Factors Associated with Recurrent Heart Failure during Incorporating SGLT2 Inhibitors in Patients Hospitalized for Acute Decompensated Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design and Data Collection
2.3. Statistical Analyses
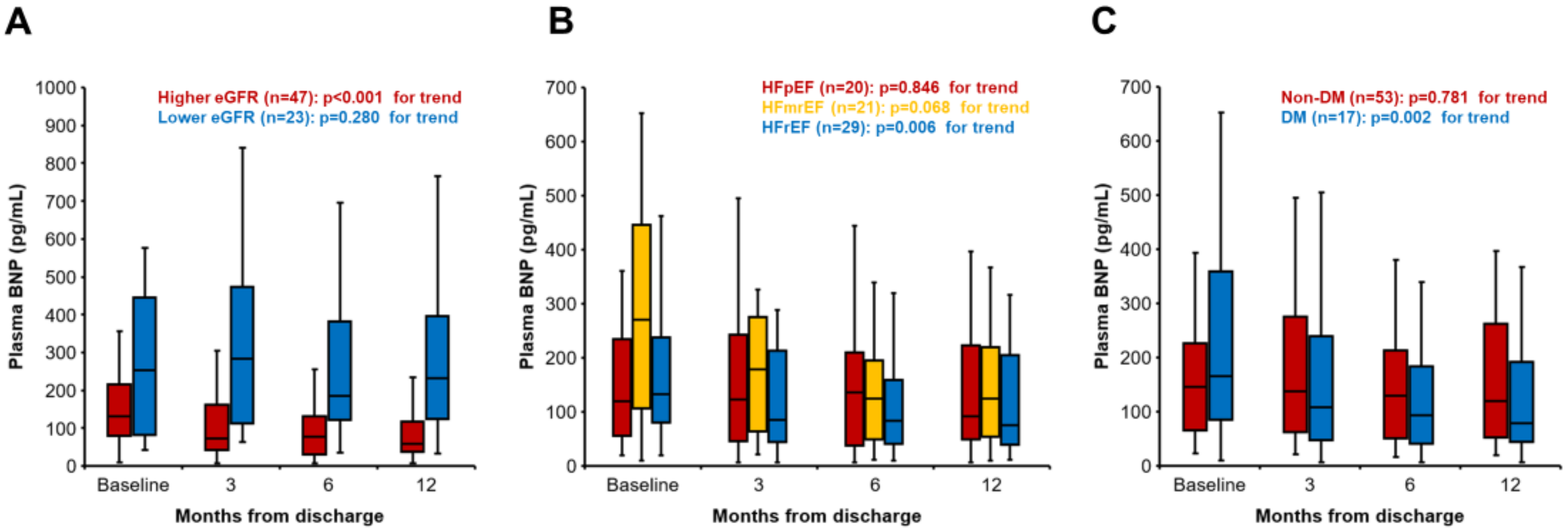
3. Results
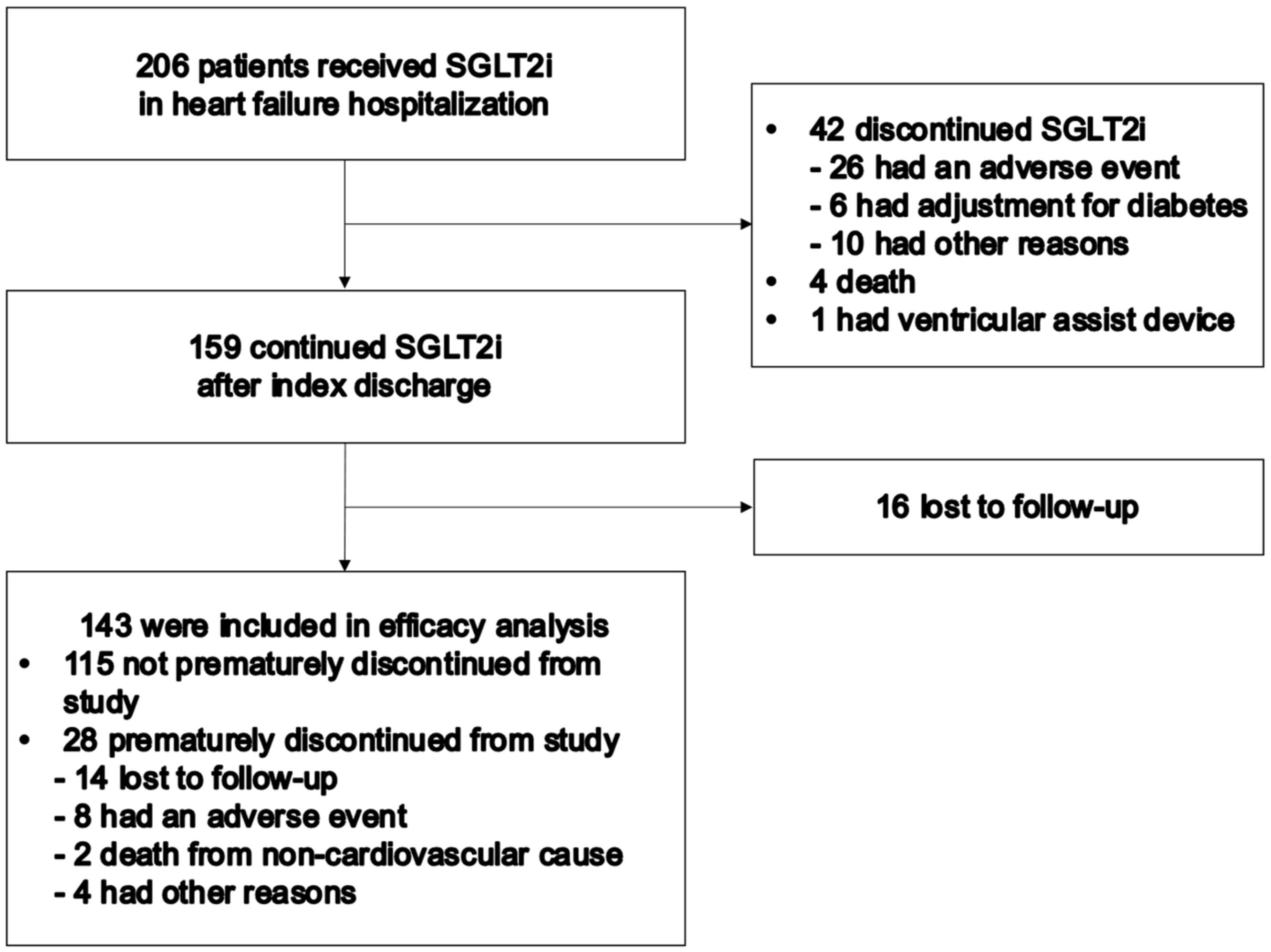
3.1. Follow-Up and Patient Characteristics
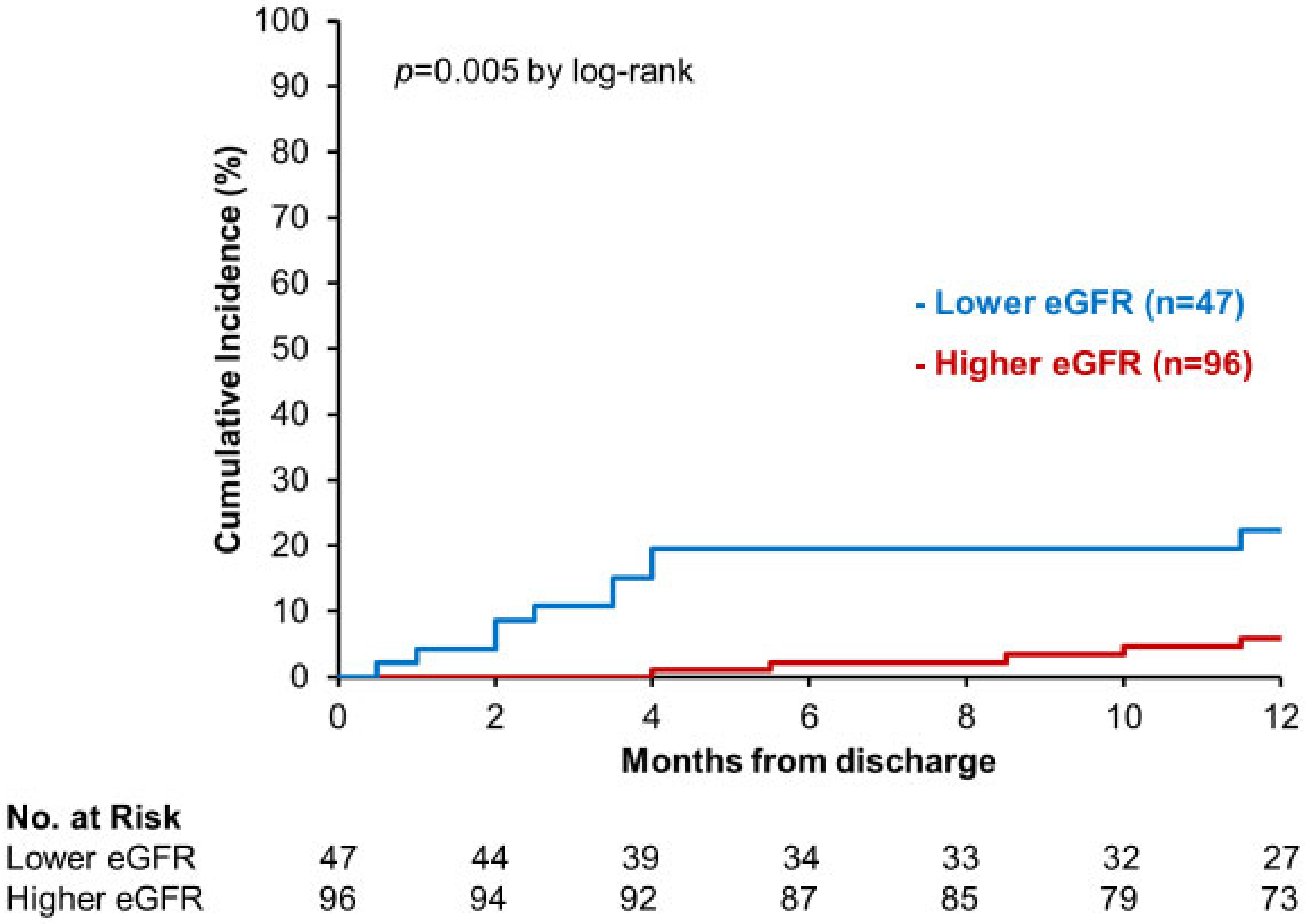
3.2. Primary Outcome
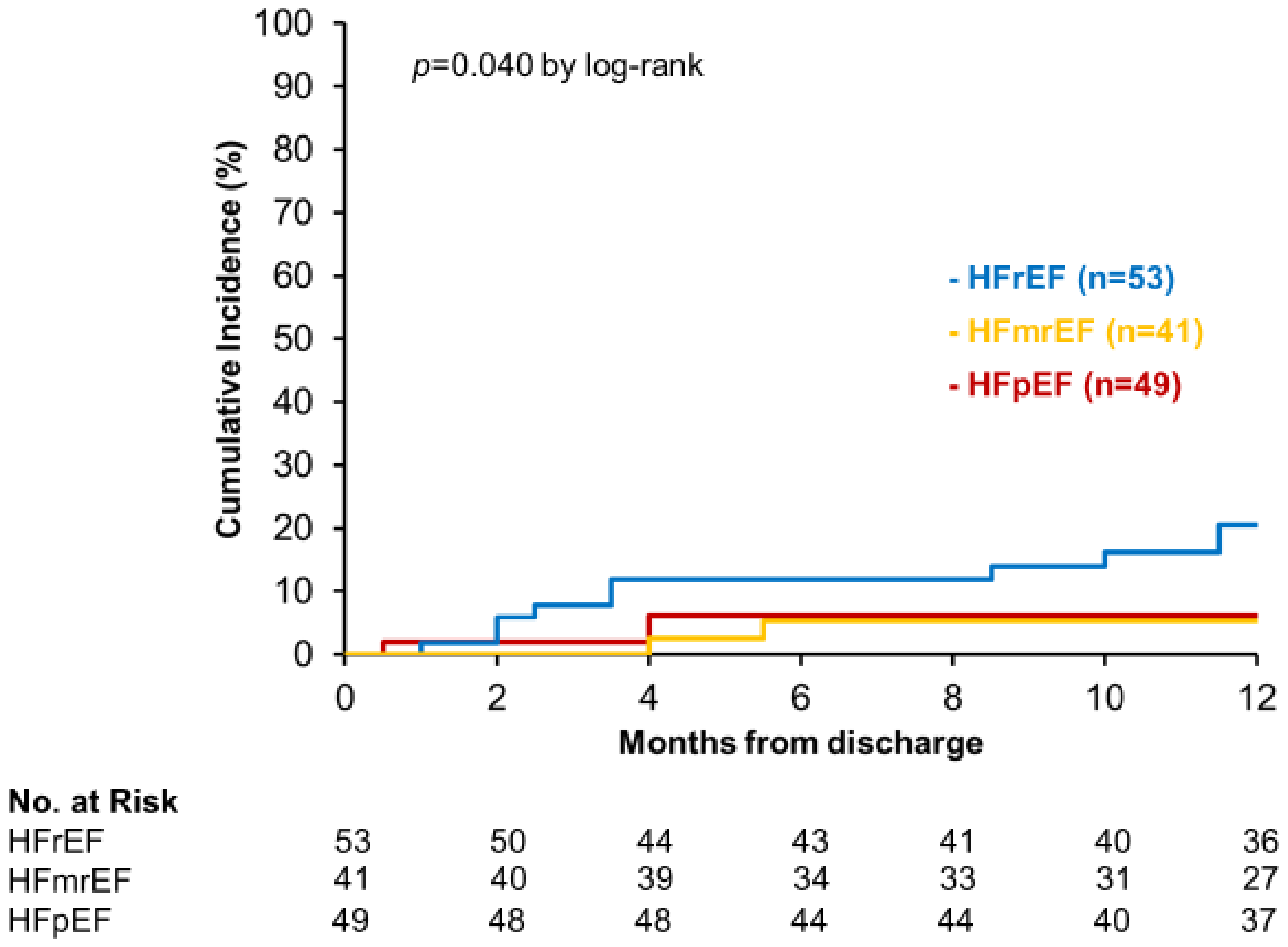
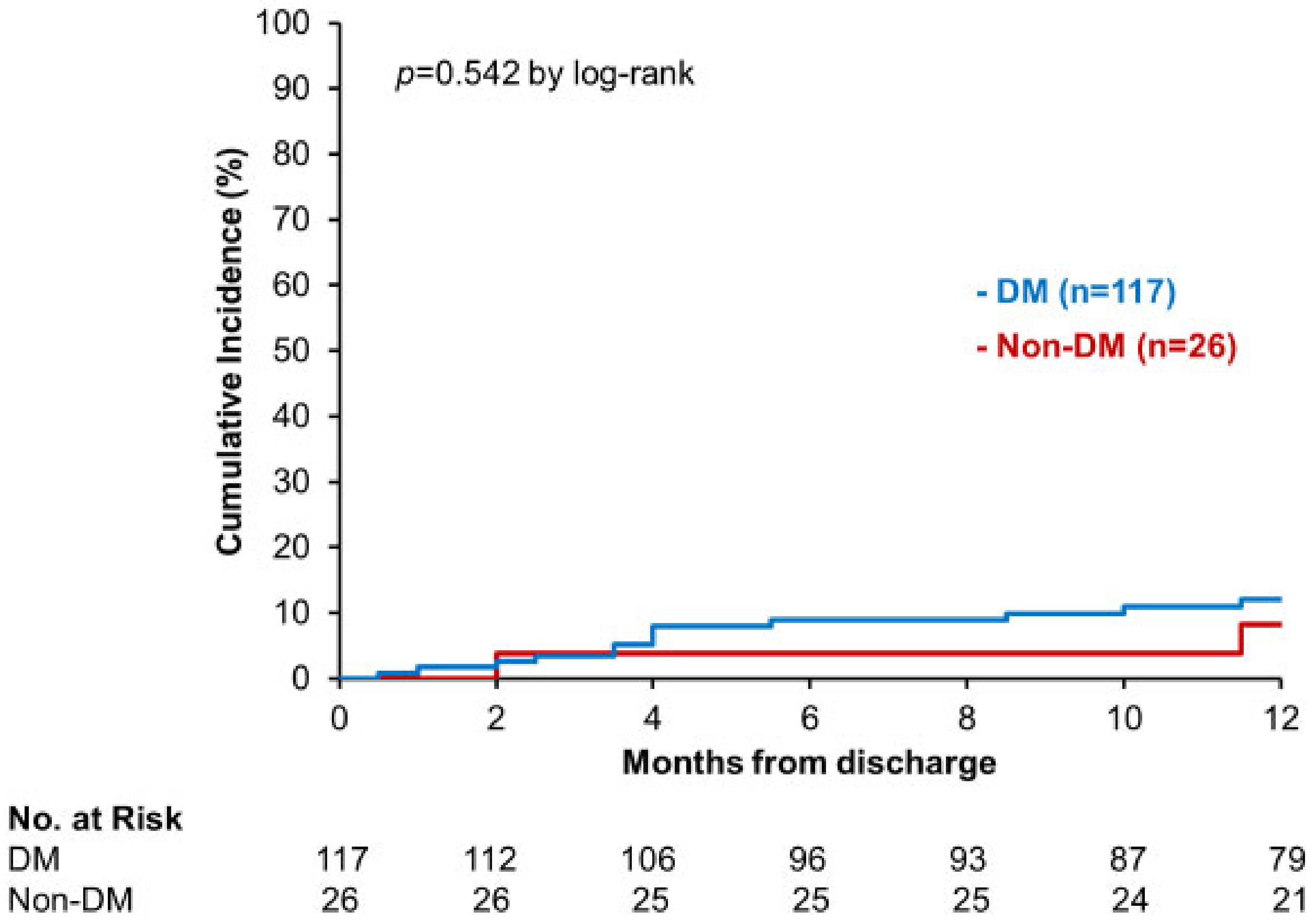
3.3. Secondary Outcome
4. Discussion
4.1. SGLT2i and Renal Function
4.2. HFrEF, HFmrEF, and HFpEF
4.3. DM and Non-DM
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Total | Lower eGFR | Higher eGFR | p Value | |
|---|---|---|---|---|
| (n = 143) | (n = 47) | (n = 96) | ||
| Age, years | 73 (65–81) | 75 (68–84) | 72 (61–79) | 0.013 * |
| Male, n (%) | 92 (64) | 30 (64) | 62 (65) | 0.930 |
| Body weight, kg | 57.8 (48.5–68.3) | 58.5 (50.7–65.3) | 57.5 (46.8–70.2) | 0.921 |
| Body mass index, kg/m2 | 22.7 (19.8–24.9) | 22.7 (20.8–24.5) | 22.7 (19.3–25.2) | 0.526 |
| Systolic blood pressure, mmHg | 107 (96–118) | 101 (94–117) | 108 (96–120) | 0.180 |
| Heart rate, beats per minute | 70 (63–878) | 71 (65–80) | 69 (63–77) | 0.329 |
| Ischemic etiology, n (%) | 59 (41) | 18 (38) | 41 (43) | 0.615 |
| Atrial fibrillation, n (%) | 42 (29) | 19 (40) | 23 (24) | 0.042 * |
| Implantable cardioverter-defibrillator, n (%) | 19 (13) | 8 (17) | 11 (12) | 0.357 |
| Cardio resynchronization therapy, n (%) | 14 (10) | 5 (11) | 9 (9) | 0.811 |
| Left ventricular ejection fraction, % | 44.0 (31.0–57.0) | 43.0 (31.0–57.0) | 44.0 (32.3–57.8) | 0.633 |
| Value of <40% (HFrEF), n (%) | 53 (37) | 19 (40) | 34 (35) | 0.734 |
| Diabetes mellitus, n (%) | 117 (82) | 38 (78) | 80 (83) | 0.502 |
| HbA1c, % | 6.8 (6.5–7.7) | 6.7 (6.4–7.7) | 6.9 (6.5–7.7) | 0.656 |
| Fasting blood sugar, mg/dL | 110 (97–130) | 121 (100–138) | 109 (95–126) | 0.083 |
