1. Introduction
Adenoid cystic carcinoma (AdCC) is a rare tumor; it accounts for 1% of all head and neck malignancies [
1,
2], but it is one of the most frequent histotypes of the salivary gland carcinomas. It has a relentless growth pattern associated with local recurrences and late distant hematogenous dissemination to the lung, liver, bone, and brain. On the other hand, lymphatic spread has long been considered not a typical figure of this disease and, differently from squamous cell carcinoma (SCC), prevalence of occult lymph node metastasis is less likely and the association between the presence of occult lymph node metastases and survival remains inconclusive. Consequently, the optimal neck management for patients affected by AdCC has been the subject of many studies in the recent literature and no definitive recommendations have been reached [
3,
4]. As surgical or radiotherapeutic neck treatment is not free from morbidity, the decision regarding whether to treat or not should be based on the estimated prevalence of occult lymph node metastases and on the expected impact of their treatment on survival. The aim of this study was to determine the prevalence of occult metastases in an attempt to establish the possible clinical impact of elective neck treatment for patients affected by head and neck AdCC.
2. Methods
This systematic review (SR) and meta-analysis (MA) followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
A systematic search of all articles published before July 2021 was performed on PubMed, Embase, and Central. Combinations of MeSH terms and free-text words were utilized to search for “Adenoid Cystic Carcinomas” AND “Salivary Gland” AND “Neck dissection” OR “Surgery”. For these keywords, all synonyms were used.
2.1. Inclusion Criteria
Two independent reviewers (J.Z. and M.C.) screened all papers on the title and the abstract, with the following inclusion criteria:
- -
Patients affected by head and neck salivary gland cancers, previously untreated and with surgery as primary therapy;
- -
Studies with complete or extractable data on the number of patients with negative neck at presentation (cN0), number of elective neck dissections performed, and pathological finding of metastases (pN0).
Excluded were papers reporting on recurrent cases, animal, cadaveric, and radiological studies; as well as unobtainable full-text studies, irrelevant studies, and studies with insufficient or aggregated data; non-original studies (i.e., reviews, editorials, and letters); papers not in English; and studies reporting on less than 10 patients.
We contacted the authors of the selected studies in order to collect missing data about an individual patient and to attempt to perform subgroup meta-analysis.
Concordance between the two reviewers was calculated with a Cohen’s k test, with a result of k = 0.71. Any disagreement between the reviewers on the eligibility of articles for inclusion was settled by discussion or, failing this, by a referral to a third author (G.B.).
2.2. Data Extraction and Statistical Analysis
The following data were extracted from each of the included studies: author, year of publication, study design, country and period of conduction, number of patients, demographic characteristics, UICC TNM stage, histology and grading of AdCC, criteria adopted for END and type of neck dissection, number of clinically negative patients who underwent END, and cases of occult metastases identified (pN+/cN0). Occult metastases was defined as the presence of nodal metastases upon END of patients with a previous clinically negative cervical lymph node.
A single-arm MA of the rate of occult metastases in cN0 patients undergoing END was performed using the R software for statistical computing (R 2.10.1; “meta” package); arcsine proportion transformation of the data was performed, and the restricted maximum likelihood method was applied for random effects meta-analysis. A 99% confidence interval (CI) was set for the analysis, which is desirable, given the observational nature of the included studies, and it should lead to more conservative results. The heterogeneity among the included studies was evaluated by I2 statistic, and the Cochrane criteria were taken as a reference to estimate the level of heterogeneity.
