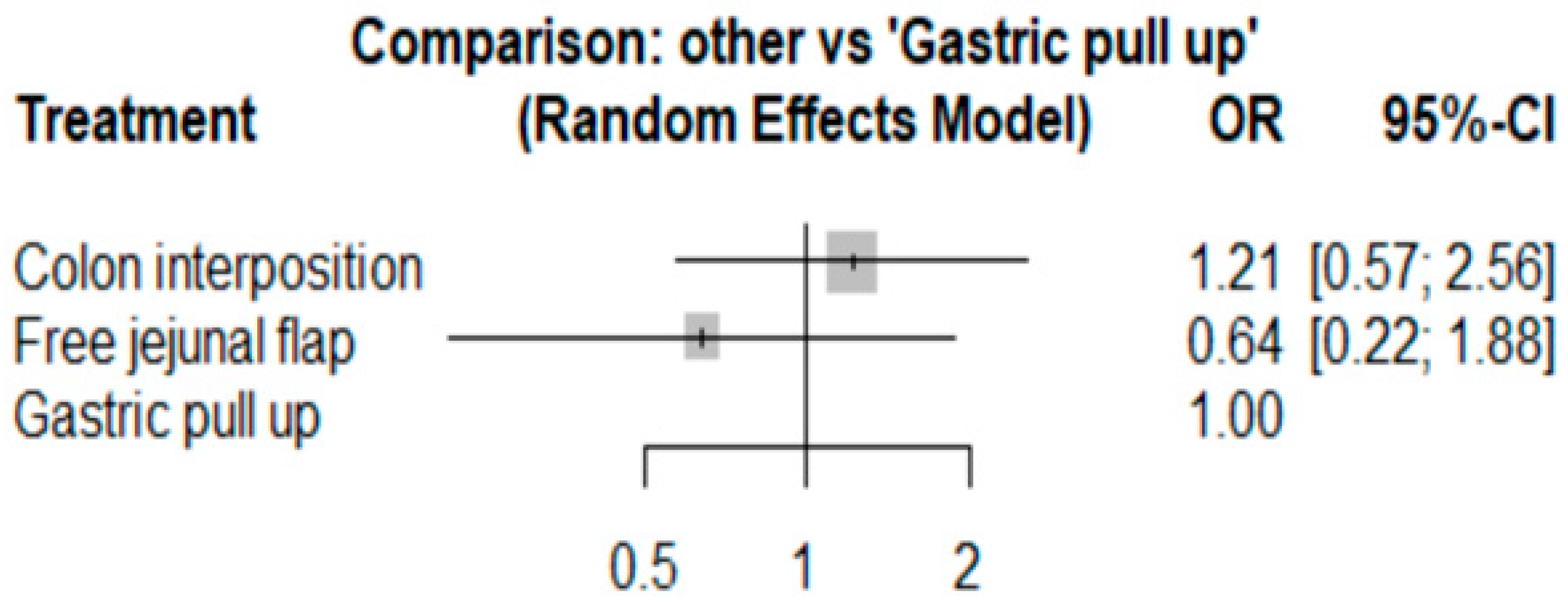
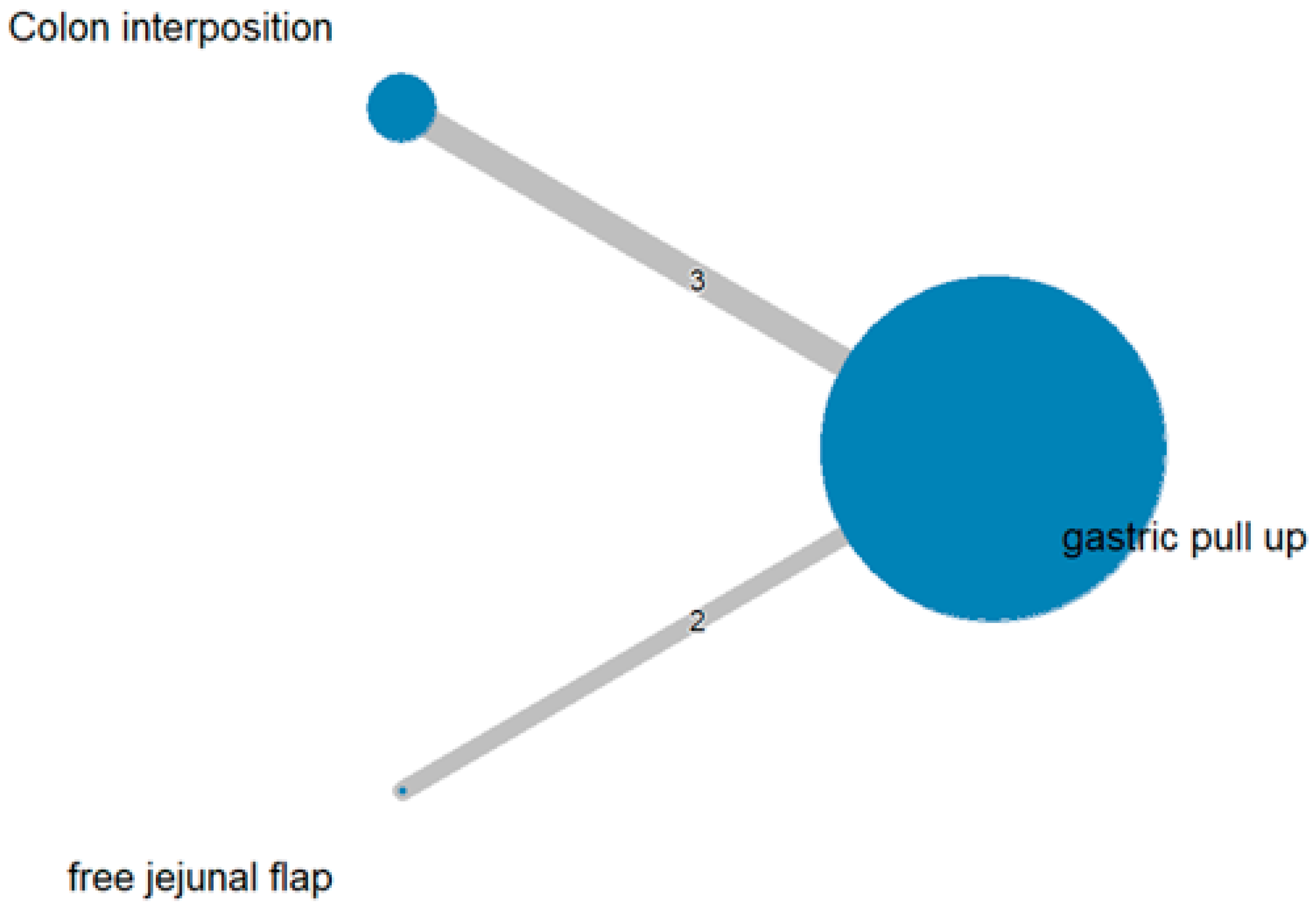
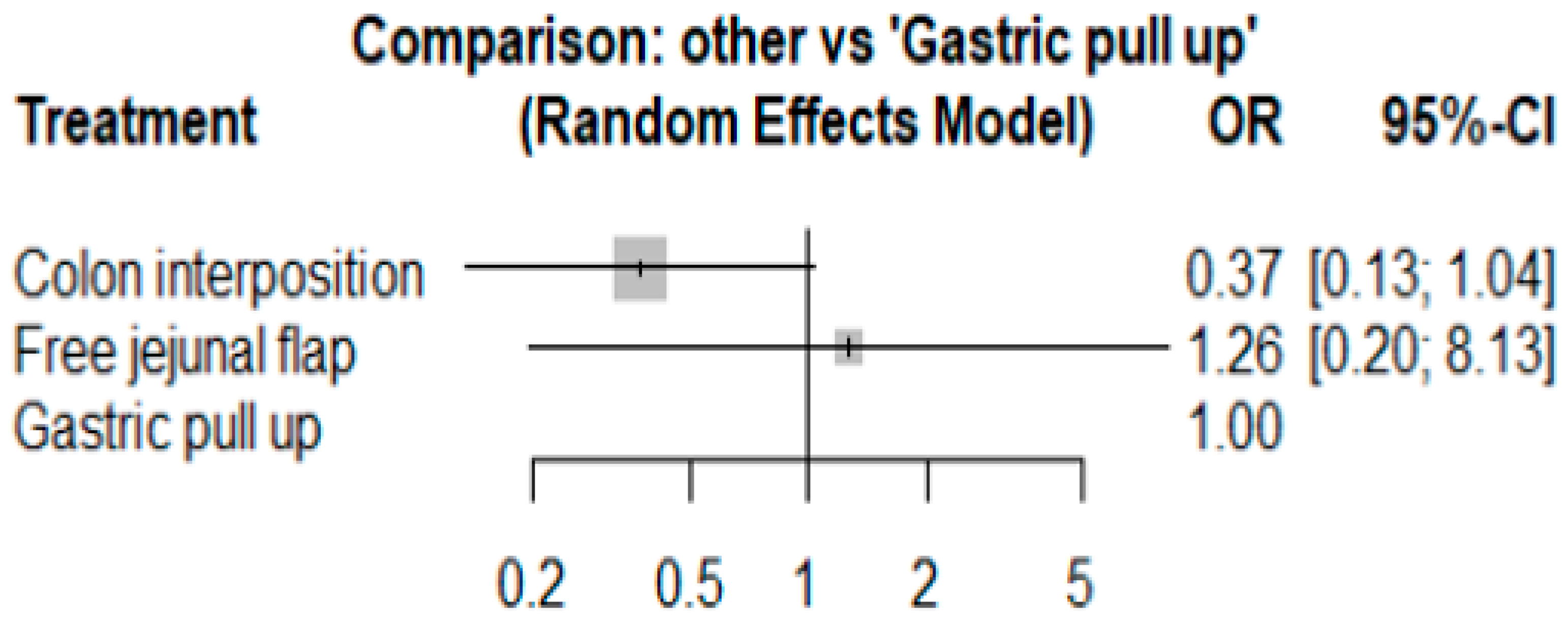
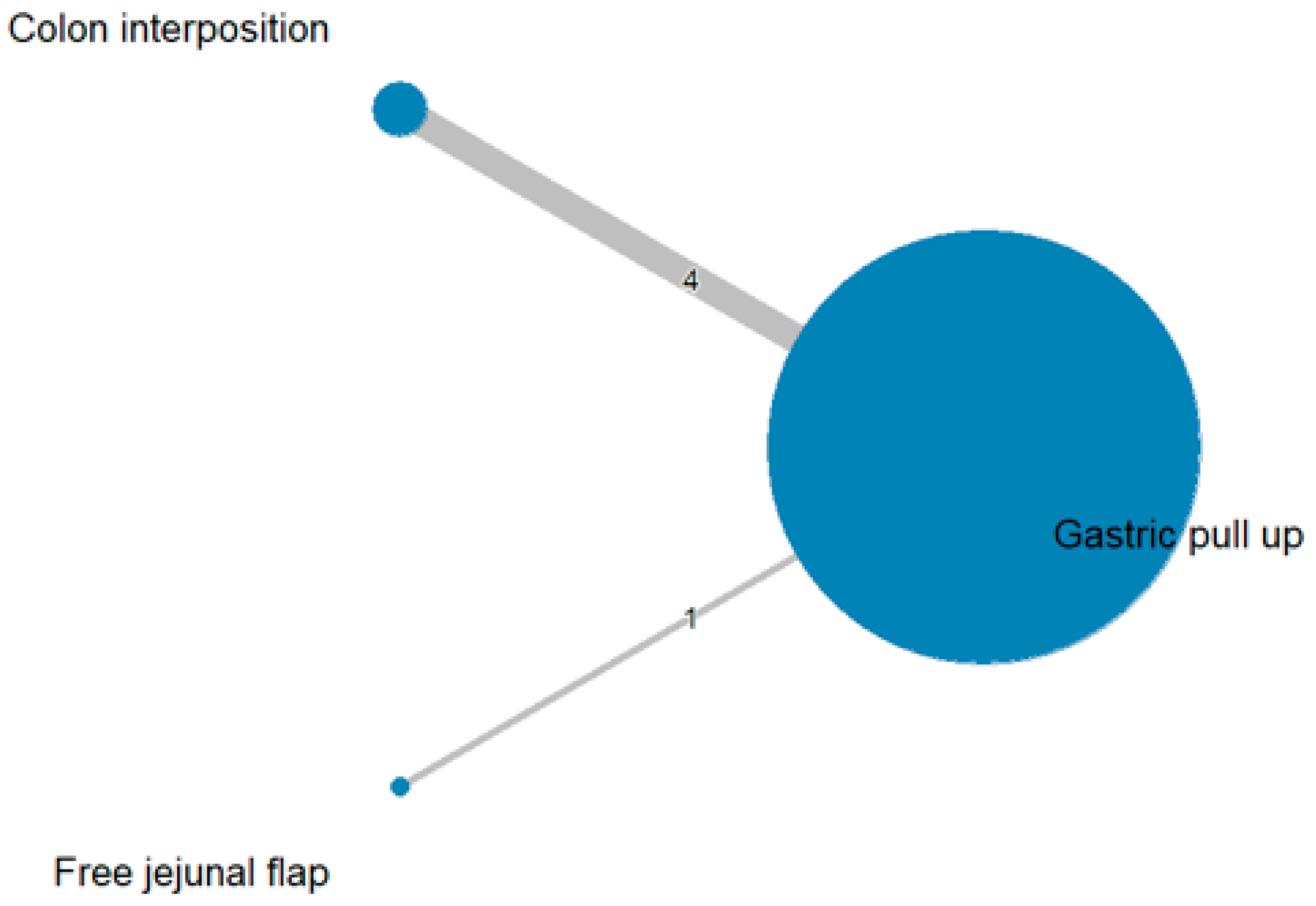
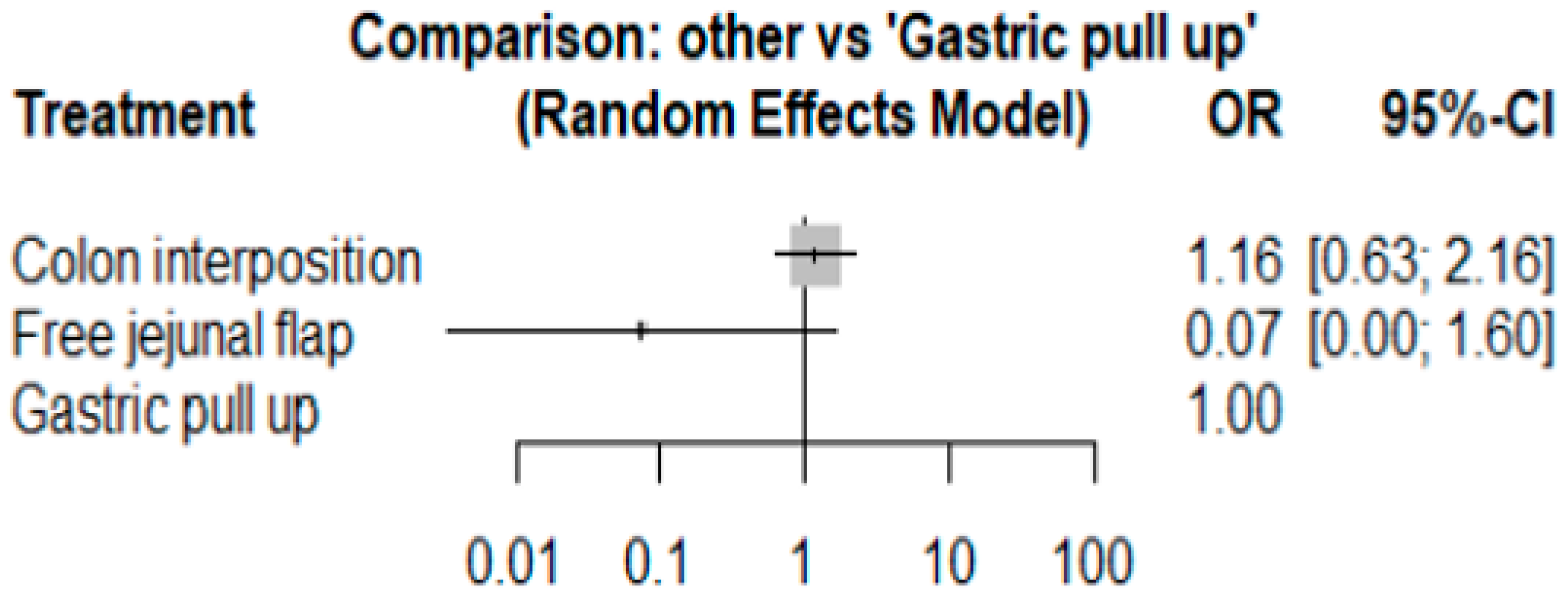
3.1. Anastomosis Leakage
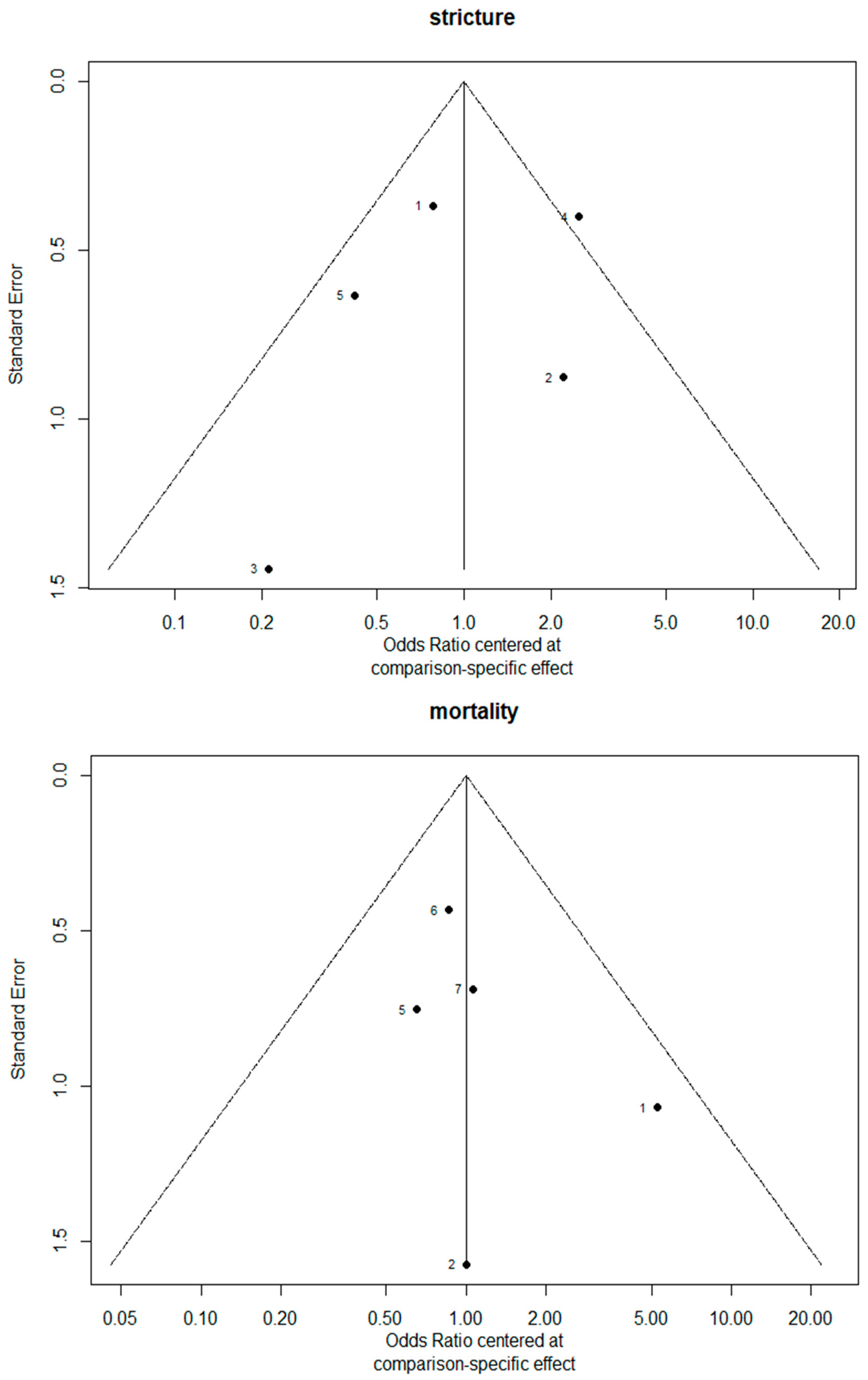
3.2. Stricture
3.3. Mortality Rate
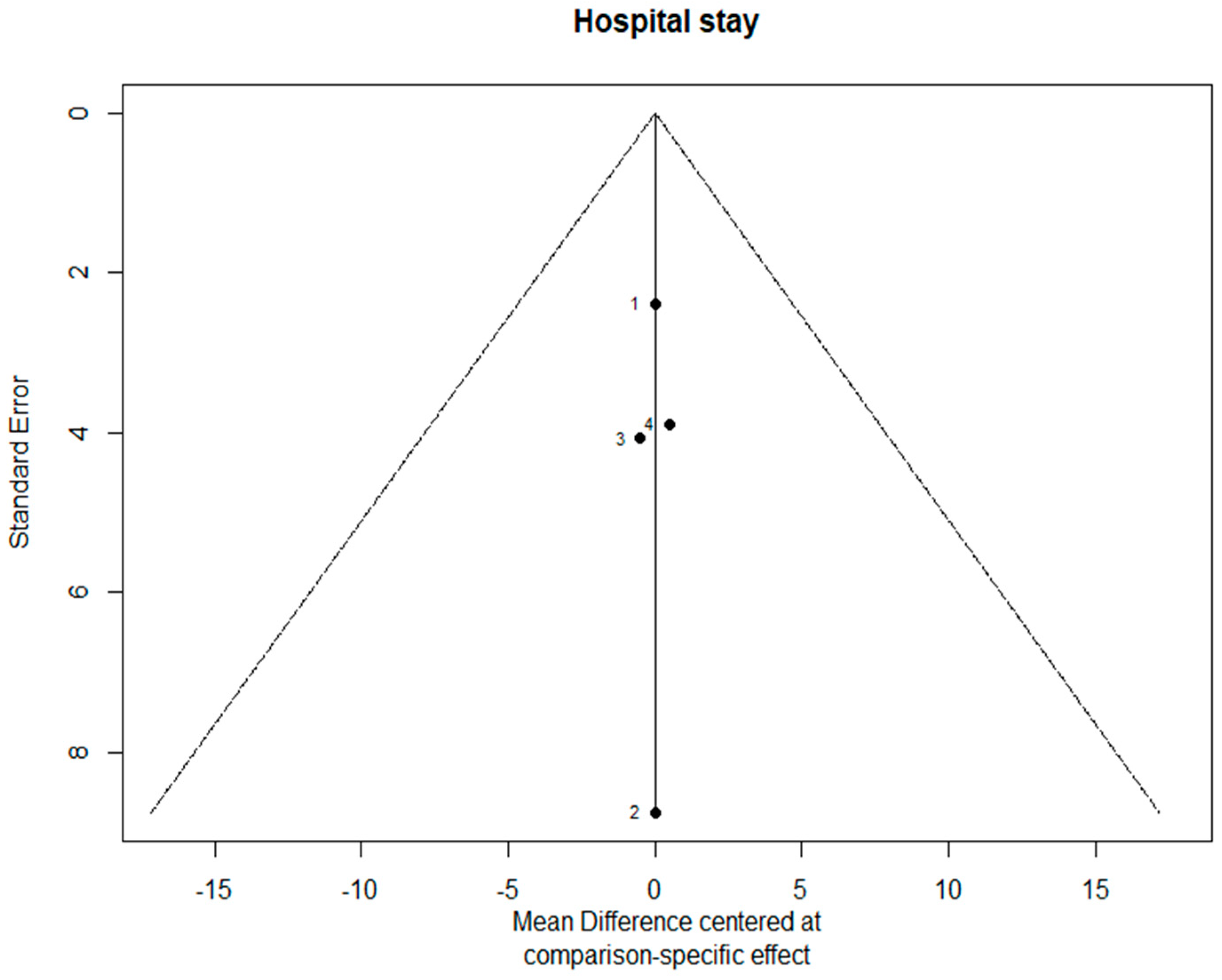
3.4. Length of Hospital Stay
3.5. Quality Assessment of Studies
3.6. Sensitivity Analysis
3.7. Bias Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Short, M.W.; Burgers, K.; Fry, V. Esophageal cancer. Am. Fam. Physician 2017, 95, 22–28. [Google Scholar]
- Zhang, Y. Epidemiology of esophageal cancer. World J. Gastroenterol. WJG 2013, 19, 5598. [Google Scholar] [CrossRef]
- Bolger, J.C.; Donohoe, C.L.; Lowery, M.; Reynolds, J.V. Advances in the curative management of oesophageal cancer. Br. J. Cancer 2022, 126, 706–717. [Google Scholar] [CrossRef]
- Rustgi, A.K.; El-Serag, H.B. Esophageal carcinoma. N. Engl. J. Med. 2014, 371, 2499–2509. [Google Scholar] [CrossRef]
- Blackmon, S.H.; Correa, A.M.; Skoracki, R.; Chevray, P.M.; Kim, M.P.; Mehran, R.J.; Rice, D.C.; Roth, J.A.; Swisher, S.G.; Vaporciyan, A.A.; et al. Supercharged pedicled jejunal interposition for esophageal replacement: A 10-year experience. Ann. Thorac. Surg. 2012, 94, 1104–1111; discussion 1111–1113. [Google Scholar] [CrossRef]
