Inodilators May Improve the In-Hospital Mortality of Patients with Cardiogenic Shock Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Outcomes
2.3. ECMO Management
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics According to the Use of Inodilators
3.2. In-Hospital Outcomes of the Propensity Score Matched Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thiele, H.; Ohman, E.M.; Desch, S.; Eitel, I.; de Waha, S. Management of cardiogenic shock. Eur. Heart J. 2015, 36, 1223–1230. [Google Scholar] [CrossRef]
- Kolte, D.; Khera, S.; Aronow, W.S.; Mujib, M.; Palaniswamy, C.; Sule, S.; Jain, D.; Gotsis, W.; Ahmed, A.; Frishman, W.H. Trends in Incidence, Management, and Outcomes of Cardiogenic Shock Complicating ST-Elevation Myocardial Infarction in the United States. J. Am. Heart Assoc. 2014, 3, e000590. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Hochman, J.S. Cardiogenic shock: Current concepts and improving outcomes. Circulation 2008, 117, 686–697. [Google Scholar] [CrossRef]
- Takayama, H.; Truby, L.; Koekort, M.; Uriel, N.; Colombo, P.; Mancini, D.M.; Jorde, U.P.; Naka, Y. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J. Heart Lung Transplant. 2013, 32, 106–111. [Google Scholar] [CrossRef]
- Schrage, B.; Becher, P.M.; Goßling, A.; Savarese, G.; Dabboura, S.; Yan, I.; Beer, B.; Söffker, G.; Seiffert, M.; Kluge, S. Temporal trends in incidence, causes, use of mechanical circulatory support and mortality in cardiogenic shock. ESC Heart Fail. 2021, 8, 1295–1303. [Google Scholar] [CrossRef]
- Patricio, D.; Peluso, L.; Brasseur, A.; Lheureux, O.; Belliato, M.; Vincent, J.-L.; Creteur, J.; Taccone, F.S. Comparison of extracorporeal and conventional cardiopulmonary resuscitation: A retrospective propensity score matched study. Crit. Care 2019, 23, 27. [Google Scholar] [CrossRef]
- Ouweneel, D.M.; Schotborgh, J.V.; Limpens, J.; Sjauw, K.D.; Engström, A.; Lagrand, W.K.; Cherpanath, T.G.; Driessen, A.H.; de Mol, B.A.; Henriques, J.P. Extracorporeal life support during cardiac arrest and cardiogenic shock: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 1922–1934. [Google Scholar] [CrossRef]