| Hemoglobin, g/dL | 12.7 (11.2–14.1) | 12.1 (10.8–13.7) | 12.8 (11.7–14.2) | 0.027 * |
| Hematocrit, % | 38.0 (34.1–41.6) | 35.9 (32.6–41.5) | 39.0 (35.3–41.9) | 0.032 * |
| Serum albumin, g/dL | 3.6 (3.4–3.9) | 3.6 (3.4–3.8) | 3.7 (3.4–4.0) | 0.261 |
| Serum sodium, mEq/L | 138 (136–140) | 137 (135–140) | 138 (136–140) | 0.659 |
| Serum potassium, mEq/L | 4.4 (4.1–4.7) | 4.6 (4.1–4.9) | 4.3 (4.1–4.5) | 0.049 * |
| eGFR, mL/minute/1.73 m2 | 50.5 (36.9–64.2) | 31.9 (26.3–36.9) | 58.0 (50.3–73.5) | <0.001 * |
| Plasma BNP, pg/mL | 142 (63–316) | 195 (67–376) | 128 (63–249) | 0.082 |
| Heart failure therapies | ||||
| Beta-blockers, n (%) | 127 (89) | 87 (91) | 40 (85) | 0.325 |
| ACEI/ARB/ARNI, n (%) | 131 (92) | 41 (87) | 90 (94) | 0.187 |
| Loop diuretics, n (%) | 93 (65) | 38 (81) | 55 (57) | 0.006 * |
| MRA, n (%) | 96 (67) | 32 (68) | 64 (67) | 0.865 |
| Thiazides, n (%) | 3 (2) | 2 (4) | 1 (1) | 0.208 |
| Antidiabetic agents | ||||
| Sulfonylureas, n (%) | 6 (4) | 3 (6) | 3 (3) | 0.361 |
| DPP-4i, n (%) | 62 (43) | 23 (49) | 39 (41) | 0.346 |
| Biguanides, n (%) | 20 (14) | 4 (9) | 16 (17) | 0.187 |
| Insulin, n (%) | 13 (9) | 6 (13) | 7 (7) | 0.285 |
| Sodium-glucose cotransporter 2 inhibitors | ||||
| Canagliflozin, n (%) | 40 (28) | 12 (25) | 28 (29) | 0.649 |
| Dapagliflozin, n (%) | 62 (43) | 22 (47) | 40 (42) | 0.560 |
| Empagliflozin, n (%) | 41 (29) | 13 (28) | 28 (29) | 0.852 |
| Total | HFrEF | HFmrEF | HFpEF | p Value | |
|---|---|---|---|---|---|
| (n = 143) | (n = 53) | (n = 41) | (n = 49) | ||
| Age, years | 73 (65–81) | 72 (61–78) | 72 (68–78) | 77 (67–83) | 0.177 |
| Male, n (%) | 92 (64) | 35 (66) | 31 (76) | 26 (53) | 0.808 |
| Body weight, kg | 57.8 (48.5–68.3) | 57.6 (45.0–66.6) | 58.5 (51.2–70.7) | 58.3 (48.1–68.9) | 0.467 |
| Body mass index, kg/m2 | 22.7 (19.8–24.9) | 22.2 (19.2–24.5) | 22.7 (19.8–24.8) | 23.1 (20.9–26.2) | 0.183 |
| Systolic blood pressure, mmHg | 107 (96–118) | 100 (93–113) | 108 (99–120) | 110 (98–123) | 0.016 * |