- Poh, M.; Selber, J.C.; Skoracki, R.; Walsh, G.L.; Yu, P. Technical challenges of total esophageal reconstruction using a supercharged jejunal flap. Ann. Surg. 2011, 253, 1122–1129. [Google Scholar] [CrossRef]
- Mays, A.C.; Yu, P.; Hofstetter, W.; Liu, J.; Xue, A.; Klebuc, M.; Chevray, P.; Selber, J. The Supercharged Pedicled Jejunal Flap for Total Esophageal Reconstruction: A Retrospective Review of 100 Cases. Plast. Reconstr. Surg. 2019, 144, 1171–1180. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hirai, T.; Kubota, H.; Murakami, H.; Higashida, M.; Hirabayashi, Y. Safe esophageal reconstruction by ileocolic interposition. Dis. Esophagus 2012, 25, 195–200. [Google Scholar] [CrossRef]
- Okazaki, M.; Asato, H.; Takushima, A.; Nakatsuka, T.; Ueda, K.; Harii, K. Secondary Reconstruction of Failed Esophageal Reconstruction. Ann. Plast. Surg. 2005, 54, 530–537. [Google Scholar] [CrossRef]
- Gujjuri, R.R.; Kamarajah, S.K.; Markar, S.R. Effect of anastomotic leaks on long-term survival after oesophagectomy for oesophageal cancer: Systematic review and meta-analysis. Dis. Esophagus 2021, 34, doaa085. [Google Scholar] [CrossRef]
- Briel, J.W.; Tamhankar, A.P.; Hagen, J.A.; DeMeester, S.R.; Johansson, J.; Choustoulakis, E.; Peters, J.H.; Bremner, C.G.; DeMeester, T.R. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: Gastric pull-up versus colon interposition. J. Am. Coll. Surg. 2004, 198, 536–541; discussion 541–542. [Google Scholar] [CrossRef]