3. Results
3.1. Study Selection
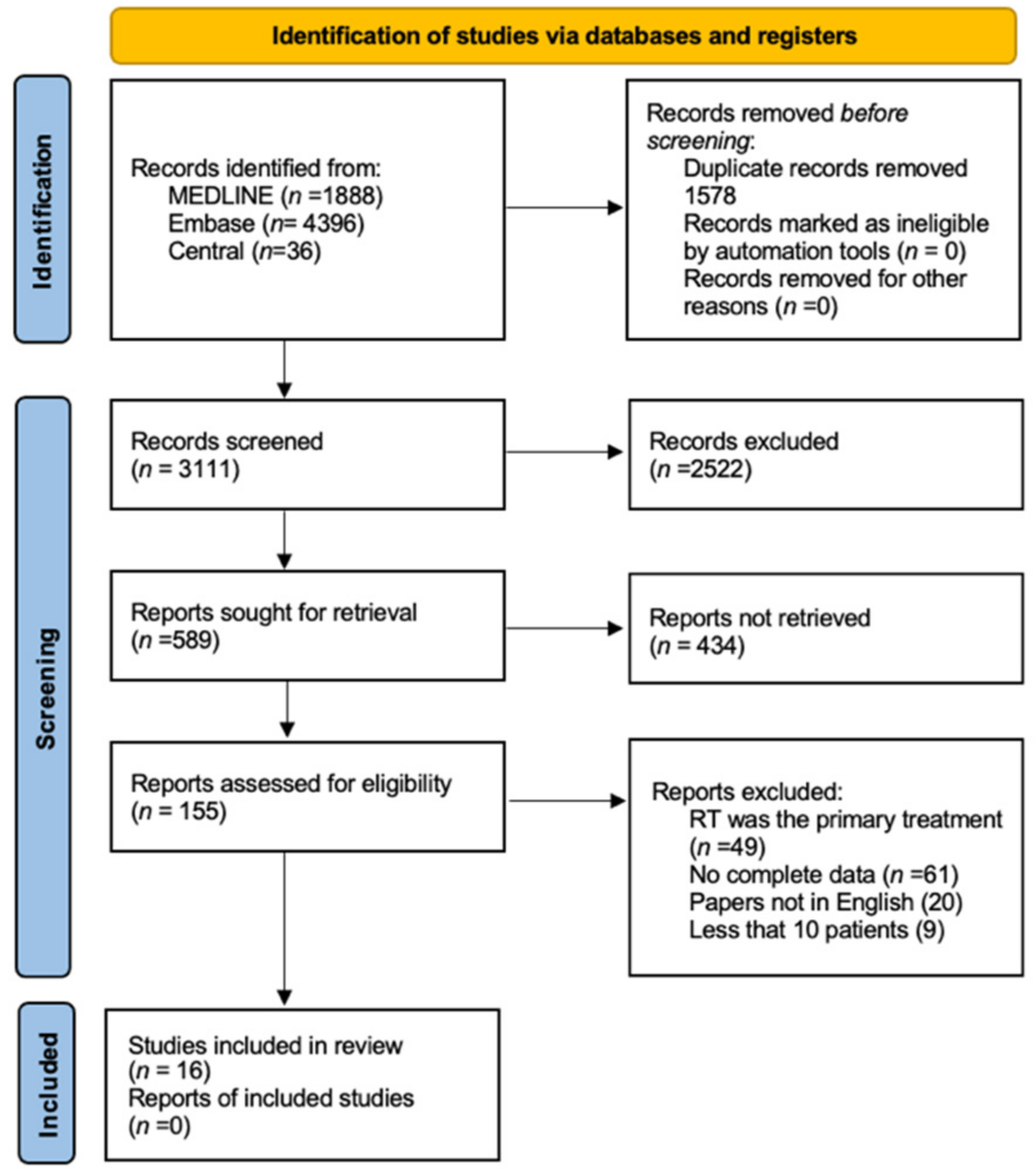
The bibliographic research led to the initial identification of 6317 studies. Details on the literature search process are shown in the flow chart of
Figure 1. By reading the titles and abstracts, 155 full-text articles that reported on primary surgical treatment of untreated head and neck AdCC were analyzed. Of these, 138 were excluded as they did not meet the inclusion criteria. Finally, 16 articles fully satisfied the inclusion criteria [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20]. Reasons for study exclusions are detailed in the flow diagram (
Figure 1), with a comprehensive population of 7497 patients.
3.2. Included Studies
The 16 articles that satisfied the criteria for inclusion in the SR and MA were all retrospective case series published between 1995 and 2020. Patient demographics were available in 15 studies. The 7481 patients included in these comprised 3173 (42.4%) males and 4308 (57.6%) females, whilst the mean age was 55.39 years (4.53 SD, 18–91 range years). Tumor location was extractable from all the studies, and it was distributed as follows: 4460 (59.5%) in the major salivary glands and 2885 (38.5%) in the minor salivary glands; the site was unknown in 152 patients (2%). In particular, AdCC was located in the parotid gland in 1416 (18.79%) patients, 129 (1.71%) in the sublingual gland in, and 1220 (16.19%) in the submandibular gland. Oral cavity minor salivary glands were the primary site in 1362 (18.07%) patients, sinonasal in 644 (8.54%), and “other site” in 2763 patients (36.67%). The tumor grade was available for 427 (5.66%) patients, with low and intermediate-grade AdCC being reported in 353 (4.68%) and high-grade AdCC in 74 (0.98%). Of the 7087 (94.5%) patients in which T-classification was reported, 4020 (56.7.%) were T1/T2 and 3067 (43.3%) T3/T4. The demographic and the histological features of the included studies are summarized in
Table 1.