- Peura, J.L.; Colvin-Adams, M.; Francis, G.S.; Grady, K.L.; Hoffman, T.M.; Jessup, M.; John, R.; Kiernan, M.S.; Mitchell, J.E.; O’Connell, J.B. Recommendations for the use of mechanical circulatory support: Device strategies and patient selection: A scientific statement from the American Heart Association. Circulation 2012, 126, 2648–2667. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, 1810–1852. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Coons, J.C.; Link, C.B.; Schmidhofer, M. Pharmacotherapy update on the use of vasopressors and inotropes in the intensive care unit. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.S.; Bartos, J.A.; Adatya, S. Inotropes. J. Am. Coll. Cardiol. 2014, 63, 2069–2078. [Google Scholar] [CrossRef]
- Harjola, V.P.; Mebazaa, A.; Čelutkienė, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Feneck, R.O.; Sherry, K.M.; Withington, P.S.; Oduro-Dominah, A.; European Milrinone Multicenter Trial Group. Comparison of the hemodynamic effects of milrinone with dobutamine in patients after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2001, 15, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Carmona, M.J.C.; Martins, L.M.; Vane, M.F.; Longo, B.A.; Paredes, L.S.; Malbouisson, L.M.S. Comparison of the effects of dobutamine and milrinone on hemodynamic parameters and oxygen supply in patients undergoing cardiac surgery with low cardiac output after anesthetic induction. Rev. Bras. Anestesiol. 2010, 60, 237–246. [Google Scholar] [CrossRef][Green Version]
- Karlsberg, R.P.; DeWood, M.A.; DeMaria, A.N.; Berk, M.R.; Lasher, K.P.; Group, M.D.S. Comparative efficacy of short-term intravenous infusions of milrinone and dobutamine in acute congestive heart failure following acute myocardial infarction. Clin. Cardiol. 1996, 19, 21–30. [Google Scholar] [CrossRef]
- Burkhoff, D.; Sayer, G.; Doshi, D.; Uriel, N. Hemodynamics of mechanical circulatory support. J. Am. Coll. Cardiol. 2015, 66, 2663–2674. [Google Scholar] [CrossRef]
- Donker, D.W.; Brodie, D.; Henriques, J.P.; Broomé, M. Left ventricular unloading during veno-arterial ECMO: A simulation study. ASAIO J. 2019, 65, 11. [Google Scholar] [CrossRef]
- Rupprecht, L.; Flörchinger, B.; Schopka, S.; Schmid, C.; Philipp, A.; Lunz, D.; Müller, T.; Camboni, D. Cardiac decompression on extracorporeal life support: A review and discussion of the literature. ASAIO J. 2013, 59, 547–553. [Google Scholar] [CrossRef]
- Ostadal, P.; Mlcek, M.; Kruger, A.; Hala, P.; Lacko, S.; Mates, M.; Vondrakova, D.; Svoboda, T.; Hrachovina, M.; Janotka, M.; et al. Increasing venoarterial extracorporeal membrane oxygenation flow negatively affects left ventricular performance in a porcine model of cardiogenic shock. J. Transl. Med. 2015, 13, 266. [Google Scholar] [CrossRef]