- Kassis, E.S.; Kosinski, A.S.; Ross, P.; Koppes, K.E.; Donahue, J.M.; Daniel, V.C. Predictors of anastomotic leak after esophagectomy: An analysis of the society of thoracic surgeons general thoracic database. Ann. Thorac. Surg. 2013, 96, 1919–1926. [Google Scholar] [CrossRef]
- Hagens, E.R.; Reijntjes, M.A.; Anderegg, M.C.J.; Eshuis, W.J.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Risk factors and consequences of anastomotic leakage after esophagectomy for cancer. Ann. Thorac. Surg. 2021, 112, 255–263. [Google Scholar] [CrossRef]
- Verstegen, M.H.; Slaman, A.E.; Klarenbeek, B.R.; Henegouwen, M.I.V.B.; Gisbertz, S.S.; Rosman, C.; van Workum, F. Outcomes of Patients with Anastomotic Leakage After Transhiatal, McKeown or Ivor Lewis Esophagectomy: A Nationwide Cohort Study. World J. Surg. 2021, 45, 3341–3349. [Google Scholar] [CrossRef]
- Markar, S.R.; Arya, S.; Karthikesalingam, A.; Hanna, G.B. Technical factors that affect anastomotic integrity following esophagectomy: Systematic review and meta-analysis. Ann. Surg. Oncol. 2013, 20, 4274–4281. [Google Scholar] [CrossRef]
- Fabbi, M.; Hagens, E.R.C.; Henegouwen, M.I.V.B.; Gisbertz, S.S. Anastomotic leakage after esophagectomy for esophageal cancer: Definitions, diagnostics, and treatment. Dis. Esophagus 2021, 34, doaa039. [Google Scholar] [CrossRef]
- Doty, J.R.; Salazar, J.D.; Forastiere, A.A.; Heath, E.I.; Kleinberg, L.; Heitmiller, R.F. Postesophagectomy morbidity, mortality, and length of hospital stay after preoperative chemoradiation therapy. Ann. Thorac. Surg. 2002, 74, 227–231; discussion 231. [Google Scholar] [CrossRef]