3.3. Meta-Analysis
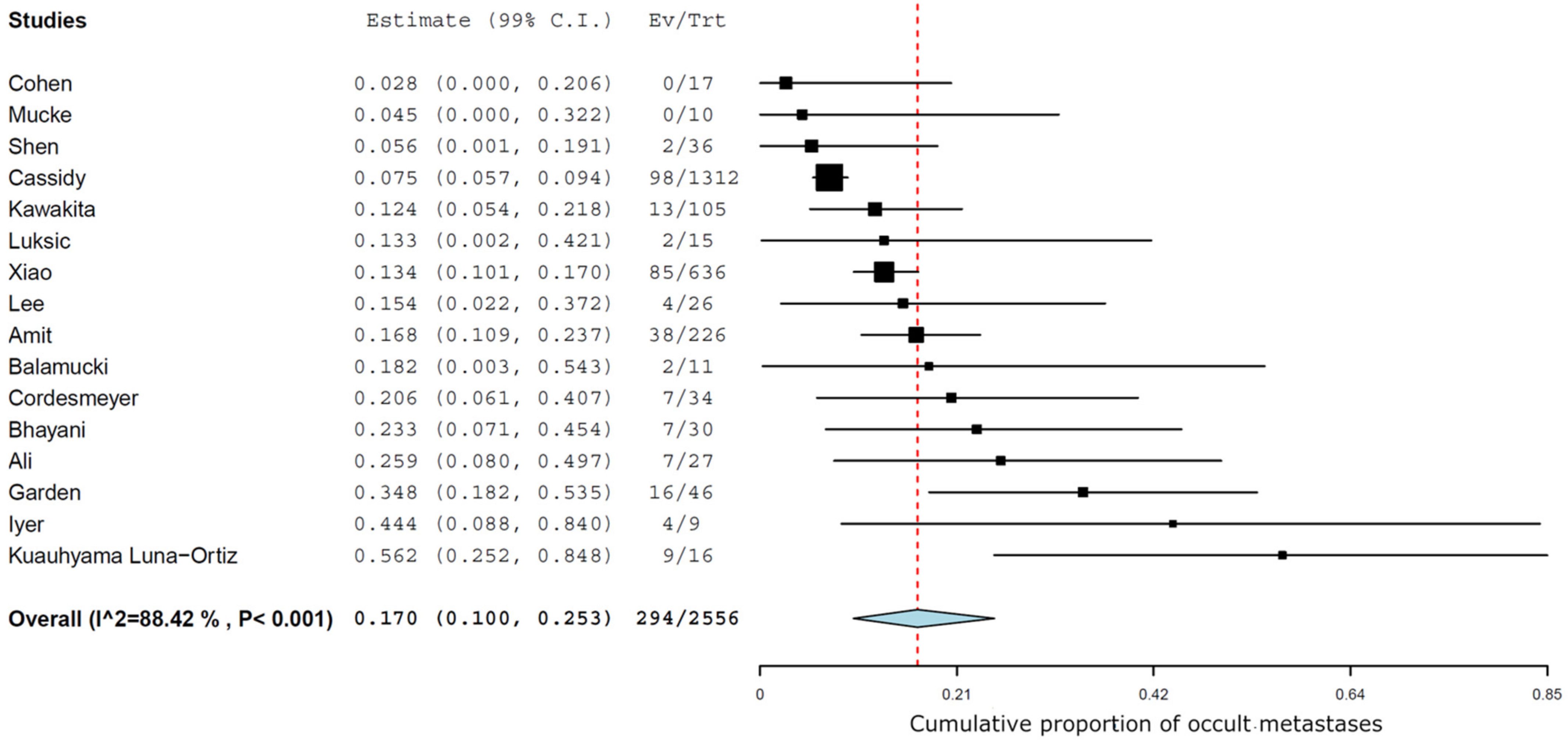
The management of the neck was an END in 2556 cN0 patients of the 7534 with head and neck salivary gland AdCC, which led to the diagnosis of 294/2556 cases of occult metastases (pN+/cN0). The MA of the results of END in the 16 studies estimated an overall prevalence of occult metastases of 17% (99% CI 10-25.3) (
Figure 2). The I2 was 88.4%, indicating that the heterogeneity between the 16 studies included in the statistical analysis was high.
Indications for END were reported in 7/16 studies: most of these report “surgeon’s preference” or “institution’s protocol” as the main factor to carry out END [
6,
7,
11,
19]; more detailed factors were: advanced T-classification of tumors and patients where the neck had to be opened as part of a transcervical approach for access to the tumor or for reconstruction of the primary defect using a free flap [
5]; invasion into the bone tumor location in the retromolar trigone or lip [
18]; patients with high-grade malignancy or dedifferentiated AdCC [
14]. Only five studies specified the extent of prophylactic surgical neck treatment, with selective dissection of levels I-III as the most reported [
6,
11].
Only two studies performed a subgroup analysis based on the tumor site [
6,
20], and only one [
20] upon T classification at presentation, thus preventing a possible subgroup meta-analysis.