- Pirracchio, R.; Parenica, J.; Resche Rigon, M.; Chevret, S.; Spinar, J.; Jarkovsky, J.; Zannad, F.; Alla, F.; Mebazaa, A.; GREAT network. The effectiveness of inodilators in reducing short term mortality among patient with severe cardiogenic shock: A propensity-based analysis. PLoS ONE 2013, 8, e71659. [Google Scholar] [CrossRef]
- Zotzmann, V.; Rilinger, J.; Lang, C.N.; Kaier, K.; Benk, C.; Duerschmied, D.; Biever, P.M.; Bode, C.; Wengenmayer, T.; Staudacher, D.L. Epinephrine, inodilator, or no inotrope in venoarterial extracorporeal membrane oxygenation implantation: A single-center experience. Crit. Care 2019, 23, 320. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Di Santo, P.; Jung, R.G.; Marbach, J.A.; Hutson, J.; Simard, T.; Ramirez, F.D.; Harnett, D.T.; Merdad, A.; Almufleh, A.; et al. Milrinone as compared with dobutamine in the treatment of cardiogenic shock. N. Engl. J. Med. 2021, 385, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.C.; Aberle, C.; Altshuler, D.; Piper, G.L.; Papadopoulos, J. Comparative effectiveness and safety between milrinone or dobutamine as initial inotrope therapy in cardiogenic shock. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 130–138. [Google Scholar] [CrossRef]
- Bayram, M.; De Luca, L.; Massie, M.B.; Gheorghiade, M. Reassessment of dobutamine, dopamine, and milrinone in the management of acute heart failure syndromes. Am. J. Cardiol. 2005, 96, 47G–58G. [Google Scholar] [CrossRef]

| Total Cohort (n = 596) | Propensity Score Matched Cohort (n = 382) | |||||
|---|---|---|---|---|---|---|
| Inodilators (n = 270) | No Inodilators (n = 226) | p Value | Inodilators (n = 191) | No Inodilators (n = 191) | p Value | |
| Age (year) | 60.9 ± 14.8 | 62.9 ± 13.7 | 0.134 | 60.9 ± 14.4 | 61.7 ± 14.0 | 0.134 |
| Gender (male) | 178 (65.8) | 165 (73.0) | 0.089 | 130 (68.1) | 135 (70.7) | 0.579 |
| Body mass index (kg/m2) | 23.2 ± 3.4 | 23.4 ± 3.4 | 0.505 | 23.4 ± 3.4 | 23.3 ± 3.2 | 0.079 |
| Hypertension | 122 (45.2) | 112 (49.6) | 0.331 | 84 (44.0) | 88 (46.1) | 0.681 |
| Diabetes mellitus | 83 (30.7) | 96 (42.5) | 0.007 | 63 (33.0) | 72 (37.7) | 0.335 |
| Dyslipidemia | 47 (17.4) | 56 (24.8) | 0.044 | 38 (19.9) | 42 (22.0) | 0.615 |
| Current smoking | 67 (24.8) | 68 (30.1) | 0.189 | 47 (24.6) | 60 (31.4) | 0.139 |