| Heart rate, beats per minute | 70 (63–878) | 69 (60–78) | 69 (64–75) | 73 (64–79) | 0.258 |
| Ischemic etiology, n (%) | 59 (41) | 21 (39) | 21(51) | 17 (35) | 0.271 |
| Atrial fibrillation, n (%) | 42 (29) | 17 (32) | 8 (20) | 17 (35) | 0.249 |
| Implantable cardioverter-defibrillator, n (%) | 19 (13) | 11 (21) | 6 (15) | 2 (4) | 0.044 * |
| Cardio resynchronization therapy, n (%) | 14 (10) | 8 (15) | 6 (15) | 0 (0) | 0.018 * |
| Left ventricular ejection fraction, % | 44.0 (31.0–57.0) | 29.0 (25.0–33.5) | 44 (42.0–47.0) | 61.0 (56.5–68.5) | <0.001 * |
| Diabetes mellitus, n (%) | 117 (82) | 39 (74) | 34 (83) | 44 (90) | 0.103 |
| HbA1c, % | 6.7 (6.4–7.4) | 6.7 (6.4–7.4) | 6.7 (6.5–7.3) | 7.3 (6.6–8.1) | 0.022 * |
| Fasting blood sugar, mg/dL | 105 (90–122) | 107 (92–123) | 119 (101–130) | 113 (100–143) | 0.029 * |
| Hemoglobin, g/dL | 12.9 (11.8–14.1) | 12.9 (11.9–14.0) | 12.7 (11.2–14.1) | 12.2 (11.0–14.2) | 0.405 |
| Hematocrit, % | 39.3 (35.4–41.9) | 39.4 (35.6–41.8) | 37.3 (33.4–41.5) | 36.4 (33.6–41.7) | 0.367 |
| Serum albumin, g/dL | 3.6 (3.4–4.0) | 3.7 (3.4–4.0) | 3.6 (3.4–3.9) | 3.7 (3.5–3.9) | 0.771 |
| Serum sodium, mEq/L | 138 (136–140) | 138 (136–140) | 137 (136–140) | 138 (135–140) | 0.981 |
| Serum potassium, mEq/L | 4.4 (4.0–4.7) | 4.4 (4.0–4.7) | 4.4 (4.1–4.6) | 4.4 (4.0–4.7) | 0.957 |
| eGFR, mL/minute/1.73 m2 | 50.2 (36.5–60.1) | 50.6 (37.2–61.4) | 51.0 (36.7–63.2) | 53.6 (37.8–68.9) | 0.712 |
| Plasma BNP, pg/mL | 163 (86–375) | 149 (82–334) | 166 (61–394) | 93 (50–213) | 0.013 * |
| Heart failure therapies | |||||
| Beta-blockers, n (%) | 127 (89) | 52 (98) | 38 (93) | 37 (76) | <0.001 * |
| ACEI/ARB/ARNI, n (%) | 131 (92) | 50 (94) | 39 (95) | 42 (86) | 0.184 |
| Loop diuretics, n (%) | 93 (65) | 47 (89) | 23 (56) | 23 (47) | <0.001 * |
| MRA, n (%) | 96 (67) | 45 (85) | 26 (63) | 25 (51) | 0.001 * |
| Thiazides, n (%) | 3 (2) | 3 (6) | 0 (0) | 0 (0) | 0.074 |
| Antidiabetic agents | |||||