Only one study analyzed the possible relationship between pathological adverse features (high-grade histology or perineural invasion) and occult metastases, without finding a significant correlation [
6].
4. Discussion
Historically, a 20% rate of occult neck metastases has been proposed as a sensible cut off of whether to perform an END or not for SCC, balancing gain in oncological control with possible treatment-related complications. Concerning AdCC, occult lymph node metastases have not been confirmed as a definitive factor impacting the course of the disease. Clinically evident metastases at diagnosis have been associated with a worse outcome and a prevalence between 19% and 37% has been found, depending on the series and site of origin of the tumor [
21,
22]. On the other side, the prevalence of occult neck metastases has been scarcely reported in the literature.
Despite this uncertainty, neck dissection is frequently performed in association with primary tumor removal. In a series of definitively treated AdCC by Cassidy et al. based on the National Cancer Database, 70.4% of the population studied (2209 patients) underwent neck dissection [
9]. In another work based on the same database, END was mostly performed in academic centers compared to community facilities and also for patients that had been transferred between facilities for treatment [
20]. These data show that END is frequently performed when dealing with head and neck AdCC, especially by head and neck surgeons at more specialized centers that include END as part of the treatment regimen [
6].
In this setting, more evidence to justify a surgical procedure that is not free of morbidity is desirable. Our analysis focuses upon AdCC as a whole. The material did not allow subgroup-analysis by site, UICC staging, or histopathological features. Nevertheless, AdCC across sites and stages is marked by a constant relentless aggressivity, with differing speed of development according to the grade of the disease. Indeed, most of the series do not study the association of clinical or pathological factors with the presence of occult neck metastases, preventing a more in-depth subgroup meta-analysis.
Our systematic review concludes that in a population of 2530 AdCC patients who underwent END, the pooled prevalence of occult metastases was 17%. This is a not neglectable prevalence but a borderline result for justifying an elective procedure per se. Going beyond a pure prevalence, other factors have to be taken into consideration in order to interpret this overall number of 17% and link it to the decision on whether to proceed to END.
5. Site of Disease
AdCC can originate from major and minor head and neck salivary glands. Different tumor origin seems associated with a different probability for lymphatic dissemination, intrinsically influencing the necessity of an END, because of a different lymphatic density and/or permeability. In their study upon incidence of cervical lymph node metastasis, Amit et al. report an overall rate (cN+ pN+) of neck metastases of 29%. The rate observed in the oral cavity (37%) was significantly higher than that of major salivary glands, 19% (
p = 0.001) [
21]. In the population of our study, the major salivary gland was the most represented tumor site (58% of case), while the oral cavity was the most frequent between minor salivary glands (18%). Only two studies in our MA correlated site and occurrence of occult neck metastases in their analysis: one reported that the major salivary glands and tongue are most distinctive with regard to the frequency of both END and subsequent identification of occult nodal metastases. In this study, END for tongue AdCC repeatedly uncovered occult nodal metastasis more than 20% of the time, regardless of the staging. In contrast, patients with AdCC of the nasal cavity/nasopharynx (12.0%, 3 of 25), hard/soft palate (9.5%, 2 of 21), oral cavity (15.4%, 4 of 26), floor of mouth (11.1%, 2 of 18), and larynx (15.4%, 2 of 13) did not exceed this threshold [
20].
The second study reported an overall prevalence of occult nodal metastases among the patients who underwent END of 17% (38/226), a result that is higher among patients with AdCC of the oral cavity (22%, 25/116 patients), lower among those with sinonasal AdCC (17%, 4/24), and the lowest among patients with a major salivary gland AdCC (11%, 9/85 patients). This difference was not statistically analyzed, but it was noticed that the decision to perform the elective neck dissection was significantly influenced by the tumor site [
6]. Neck metastases were retrieved in 4 of 9 patients (44%) in a series of patients affected by oropharyngeal and oral cavity minor salivary glands AdCC [
13] and only in 2 of 19 (10%) patients with submandibular gland AdCC [
10]. In the study by Mücke et al., none of the ten patients with minor salivary glands AdCC who underwent END presented with occult neck metastases, but 8 of 33 presented (24%) with a clinically positive neck node [
18]. Looking at the literature, the submandibular gland is slightly burdened by node metastasis (22.5%) compared to the parotid gland (14.5%) [
4]; oral cavity and oropharyngeal minor salivary glands are confirmed to have a major tendency for node metastasis, with the series reporting up to 43% of cases [
22]. This behavior could be explained by their advanced stage at presentation and the extensive lymphatic network in this site [
20,
22,
23]. Sinonasal AdCC seems to manifest a minor tendency for neck node metastases both at presentation [
24,
25] and long-term distance [
26]. A collective international review estimated a prevalence of 12.1% nodal metastases in a population of 91 laryngeal AdCC patients [
27].