- Biere, S.S.; Maas, K.; Cuesta, M.; van der Peet, D. Cervical or thoracic anastomosis after esophagectomy for cancer: A systematic review and meta-analysis. Dig. Surg. 2011, 28, 29–35. [Google Scholar] [CrossRef]
- Yoon, S.W.; Kang, H.; Choi, G.J.; Ryu, C.; Park, Y.H.; Baek, C.W.; Jung, Y.H.; Woo, Y.C. Comparison of supraglottic airway devices in laparoscopic surgeries: A network meta-analysis. J. Clin. Anesth. 2019, 55, 52–66. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Kolh, P.; Honore, P.; Degauque, C.; Gielen, J.L.; Gerard, P.; Jacquet, N. Early stage results after oesophageal resection for malignancy—Colon interposition vs. gastric pull-up. Eur. J. Cardio-Thorac. Surg. 2000, 18, 293–300. [Google Scholar] [CrossRef][Green Version]
- DeMeester, S.R. Colon interposition following esophagectomy. Dis. Esophagus 2001, 14, 169–172. [Google Scholar] [CrossRef]
- Huttl, T.P.; Wichmann, M.; Geiger, T.; Schildberg, F.; Fürst, H. Techniques and results of esophageal cancer surgery in Germany. Langenbeck’s Arch. Surg. 2002, 387, 125–129. [Google Scholar] [CrossRef]
- Davis, P.A.; Law, S.; Wong, J. Colonic interposition after esophagectomy for cancer. Arch. Surg. 2003, 138, 303–308. [Google Scholar] [CrossRef]
- Daiko, H.; Hayashi, R.; Saikawa, M.; Sakuraba, M.; Yamazaki, M.; Miyazaki, M.; Ugumori, T.; Asai, M.; Oyama, W.; Ebihara, S. Surgical management of carcinoma of the cervical esophagus. J. Surg. Oncol. 2007, 96, 166–172. [Google Scholar] [CrossRef]