| Sulfonylureas, n (%) | 6 (4) | 2 (4) | 0 (0) | 4 (8) | 0.154 |
| DPP-4i, n (%) | 62 (43) | 19 (36) | 15 (37) | 28 (57) | 0.056 |
| Biguanides, n (%) | 20 (14) | 6 (11) | 8 (20) | 6 (12) | 0.478 |
| Insulin, n (%) | 13 (9) | 5 (9) | 2 (5) | 6 (12) | 0.478 |
| Sodium-glucose cotransporter 2 inhibitors | |||||
| Canagliflozin, n (%) | 40 (28) | 14 (26) | 9 (22) | 17 (35) | 0.387 |
| Dapagliflozin, n (%) | 62 (43) | 27 (51) | 17 (42) | 18 (37) | 0.337 |
| Empagliflozin, n (%) | 41 (29) | 12 (23) | 15 (37) | 14 (29) | 0.333 |
| Total | DM | Non-DM | p Value | |
|---|---|---|---|---|
| (n = 143) | (n = 117) | (n = 26) | ||
| Age, years | 73 (65–81) | 74 (67–82) | 72 (61–79) | 0.414 |
| Male, n (%) | 92 (64) | 77 (66) | 15 (58) | 0.434 |
| Body weight, kg | 57.8 (48.5–68.3) | 58.7 (50.0–69.5) | 53.2 (46.1–62.3) | 0.135 |
| Body mass index, kg/m2 | 22.7 (19.8–24.9) | 22.9 (20.1–25.6) | 20.8 (19.0–24.5) | 0.051 |
| Systolic blood pressure, mmHg | 107 (96–118) | 108 (97–119) | 100 (91–108) | 0.008 * |
| Heart rate, beats per minute | 70 (63–878) | 71 (64–78) | 67 (60–70) | 0.010 * |
| Ischemic etiology, n (%) | 59 (41) | 49 (42) | 10 (39) | 0.749 |
| Atrial fibrillation, n (%) | 42 (29) | 35 (30) | 7 (27) | 0.762 |
| Implantable cardioverter-defibrillator, n (%) | 19 (13) | 14 (12) | 5 (19) | 0.324 |
| Cardio resynchronization therapy, n (%) | 14 (10) | 12 (10) | 2 (8) | 0.691 |
| Left ventricular ejection fraction, % | 44.0 (31.0–57.0) | 45.0 (33.5–58.0) | 36.0 (25.0–45.3) | 0.016 * |
| Value of <40% (HFrEF), n (%) | 53 (37) | 39 (33) | 14 (54) | 0.050 * |
| HbA1c, % | 6.8 (6.5–7.7) | 7.1 (6.6–7.8) | 5.9 (5.6–6.3) | <0.001 * |
| Fasting blood sugar, mg/dL | 110 (97–130) | 117 (99–137) | 98 (92–108) | <0.001 * |
| Hemoglobin, g/dL | 12.7 (11.2–14.1) | 12.4 (11.1–14.1) | 13.2 (11.5–14.0) | 0.281 |
| Hematocrit, % | 38.0 (34.1–41.6) | 37.5 (33.8–41.5) | 40.0 (35.3–42.3) | 0.198 |
| Serum albumin, g/dL | 3.6 (3.4–3.9) | 3.6 (3.4–3.9) | 3.7 (3.6–4.0) | 0.037 * |