The only study to compare the impact of END on outcome according to the primary tumor site (oral cavity, major salivary glands, or sinonasal salivary glands) showed survival rates similar to those for the patients with and without END [
6].
T-Classification
Local extension of the primary lesion should logically predict occult neck metastases occurrence. The only study to perform this kind of analysis reports that the odds of performing END paralleled increasing clinical T classification. Compared to T1 patients, T2 (OR 1.57, 95% CI 1.22–2.02), T3 (OR 2.17, 95% CI 1.61–2.91), and T4 (OR 3.02, 95% CI 2.24–4.08) were all associated with an increased rate of END (
p < 0.001). Using logistic regression, clinical T-classification significantly predicted occult nodal metastasis among END patients. If a higher stage has been reported as a criterion to perform END [
18], quite surprisingly Bhayani et al. report that of 30 early-stage AdCC who underwent elective neck dissection, 7 had occult neck metastases (23%) and 6 had extracapsular spread (ECS), encouraging END also for this population [
8].
Again, Xiao highlights that END did not lead to an advantage in OS when dealing with T1–T2 lesions, while it did for T3-T4 lesions (5-year OS 78.1% vs. 70.4%, p = 0.041).
6. Prognostic Role of Occult Metastases
Between the studies selected in our meta-analysis, seven questioned the association between lymph node involvement and survival: three found an association with a worse survival [
6,
15,
18], whereas four studies did not [
11,
13,
14,
17]. Lee et al., studying 61 patients with head and neck AdCC, found an overall survival rate of 85% at 5 years and 81.1% at 10 years in patients with an overall negative N-status [
15] against 56.8% at 5 years and 28.4% at 10 years in patients with an overall positive N-status. On the other hand, there was no significant difference for OS between pN0 and pN+ (
p = 0.366) in the study by Cordesmeyer [
11]. In particular, these authors found a DFS of 85.7% at 5 and 10 years for the group with pN+, whereas the amount was 58.6% after 5 and even at 10 years in the group with pN0. Despite the findings in this one study, pathological lymph node involvement with or without extracapsular spread at diagnosis in AdCC is recognized as an independent prognostic factor in most reports [
28,
29,
30]. Lymph node involvement was the only factor associated with decreased OS on multivariate analysis in an American study of 110 patients [
31] and in a Japanese study of 42 patients [
32].
Moreover, most authors report lymph node involvement as a risk factor for subsequent occurrence of distant metastasis. In the study of Bhayany et al., ECS and solid tumor subtypes were independently associated with the development of DM [
8]. This is confirmed by the work of Kawakita et al. that, even without noting an association between pN+ and survival, demonstrates an impact on distant metastasis free survival [
14]. Once more, a 5-year distant metastasis rate was significantly higher among patients with pN+ than among those without (40% and 27%, respectively) in the work by Amit et al. [
6]. According to Ko et al., 75% of patients with an initial nodal involvement eventually developed distant metastasis [
32].
7. Therapeutic Role of END
Besides the prevalence of occult metastases, more importantly, the effectiveness of a treatment should rely on a proven oncological advantage. Unfortunately, there remains uncertainty about the impact of END on locoregional control (LRC), disease free survival (DFS), and overall survival (OS), due to conflicting results reported in the current literature.
Among the studies included in our MA, only one reports a benefit from an END as compared to three that do not see an advantage (see
Table 2). As already noted, Xiao reports that patients with advanced-stage disease who underwent surgery alone experienced a significantly inferior OS compared to those who underwent surgery with END (5-year OS 78.1% vs. 70.4%,
p = 0.041) [
20]. On the other hand, Amit reports a 5-year OS of 72% for the patients who underwent END, compared with 79% for the patients who did not (not statistically different) and also the 5-year regional control and distant metastasis rates did not differ significantly between the two groups [
6]. The same result was found by Cordesmeyer et al. [
11]. Even if they found a non-statistically significant higher 15-year survival rate in the END group (86% for END and 64% for group without END,
p = 0.829), DFS between those two groups did not differ significantly (65.6% at 5 and 10 years for the END group and 81.0% and 26.7% for the group without END, respectively). In the study by Kawawita, among cN0 cases (
n = 161), neck dissection did not improve OS or LRC [
14]. The reliability of these results suffers from a likely selection bias: patients undergoing END are likely those with more advanced disease and worse prognostic factors that are incorporated in the decision to perform more extended surgery.
8. Elective Neck Irradiation
Neck disease control could take advantage of postoperative elective neck irradiation (ENI). The use and the indication for radiotherapy, however, does not seem to be homogeneous in the current literature.