- Doki, Y.; Okada, K.; Miyata, H.; Yamasaki, M.; Fujiwara, Y.; Takiguchi, S.; Yasuda, T.; Hirao, T.; Nagano, H.; Monden, M. Long-term and short-term evaluation of esophageal reconstruction using the colon or the jejunum in esophageal cancer patients after gastrectomy. Dis. Esophagus 2008, 21, 132–138. [Google Scholar] [CrossRef]
- Stephens, E.H.; Gaur, P.; Hotze, K.O.; Correa, A.M.; Kim, M.P.; Blackmon, S.H. Super-Charged Pedicled Jejunal Interposition Performance Compares Favorably With a Gastric Conduit After Esophagectomy. Ann. Thorac. Surg. 2015, 100, 407–413. [Google Scholar] [CrossRef]
- Luan, A.; Hunter, C.L.; Crowe, C.S.; Lee, G.K. Comparison of Outcomes of Total Esophageal Reconstruction With Supercharged Jejunal Flap, Colonic Interposition, and Gastric Pull-up. Ann. Plast. Surg. 2018, 80, S274–S278. [Google Scholar] [CrossRef]
- Barzin, A.; Norton, J.A.; Whyte, R.; Lee, G.K. Supercharged jejunum flap for total esophageal reconstruction: Single-surgeon 3-year experience and outcomes analysis. Plast. Reconstr. Surg. 2011, 127, 173–180. [Google Scholar] [CrossRef]
- Watanabe, M.; Mine, S.; Nishida, K.; Kurogochi, T.; Okamura, A.; Imamura, Y. Reconstruction after esophagectomy for esophageal cancer patients with a history of gastrectomy. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 457–463. [Google Scholar] [CrossRef]