| Chronic kidney disease | 18 (6.7) | 20 (8.8) | 0.363 | 15 (7.9) | 16 (8.4) | 0.851 |
| Previous myocardial infarction | 32 (11.9) | 30 (13.3) | 0.633 | 23 (12.0) | 23 (12.0) | 1.000 |
| Previous coronary revascularization | 38 (14.1) | 40 (17.7) | 0.269 | 29 (15.2) | 33 (17.3) | 0.579 |
| Previous cerebrovascular accident | 25 (9.3) | 14 (6.2) | 0.207 | 22 (11.5) | 8 (4.2) | 0.008 |
| Systolic blood pressure (mmHg) | 65.0 ± 27.8 | 68.0 ± 35.0 | 0.296 | 66.4 ± 24.8 | 65.3 ± 32.5 | 0.709 |
| Diastolic blood pressure (mmHg) | 43.8 ± 23.5 | 43.8 ± 23.5 | 0.991 | 44.4 ± 19.9 | 42.0 ± 22.0 | 0.278 |
| Heart rate (beat/min) | 83.8 ± 37.2 | 82.8 ± 39.8 | 0.771 | 83.2 ± 35.8 | 81.0 ± 40.6 | 0.573 |
| Hemoglobin (mg/dL) | 12.3 ± 2.7 | 12.5 ± 2.8 | 0.359 | 12.4 ± 2.6 | 12.5 ± 2.8 | 0.595 |
| Total bilirubin (mg/dL) | 1.4 ± 3.3 | 0.8 ± 0.9 | 0.010 | 0.9 ± 0.8 | 0.9 ± 0.9 | 0.594 |
| Creatinine clearance rate (mL/min/1.73 m2) | 53.4 ± 35.3 | 56.1 ± 30.3 | 0.357 | 54.6 ± 39.9 | 57.4 ± 31.2 | 0.448 |
| Serum glucose (mg/dL) | 241.4 ± 131.9 | 236.2 ± 117.9 | 0.658 | 246.4 ± 133.0 | 233.8 ± 118.8 | 0.348 |
| NT–proBNP (pg/mL) | 10,598.1 ± 11,784.3 | 8321.0 ± 14,234.5 | 0.147 | 11,104.2 ± 12,349.3 | 8735.5 ± 14,800.7 | 0.192 |
| Lactic acid (mmol/L) | 7.4 ± 3.7 | 7.6 ± 3.9 | 0.684 | 7.4 ± 3.7 | 7.6 ± 3.9 | 0.670 |
| Peak CK-MB (ng/mL) | 221.3 ± 558.0 | 212.9 ± 222.4 | 0.821 | 243.7 ± 650.9 | 221.5 ± 227.5 | 0.657 |
| Cardiopulmonary resuscitation | 129 (47.8) | 116 (51.3) | 0.431 | 90 (47.1) | 105 (55.0) | 0.125 |
| Shock to ECMO insertion time (min) | 434.7 ± 869.9 | 358.7 ± 787.0 | 0.309 | 389.1 ± 713.8 | 312.3 ± 673.9 | 0.280 |
| Initial pump flow (L/min) | 3.0 ± 0.8 | 2.8 ± 0.9 | 0.132 | 2.9 ± 0.8 | 2.8 ± 0.9 | 0.255 |
| Distal perfusion | 97 (35.9) | 90 (39.8) | 0.372 | 73 (38.2) | 78 (40.8) | 0.601 |
| Unloading of left ventricle | 55 (20.4) | 32 (14.2) | 0.070 | 41 (21.5) | 28 (14.7) | 0.084 |
| Left ventricular ejection fraction (%) | 26.2 ± 12.4 | 29.0 ± 15.8 | 0.034 | 27.0 ± 12.2 | 27.7 ± 15.0 | 0.596 |
| Dopamine | 161 (59.6) | 126 (55.8) | 0.384 | 105 (55.0) | 119 (62.3) | 0.146 |
| Norepinephrine | 170 (63.0) | 159 (70.4) | 0.083 | 121 (63.4) | 133 (69.6) | 0.193 |
| Epinephrine | 44 (16.3) | 25 (11.1) | 0.093 | 21 (11.0) | 24 (12.6) | 0.634 |
| Vasopressin | 41 (15.2) | 21 (9.3) | 0.048 | 28 (14.7) | 16 (8.4) | 0.054 |
| Vasoactive inotropic score | 110.3 ± 176.7 | 90.4 ± 134.8 | 0.156 | 94.3 ± 182.4 | 93.9 ± 134.1 | 0.977 |