| Serum sodium, mEq/L | 138 (136–140) | 138 (136–140) | 138 (136–140) | 0.752 |
| Serum potassium, mEq/L | 4.4 (4.1–4.7) | 4.4 (4.0–4.7) | 4.4 (4.2–4.7) | 0.105 |
| eGFR, mL/minute/1.73 m2 | 50.5 (36.9–64.2) | 50.2 (37.0–68.3) | 52.1 (34.7–58.6) | 0.697 |
| Plasma BNP, pg/mL | 142 (63–316) | 139 (63–311) | 163 (68–396) | 0.483 |
| Heart failure therapies | ||||
| Beta-blockers, n (%) | 127 (89) | 103 (88) | 24 (92) | 0.532 |
| ACEI/ARB/ARNI, n (%) | 131 (92) | 106 (91) | 25 (96) | 0.355 |
| Loop diuretics, n (%) | 93 (65) | 75 (64) | 18 (69) | 0.620 |
| MRA, n (%) | 96 (67) | 73 (62) | 23 (89) | 0.011 * |
| Thiazides, n (%) | 3 (2) | 2 (2) | 1 (4) | 0.492 |
| Sodium-glucose cotransporter 2 inhibitors | ||||
| Canagliflozin, n (%) | 40 (28) | 39 (33) | 1 (4) | 0.002 * |
| Dapagliflozin, n (%) | 62 (43) | 41 (35) | 21 (81) | <0.001 * |
| Empagliflozin, n (%) | 41 (29) | 37 (32) | 4 (15) | 0.098 |
References
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Bamadhaj, S.; Cleland, J.G.F.; Angermann, C.E.; Dahlstrom, U.; Ouwerkerk, W.; Tay, W.T.; Dickstein, K.; Ertl, G.; Hassanein, M.; et al. Post-discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT-HF): A cohort study. Lancet Glob. Health 2020, 8, e411–e422. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Zeller, C.; Anker, S.D.; Butler, J.; Filippatos, G.; Hauske, S.J.; Brueckmann, M.; Pfarr, E.; et al. Cardiac and Kidney Benefits of Empagliflozin in Heart Failure Across the Spectrum of Kidney Function: Insights From EMPEROR-Reduced. Circulation 2021, 143, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Macha, S.; Mattheus, M.; Halabi, A.; Pinnetti, S.; Woerle, H.J.; Broedl, U.C. Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment. Diabetes Obes. Metab. 2014, 16, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Packer, M.; Filippatos, G.; Ferreira, J.P.; Zeller, C.; Schnee, J.; Brueckmann, M.; Pocock, S.J.; Zannad, F.; Anker, S.D. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur. Heart J. 2022, 43, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.P.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail 2017, 19, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Nakagaito, M.; Imamura, T.; Joho, S.; Ushijima, R.; Nakamura, M.; Kinugawa, K. Relationship Between HbA1c Level and Effectiveness of SGLT2 Inhibitors in Decompensated Heart Failure Patients with Type 2 Diabetes Mellitus. Int. Heart J. 2021, 62, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Khan, M.S.; Marx, N.; Lam, C.S.P.; Schnaidt, S.; Ofstad, A.P.; Brueckmann, M.; Jamal, W.; et al. Effect of Empagliflozin on Cardiovascular and Renal Outcomes in Patients with Heart Failure by Baseline Diabetes Status: Results From the EMPEROR-Reduced Trial. Circulation 2021, 143, 337–349. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.R.; Petrie, M.C.; Varyani, F.; Ostergren, J.; Michelson, E.L.; Young, J.B.; Solomon, S.D.; Granger, C.B.; Swedberg, K.; Yusuf, S.; et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: An analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur. Heart J. 2008, 29, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kaneko, H.; Okada, A.; Itoh, H.; Matsuoka, S.; Fujiu, K.; Michihata, N.; Jo, T.; Takeda, N.; Morita, H.; et al. Comparison of cardiovascular outcomes between SGLT2 inhibitors in diabetes mellitus. Cardiovasc. Diabetol. 2022, 21, 67. [Google Scholar] [CrossRef] [PubMed]
| Age, years | 73 (65–81) |
| Male, n (%) | 92 (64) |
| Body weight, kg | 57.8 (48.5–68.3) |
| Body mass index, kg/m2 | 22.7 (19.8–24.9) |
| Systolic blood pressure, mmHg | 107 (96–118) |
| Heart rate, beats per minutes | 70 (63–878) |
| Ischemic etiology, n (%) | 59 (41) |
| Atrial fibrillation, n (%) | 42 (29) |
| Implantable cardioverter-defibrillator, n (%) | 19 (13) |
| Cardio resynchronization therapy, n (%) | 14 (10) |
| Left ventricular ejection fraction, % | 44.0 (31.0–57.0) |
| Value of <40% (HFrEF), n (%) | 53 (37) |