- Mine, S.; Udagawa, H.; Tsutsumi, K.; Kinoshita, Y.; Ueno, M.; Ehara, K.; Haruta, S. Colon interposition after esophagectomy with extended lymphadenectomy for esophageal cancer. Ann. Thorac. Surg. 2009, 88, 1647–1653. [Google Scholar] [CrossRef]
- Knežević, J.; Radovanović, N.S.; Simić, A.P.; Kotarac, M.M.; Skrobić, O.M.; Konstantinović, V.D.; Peško, P.M. Colon interposition in the treatment of esophageal caustic strictures: 40 years of experience. Dis. Esophagus 2007, 20, 530–534. [Google Scholar] [CrossRef]
- Kesler, K.A.; Pillai, S.T.; Birdas, T.J.; Rieger, K.M.; Okereke, I.C.; Ceppa, D.; Socas, J.; Starnes, S.L. “Supercharged” isoperistaltic colon interposition for long-segment esophageal reconstruction. Ann. Thorac. Surg. 2013, 95, 1162–1169. [Google Scholar] [CrossRef]
- Fujita, H.; Yamana, H.; Sueyoshi, S.; Shima, I.; Fujii, T.; Shirouzu, K.; Inoue, Y.; Kiyokawa, K.; Tanabe, H.Y.; Tai, Y.; et al. Impact on outcome of additional microvascular anastomosis—supercharge—on colon interposition for esophageal replacement: Comparative and multivariate analysis. World J. Surg. 1997, 21, 998–1003. [Google Scholar] [CrossRef]
- Slooter, M.D.; Eshuis, W.J.; Cuesta, M.A.; Gisbertz, S.S.; Henegouwen, M.I.V.B. Fluorescent imaging using indocyanine green during esophagectomy to prevent surgical morbidity: A systematic review and meta-analysis. J. Thorac. Dis. 2019, 11, S755. [Google Scholar] [CrossRef]