In the studies selected in our MA, only Balamucky et al. analyzed the role of ENI [
7]: of 102 patients presenting with an undissected clinically negative neck (cN0), 38 patients (37%) were observed and 64 patients (63%) received ENI. A better disease control was observed for the ENI arm, controlling for confounders in multivariate analysis (10y neck control 95% with ENI vs. 89% without ENI,
p = 0.0469). The authors do not specify which factors led to the use of RT, certainly patients were not randomly assigned to the two groups. Moreover, of 11 patients who received END, 9 had pathologically negative neck nodes, yet 6 of these did receive postoperative ENI, but the reasons for this choice are not reported. Of the remaining three patients, one developed a recurrent disease in the neck and lungs.
Despite a lack of standardization, adjuvant therapeutic lateral neck irradiation seems to be widely adopted: in the study of Amit, 66% of patients who underwent END, compared with 55% of patients without END (
p = 0.09) received postoperative radiotherapy, with a dose ranging from 60 to 74 Gy [
6]. A major tendency for adjuvant neck irradiation for patients who underwent END is confirmed by Xiao et al. [
20]. A total of 14 of 34 (41%) patients received adjuvant radiotherapy in the study by Cordesmeyer et al. [
11] and 21 of 50 (42%) in that of Lee et al. [
15].
Shen reports that 25 patients received ENI at the discretion of their attending radiation oncologists, mainly in patients with extensive infiltration of the primary disease [
16] as well as in the study by Agarwall in which ENI was offered selectively to the patients with primary sites rich in capillary lymphatics [
33].
Garden et al. reasonably report to include the neck in the irradiation field in case of pathological nodes [
12], and they also rightly state that the upper neck nodes will often be included in the field required to cover the primary tumor. In this study, none of the 20 irradiated patients with pathologic nodal disease recurred in the irradiated neck area.
9. Limitations
Despite adherence to PRISMA guidelines and adoption of strict inclusion and exclusion criteria, some limitations of this MA have to be highlighted: the most important is the retrospective nature of all selected studies and thus an unavoidable selection bias; and, secondly, the impossibility to perform any subgroup analysis according to site, staging, and treatment. Furthermore, criteria for selecting patients in many studies is either insufficiently stringent or inadequately described.
Finally, our SR and MA were not able to determine which therapeutical strategy (i.e., END, ENI, or observation) gives the better oncological outcome and therefore its prognostic role.
10. Conclusions
By pooling the available information in the literature on head and neck AdCC, this systematic review and meta-analysis offers a solid estimate of an overall prevalence of 17% occult regional nodal metastasis. While the correlation between clinically obvious neck metastases and survival and distant metastases is recognized, the oncological impact of treating occult neck metastases, however, remains controversial.
Until randomized prospective studies bring unbiased evidence on this topic, the indication for END remains patient-tailored. More advanced UICC-stage disease presentation, the oropharyngeal minor salivary glands as a site of origin, and high-grade transformed AdCC are probably the entities that will benefit from performing this procedure. Being in favor of END leads to the observation that it does not add significant morbidity when dealing with more extended lesions that necessitate an open neck procedure or a flap inset, and it allows for an exact pathological N status, which is important for the decision on the extent of further treatment, such as radiotherapy in the case of positive nodes. On the other hand, keeping the unproven oncological impact in consideration, refraining from END in early-stage disease and frail patients is a defendable approach. In addition, ENI can be a reliable strategy if an adjuvant radiotherapy is already foreseen for reasons related to the primary tumor or when it becomes necessary due to the pathological features of the resected primary.
Once again, we have to underline that none of the studies in the literature specify the indications for selecting patients both for END and ENI, so selection bias limits the value of these reports.
Author Contributions
Conceptualization, J.Z., F.C., S.M. and G.P.; methodology, J.Z., O.I. and P.D.M.; investigation, J.Z., M.C., G.B., F.C., S.M. and G.P.; data curation, O.I. and P.D.M.; writing—original draft preparation, J.Z., M.C. and G.B.; writing—review and editing, A.D.V., V.V.P. and R.P.; supervision, A.D.V., V.V.P. and R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
All authors declare no conflict of interest and no financial relationship requiring disclosure.