| Inotropic score | 34.1 ± 41.5 | 17.6 ± 40.9 | <0.001 | 23.6 ± 21.1 | 20.5 ± 43.8 | 0.387 |
| Ischemic cardiomyopathy | 177 (65.8) | 165 (73.0) | 0.074 | 124 (64.9) | 141 (73.8) | 0.059 |
| Continuous renal replacement therapy | 115 (42.6) | 80 (35.4) | 0.102 | 85 (44.5) | 69 (36.1) | 0.095 |
| Mechanical ventilation | 226 (83.7) | 183 (81.0) | 0.426 | 154 (80.6) | 156 (81.7) | 0.794 |
| Overall (n = 382) | Inodilators (n = 191) | No Inodilators (n = 191) | p Value | |
|---|---|---|---|---|
| In-hospital cardiac mortality | 194 (50.8%) | 91 (47.6%) | 103 (53.9%) | 0.220 |
| In-hospital mortality | 168 (44.0%) | 79 (41.4%) | 89 (46.6%) | 0.304 |
| ECMO site bleeding | 54 (14.1%) | 29 (15.2%) | 25 (13.1%) | 0.558 |
| Limb ischemia | 32 (8.4%) | 16 (8.4%) | 16 (8.4%) | 1.000 |
| Stroke | 16 (4.2%) | 9 (4.7%) | 7 (3.7%) | 0.611 |
| GI bleeding | 24 (6.3%) | 11 (5.8%) | 13 (6.8%) | 0.674 |
| Sepsis | 18 (4.7%) | 9 (4.7%) | 9 (4.7%) | 1.000 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
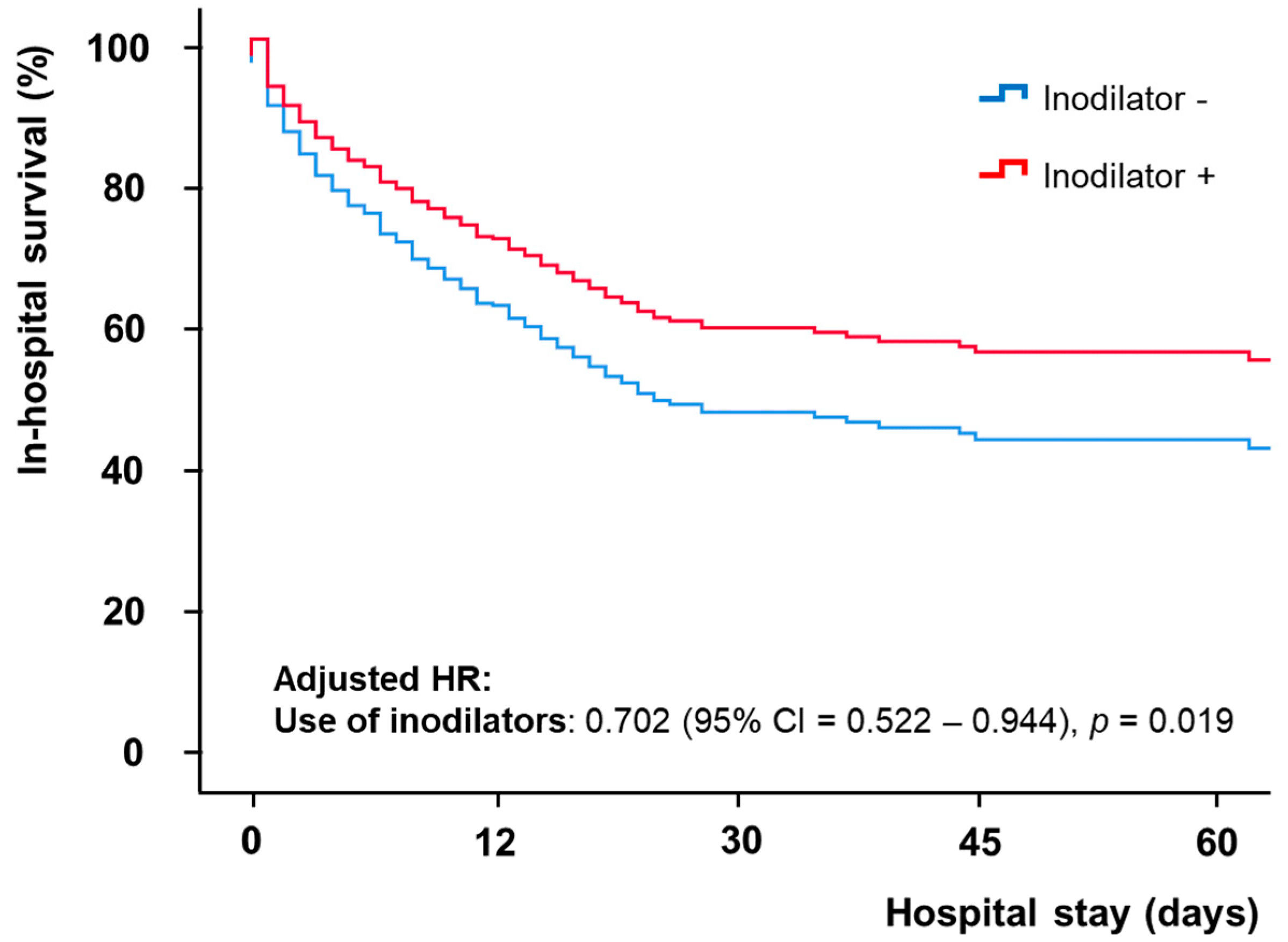
| Use of inodilators | 0.768 (0.579–1.018) | 0.066 | 0.702 (0.522–0.944) | 0.019 |
| Age (year) | 1.024 (1.013–1.034) | <0.001 | 1.019 (1.005–1.033) | 0.007 |
| Gender (male) | 0.865 (0.633–1.182) | 0.363 | ||
| Body mass index (kg/m2) | 1.035 (0.991–1.080) | 0.118 | ||
| Systolic blood pressure (mmHg) | 0.999 (0.993–1.004) | 0.595 | ||
| Heart rate | 0.995 (0.991–0.999) | 0.007 | 0.998 (0.995–1.002) | 0.392 |
| Hypertension | 1.504 (1.133–1.996) | 0.005 | 1.196 (0.868–1.646) | 0.274 |
| Diabetes mellitus | 1.161 (0.871–1.549) | 0.309 | ||
| Previous chronic kidney disease | 1.515 (0.987–2.323) | 0.057 | 0.928 (0.575–1.495) | 0.758 |
| Ischemic cardiomyopathy | 2.191 (1.546–3.107) | <0.001 | 1.131 (0.753–1.697) | 0.553 |
| Hemoglobin (g/dL) | 1.036 (0.981–1.094) | 0.204 | ||
| Creatinine (mg/dL) | 1.083 (0.993–1.180) | 0.070 | ||
| Creatinine clearance rate (mL/min/1.73 m2) | 0.994 (0.989–0.999) | 0.029 | 1.001 (0.996–1.006) | 0.773 |
| Lactic acid (mmol/L) | 1.090 (1.052–1.129) | <0.001 | 1.077 (1.037–1.119) | <0.001 |
| Peak CK-MB (ng/mL) | 1.000 (1.000–1.000) | 0.002 | 1.000 (1.000–1.000) | 0.076 |
| Left ventricular ejection fraction (%) | 0.984 (0.972–0.995) | 0.007 | 0.986 (0.974–0.999) | 0.032 |
| Cardiopulmonary resuscitation | 2.229 (1.659–2.995) | <0.001 | 1.389 (0.993–1.945) | 0.055 |