| Value of 40–49% (HFmrEF), n (%) | 41 (29) |
| Value of ≥50% (HFpEF), n (%) | 49 (34) |
| Diabetes mellitus, n (%) | 117 (82) |
| HbA1c, % | 6.8 (6.5–7.7) |
| Fasting blood sugar, mg/dL | 110 (97–130) |
| Hemoglobin, g/dL | 12.7 (11.2–14.1) |
| Hematocrit, % | 38.0 (34.1–41.6) |
| Serum albumin, g/dL | 3.6 (3.4–3.9) |
| Serum sodium, mEq/L | 138 (136–140) |
| Serum potassium, mEq/L | 4.4 (4.1–4.7) |
| eGFR, mL/minute/1.73 m2 | 50.5 (36.9–64.2) |
| Plasma BNP, pg/mL | 142 (63–316) |
| Heart failure therapies | |
| Beta-blockers, n (%) | 127 (89) |
| ACEI/ARB/ARNI, n (%) | 131 (92) |
| Loop diuretics, n (%) | 93 (65) |
| MRA, n (%) | 96 (67) |
| Thiazides, n (%) | 3 (2) |
| Antidiabetic agents | |
| Sulfonylureas, n (%) | 6 (4) |
| DPP-4i, n (%) | 62 (43) |
| Biguanides, n (%) | 20 (14) |
| Insulin, n (%) | 13 (9) |
| Sodium-glucose cotransporter 2 inhibitors | |
| Canagliflozin, n (%) | 40 (28) |
| Dapagliflozin, n (%) | 62 (43) |
| Empagliflozin, n (%) | 41 (29) |
| All Patients (n = 143) | ||||||
|---|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | |||||
| Variables | Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | p Value |
| Age, years | 1.03 | 0.99–1.09 | 0.198 | |||
| Male, yes | 1.06 | 0.36–3.09 | 0.922 | |||
| Body mass index, kg/m2 | 0.84 | 0.72–0.98 | 0.030 * | 0.88 | 0.71–1.05 | 0.206 |
| Systolic blood pressure, mmHg | 0.97 | 0.94–1.00 | 0.087 | |||
| Heart rate, bpm | 1.02 | 0.97–1.06 | 0.436 | |||
| Ischemic etiology, yes | 1.26 | 0.46–3.48 | 0.654 | |||
| Atrial fibrillation, yes | 0.87 | 0.27–2.74 | 0.813 | |||
| HFrEF, yes | 3.64 | 1.24–10.64 | 0.019 * | 2.02 | 0.61–6.65 | 0.247 |
| Diabetes mellitus, yes | 1.58 | 0.36–7.01 | 0.547 | |||
| Fasting blood sugar, mg/dL | 0.99 | 0.96–1.00 | 0.178 | |||
| Hematocrit, % | 0.90 | 0.80–1.00 | 0.073 | |||
| Serum albumin, g/dL | 0.28 | 0.08–0.93 | 0.039 * | 0.60 | 0.16–2.47 | 0.469 |
| Serum sodium, mEq/L | 0.99 | 0.88–1.15 | 0.911 | |||