References
- Bjorndal, K.; Krogdahl, A.; Therkildsen, M.H.; Overgaard, J.; Johansen, J.; Kristensen, C.A.; Homøe, P.; Sørensen, C.H.; Andersen, E.; Bundgaard, T.; et al. Salivary gland carcinoma in Denmark 1990–2005: A national study of incidence, site and histology. Results of the danish head and neck cancer group (DAHANCA). Oral Oncol. 2011, 47, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Coca-Pelaz, A.; Rodrigo, J.P.; Bradley, P.J.; Vander Poorten, V.; Triantafyllou, A.; Hunt, J.L.; Strojan, P.; Rinaldo, A.; Haigentz Jr, M.; Takes, R.P. Adenoid cystic carcinoma of the head and neck–an update. Oral Oncol. 2015, 51, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Hellquist, H.; Skálová, A.; Barnes, L.; Cardesa, A.; Thompson, L.D.; Triantafyllou, A.; Williams, M.D.; Devaney, K.O.; Gnepp, D.R.; Bishop, J.A.; et al. Cervical lymph node metastasis in high-grade transformation of head and neck adenoid cystic carcinoma: A collective international review. Adv. Ther. 2016, 33, 357–368. [Google Scholar] [CrossRef] [PubMed]
- International Head and Neck Scientific Group. Cervical lymph node metastasis in adenoid cystic carcinoma of the major salivary glands. J. Laryngol. Otol. 2017, 131, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Palmer, F.L.; Katabi, N.; Lee, N.; Shah, J.P.; Patel, S.G.; Ganly, I. Long-term local control rates of patients with adenoid cystic carcinoma of the head and neck managed by surgery and postoperative radiation. Laryngoscope 2017, 127, 2265–2269. [Google Scholar] [CrossRef]
- Amit, M.; Na’ara, S.; Sharma, K.; Ramer, N.; Ramer, I.; Agbetoba, A.; Glick, J.; Yang, X.; Lei, D.; Bjoerndal, K.; et al. Elective neck dissection in patients with head and neck adenoid cystic carcinoma: An international collaborative study. Ann. Surg. Oncol. 2015, 22, 1353–1359. [Google Scholar] [CrossRef]
- Balamucki, C.J.; Amdur, R.J.; Werning, J.W.; Vaysberg, M.; Morris, C.G.; Kirwan, J.M.; Mendenhall, W.M. Adenoid cystic carcinoma of the head and neck. Am. J. Otolaryngol.-Head Neck Med. Surg. 2012, 33, 510–518. [Google Scholar] [CrossRef]
- Bhayani, M.K.; Yener, M.; El-Naggar, A.; Garden, A.; Hanna, E.Y.; Weber, R.S.; Kupferman, M.E. Prognosis and risk factors for early-stage adenoid cystic carcinoma of the major salivary glands. Cancer 2012, 118, 2872–2878. [Google Scholar] [CrossRef]
- Cassidy, R.J.; Switchenko, J.M.; El-Deiry, M.W.; Belcher, R.H.; Zhong, J.; Steuer, C.E.; Saba, N.F.; McDonald, M.W.; Yu, D.S.; Gillespie, T.W.; et al. Disparities in postoperative therapy for salivary gland adenoid cystic carcinomas. Laryngoscope 2019, 129, 377–386. [Google Scholar] [CrossRef]
- Cohen, A.N.; Damrose, E.J.; Huang, R.Y.; Nelson, S.D.; Blackwell, K.E.; Calcaterra, T.C. Adenoid cystic carcinoma of the submandibular gland: A 35-year review. Otolaryngol.-Head Neck Surg. 2004, 131, 994–1000. [Google Scholar] [CrossRef]
- Cordesmeyer, R.; Kauffmann, P.; Laskawi, R.; Rau, A.; Bremmer, F. The incidence of occult metastasis and the status of elective neck dissection in salivary adenoid cystic carcinoma: A single center study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Garden, A.S.; Weber, R.S.; Morrison, W.H.; Kian Ang, K.; Peters, L.J. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 619–626. [Google Scholar] [CrossRef]
- Iyer, N.G.; Kim, L.; Nixon, I.J.; Palmer, F.; Kraus, D.; Shaha, A.R.; Shah, J.P.; Patel, S.G.; Ganly, I. Factors predicting outcome in malignant minor salivary gland tumors of the oropharynx. Arch. Otolaryngol.–Head Neck Surg. 2010, 136, 1240–1247. [Google Scholar] [CrossRef]