- Degett, T.H.; Andersen, H.S.; Gögenur, I. Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: A systematic review of clinical trials. Langenbeck’s Arch. Surg. 2016, 401, 767–775. [Google Scholar] [CrossRef]
- Campbell, C.; Reames, M.K.; Robinson, M.; Symanowski, J.; Salo, J.C. Conduit vascular evaluation is associated with reduction in anastomotic leak after esophagectomy. J. Gastrointest. Surg. 2015, 19, 806–812. [Google Scholar] [CrossRef]
- Butskiy, O.; Rahmanian, R.; White, R.A.; Durham, S.; Anderson, D.W.; Prisman, E. Revisiting the gastric pull-up for pharyngoesophageal reconstruction: A systematic review and meta-analysis of mortality and morbidity. J. Surg. Oncol. 2016, 114, 907–914. [Google Scholar] [CrossRef]
- Takeda, F.R.; Tutihashi, R.; Tustumi, F.; Sallum, R.A.A.; Busnardo, F.D.F.; Ribeiro, U.; Cecconello, I. Supercharged cervical anastomosis for esophagectomy and gastric pull-up. J. Thorac. Cardiovasc. Surg. 2021, 162, 688–697.e3. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]


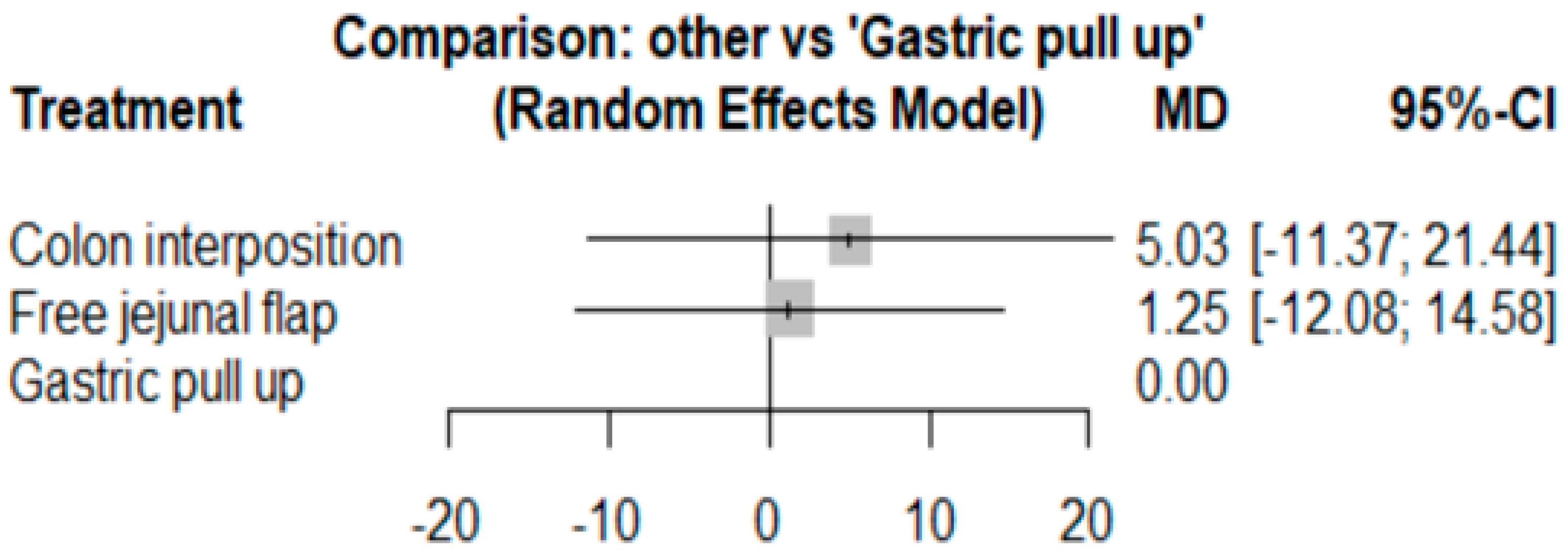
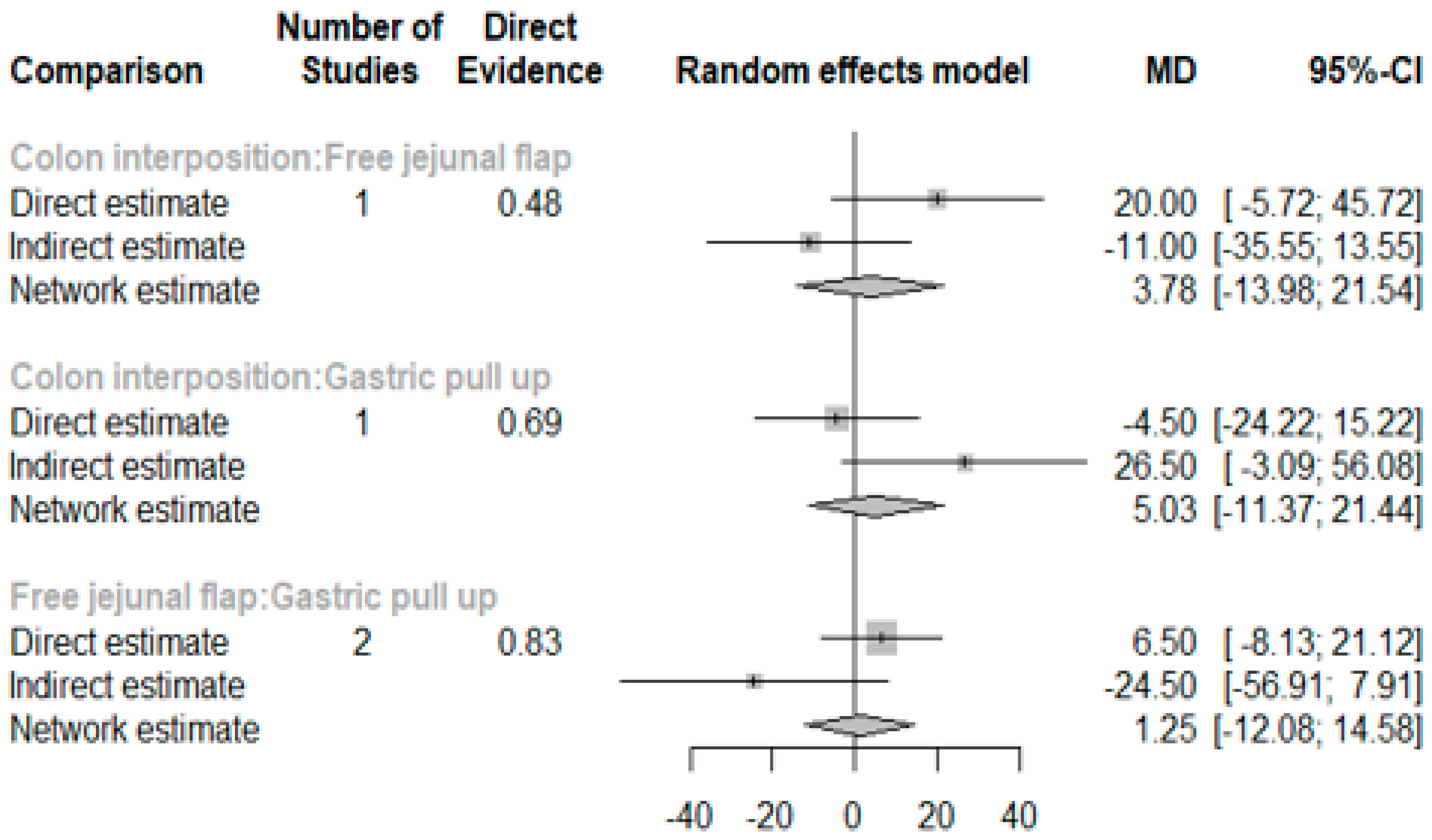
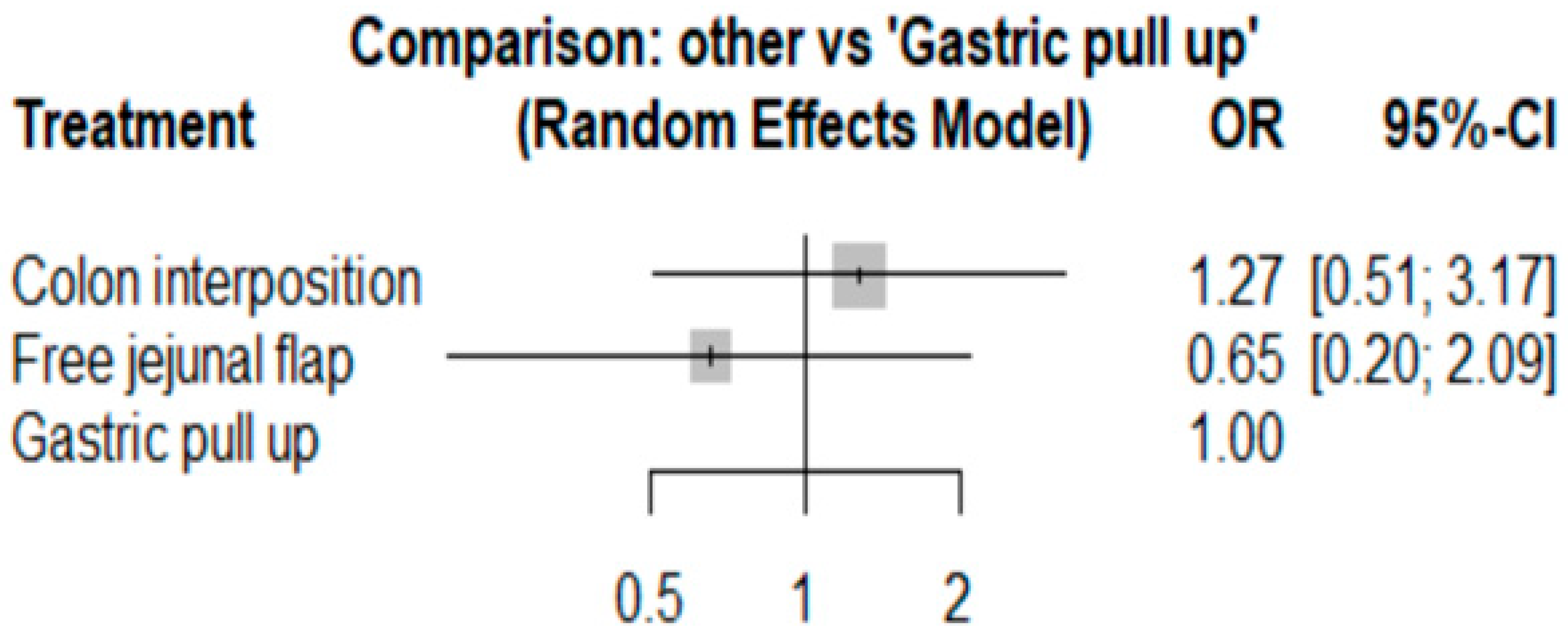
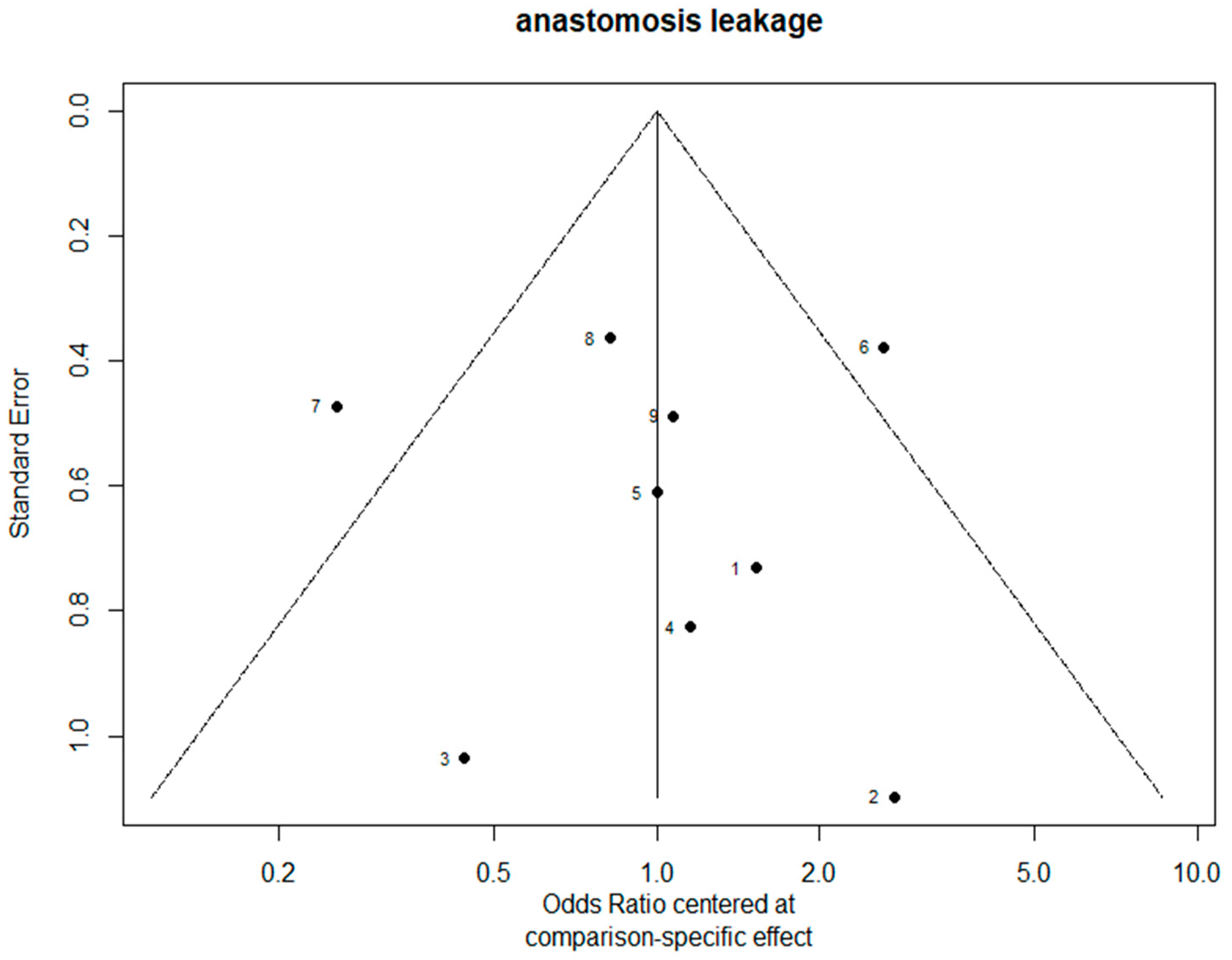
| Author (Ref.) | Selection of Cohorts | Comparability of Cohorts | Outcome | Overall | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest Was Not Present at Start of Study | Assessment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | |||
| Kolh et al. [24] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| DeMeester et al. [22] * | ★ | 1 | |||||||