| Serum potassium, mEq/L | 0.21 | 0.06–0.74 | 0.018 * | 0.32 | 0.09–1.01 | 0.059 |
| eGFR, mL/min/1.73 m2 | 0.95 | 0.92–0.98 | 0.003 * | 0.94 | 0.90–0.98 | 0.007 * |
| ln BNP | 2.57 | 1.42–4.90 | 0.003 * | 1.50 | 0.80–3.05 | 0.233 |
| Beta-blockers, yes | NA | NA | 0.999 | |||
| ACEI/ARB/ARNI, yes | 0.56 | 0.13–2.50 | 0.450 | |||
| Loop diuretics, yes | 3.81 | 0.86–16.89 | 0.078 | |||
| MRA, yes | 1.87 | 0.53–6.63 | 0.332 | |||
| Thiazides, yes | NA | NA | 0.999 | |||
| Sulfonylureas, yes | NA | NA | 0.999 | |||
| DPP-4i, yes | 2.10 | 0.75–5.89 | 0.161 | |||
| Biguanides, yes | 1.55 | 0.44–5.49 | 0.497 | |||
| Insulin, yes | 0.73 | 0.10–5.52 | 0.757 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagaito, M.; Imamura, T.; Joho, S.; Ushijima, R.; Nakamura, M.; Kinugawa, K. Factors Associated with Recurrent Heart Failure during Incorporating SGLT2 Inhibitors in Patients Hospitalized for Acute Decompensated Heart Failure. J. Clin. Med. 2022, 11, 5027. https://doi.org/10.3390/jcm11175027
Nakagaito M, Imamura T, Joho S, Ushijima R, Nakamura M, Kinugawa K. Factors Associated with Recurrent Heart Failure during Incorporating SGLT2 Inhibitors in Patients Hospitalized for Acute Decompensated Heart Failure. Journal of Clinical Medicine. 2022; 11(17):5027. https://doi.org/10.3390/jcm11175027
Chicago/Turabian StyleNakagaito, Masaki, Teruhiko Imamura, Shuji Joho, Ryuichi Ushijima, Makiko Nakamura, and Koichiro Kinugawa. 2022. "Factors Associated with Recurrent Heart Failure during Incorporating SGLT2 Inhibitors in Patients Hospitalized for Acute Decompensated Heart Failure" Journal of Clinical Medicine 11, no. 17: 5027. https://doi.org/10.3390/jcm11175027
APA StyleNakagaito, M., Imamura, T., Joho, S., Ushijima, R., Nakamura, M., & Kinugawa, K. (2022). Factors Associated with Recurrent Heart Failure during Incorporating SGLT2 Inhibitors in Patients Hospitalized for Acute Decompensated Heart Failure. Journal of Clinical Medicine, 11(17), 5027. https://doi.org/10.3390/jcm11175027