| Shock to ECMO insertion time (min) | 1.000 (1.000–1.000) | 0.087 | 1.000 (1.000–1.000) | 0.070 |
| Continuous renal replacement therapy | 1.910 (1.436–2.540) | <0.001 | 1.558 (1.138–2.134) | 0.006 |
| Mechanical ventilation | 6.412 (3.280–12.534) | <0.001 | 3.266 (1.612–6.616) | 0.001 |
| Initial pump flow (L/min) | 0.804 (0.663–0.973) | 0.025 | 0.842 (0.689–1.029) | 0.094 |
| Distal perfusion | 0.644 (0.478–0.866) | 0.004 | 0.692 (0.506–0.946) | 0.021 |
| Unloading of left ventricle | 1.229 (0.873–1.730) | 0.238 | ||
| Vasoactive inotropic score | 1.001 (1.000–1.001) | 0.001 | 1.001 (1.000–1.002) | 0.001 |
| Inotropic score | 1.006 (1.004–1.009) | <0.001 | ||
| Dopamine | 2.033 (1.499–2.756) | <0.001 | ||
| Norepinephrine | 1.791 (1.292–2.484) | <0.001 | ||
| Epinephrine | 1.401 (0.949–2.068) | 0.090 | ||
| Vasopressin | 1.531 (1.037–2.261) | 0.032 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Seong, S.-W.; Song, P.S.; Jeong, J.-O.; Yang, J.H.; Gwon, H.-C.; Ko, Y.-G.; Yu, C.W.; Chun, W.J.; Jang, W.J.; et al. Inodilators May Improve the In-Hospital Mortality of Patients with Cardiogenic Shock Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation. J. Clin. Med. 2022, 11, 4958. https://doi.org/10.3390/jcm11174958
Kim M, Seong S-W, Song PS, Jeong J-O, Yang JH, Gwon H-C, Ko Y-G, Yu CW, Chun WJ, Jang WJ, et al. Inodilators May Improve the In-Hospital Mortality of Patients with Cardiogenic Shock Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation. Journal of Clinical Medicine. 2022; 11(17):4958. https://doi.org/10.3390/jcm11174958
Chicago/Turabian StyleKim, Mijoo, Seok-Woo Seong, Pil Sang Song, Jin-Ok Jeong, Jeong Hoon Yang, Hyeon-Cheol Gwon, Young-Guk Ko, Cheol Woong Yu, Woo Jung Chun, Woo Jin Jang, and et al. 2022. "Inodilators May Improve the In-Hospital Mortality of Patients with Cardiogenic Shock Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation" Journal of Clinical Medicine 11, no. 17: 4958. https://doi.org/10.3390/jcm11174958
APA StyleKim, M., Seong, S.-W., Song, P. S., Jeong, J.-O., Yang, J. H., Gwon, H.-C., Ko, Y.-G., Yu, C. W., Chun, W. J., Jang, W. J., Kim, H.-J., Bae, J.-W., Kwon, S. U., Lee, H.-J., Lee, W. S., Park, S.-D., Cho, S. S., & Park, J.-H. (2022). Inodilators May Improve the In-Hospital Mortality of Patients with Cardiogenic Shock Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation. Journal of Clinical Medicine, 11(17), 4958. https://doi.org/10.3390/jcm11174958