| Huttl et al. [23] | ★ | ★ | ★ | ★ | 4 | ||||
| Davis et al. [24] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | 8 | |
| Briel et al. [11] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Daiko et al. [25] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | |
| Doki et al. [26] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Stephens et al. [27] | ★ | ★ | ★ | ★ | 4 | ||||
| Luan et al. [28] | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, P.-C.; Chen, H.-Y.; Tu, Y.-K.; Kao, Y.-S. A Comparison of Different Types of Esophageal Reconstructions: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2022, 11, 5025. https://doi.org/10.3390/jcm11175025
Hung P-C, Chen H-Y, Tu Y-K, Kao Y-S. A Comparison of Different Types of Esophageal Reconstructions: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine. 2022; 11(17):5025. https://doi.org/10.3390/jcm11175025
Chicago/Turabian StyleHung, Pang-Chieh, Hsuan-Yu Chen, Yu-Kang Tu, and Yung-Shuo Kao. 2022. "A Comparison of Different Types of Esophageal Reconstructions: A Systematic Review and Network Meta-Analysis" Journal of Clinical Medicine 11, no. 17: 5025. https://doi.org/10.3390/jcm11175025
APA StyleHung, P.-C., Chen, H.-Y., Tu, Y.-K., & Kao, Y.-S. (2022). A Comparison of Different Types of Esophageal Reconstructions: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine, 11(17), 5025. https://doi.org/10.3390/jcm11175025