- Kawakita, D.; Murase, T.; Ueda, K.; Kano, S.; Tada, Y.; Tsukahara, K.; Okami, K.; Onitsuka, T.; Fujimoto, Y.; Matoba, T.; et al. The impact of clinicopathological factors on clinical outcomes in patients with salivary gland adenoid cystic carcinoma: A multi-institutional analysis in Japan. Int. J. Clin. Oncol. 2020, 25, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, B.H.; Choi, E.C. Nineteen-year oncologic outcomes and the benefit of elective neck dissection in salivary gland adenoid cystic carcinoma. Head Neck 2014, 36, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Lukšić, I.; Baranović, S.; Suton, P.; Gerbl, D. Adenoid cystic carcinoma of the head and neck: A single-institution’s analysis of 45 consecutive cases over a 29-year period. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Luna-Ortiz, K.; Villavicencio-Valencia, V.; Rodríguez-Falconi, A.; Peteuil, N.; Mosqueda-Taylor, A. Adenoid cystic carcinoma in a mexican population. J. Maxillofac. Oral Surg. 2016, 15, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Mücke, T.; Tannapfel, A.; Kesting, M.R.; Wagenpfeil, S.; Robitzky, L.K.; Wolff, K.D.; Hölzle, F. Adenoid cystic carcinomas of minor salivary glands. Auris Nasus Larynx 2010, 37, 615–620. [Google Scholar] [CrossRef]
- Shen, C.; Xu, T.; Huang, C.; Hu, C.; He, S. Treatment outcomes and prognostic features in adenoid cystic carcinoma originated from the head and neck. Oral Oncol. 2012, 48, 445–449. [Google Scholar] [CrossRef]
- Xiao, R.; Sethi, R.K.V.; Feng, A.L.; Fontanarosa, J.B.; Deschler, D.G. The role of elective neck dissection in patients with adenoid cystic carcinoma of the head and neck. Laryngoscope 2019, 129, 2094–2104. [Google Scholar] [CrossRef]
- Amit, M.; Binenbaum, Y.; Sharma, K.; Ramer, N.; Ramer, I.; Agbetoba, A.; Glick, J.; Yang, X.; Lei, D.; Bjørndal, K.; et al. Incidence of cervical lymph node metastasis and its association with outcomes in patients with adenoid cystic carcinoma. An international collaborative study. Head Neck 2015, 37, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Suárez, C.; Barnes, L.; Silver, C.E.; Rodrigo, J.P.; Shah, J.P.; Triantafyllou, A.; Rinaldo, A.; Cardesa, A.; Pitman, K.T.; Kowalski, L.P.; et al. Cervical lymph node metastasis in adenoid cystic carcinoma of oral cavity and oropharynx: A collective international review. Auris Nasus Larynx 2016, 43, 477–484. [Google Scholar] [CrossRef] [PubMed]
- International Head and Neck Scientific Group. Cervical lymph node metastasis in adenoid cystic carcinoma of the sinonasal tract, nasopharynx, lacrimal glands and external auditory canal: A collective international review. J. Laryngol. Otol. 2016, 130, 1093–1097. [Google Scholar] [CrossRef]
- Min, R.; Siyi, L.; Wenjun, Y.; Ow, A.; Lizheng, W.; Minjun, D.; Chenping, Z. Salivary gland adenoid cystic carcinoma with cervical lymph node metastasis: A preliminary study of 62 cases. Int. J. Oral Maxillofac. Surg. 2012, 41, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Volpi, L.; Bignami, M.; Lepera, D.; Karligkiotis, A.; Pistochini, A.; Ottini, G.; Grigioni, E.; Lombardi, D.; Nicolai, P.; Castelnuovo, P. Endoscopic endonasal resection of adenoid cystic carcinoma of the sinonasal tract and skull base. Laryngoscope 2019, 129, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, J.; Pietrobon, G.; Campomagnani, I.; Riggi, E.; Veronesi, G.; Borchini, R.; Pellini, R.; Volpi, L.; Bignami, M.; Castelnuovo, P. The role of a post therapeutic surveillance program for sinonasal malignancies: Analysis of 417 patients. Head Neck 2020, 42, 963–973. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Barnes, L.; Rinaldo, A.; Cardesa, A.; Shah, J.P.; Rodrigo, J.P.; Suárez, C.; Eloy, J.A.; Bishop, J.A.; Devaney, K.O.; et al. Cervical lymph node metastasis in adenoid cystic carcinoma of the larynx: A collective international review. Adv. Ther. 2016, 33, 553–579. [Google Scholar] [CrossRef][Green Version]
- Lloyd, S.; James, B.Y.; Wilson, L.D.; Decker, R.H. Determinants and patterns of survival in adenoid cystic carcinoma of the head and neck, including an analysis of adjuvant radiation therapy. Am. J. Clin. Oncol. 2011, 34, 76–81. [Google Scholar] [CrossRef]
- Bianchi, B.; Copelli, C.; Cocchi, R.; Ferrari, S.; Pederneschi, N.; Sesenna, E. Adenoid cystic carcinoma of intraoral minor salivary glands. Oral Oncol. 2008, 44, 1026–1031. [Google Scholar] [CrossRef]
- Megwalu, U.C.; Sirjani, D. Risk of nodal metastasis in major salivary gland adenoid cystic carcinoma. Otolaryngol.-Head Neck Surg. U.S. 2017, 156, 660–664. [Google Scholar] [CrossRef]
- Gomez, D.R.; Hoppe, B.S.; Wolden, S.L.; Zhung, J.E.; Patel, S.G.; Kraus, D.H.; Shah, J.P.; Ghossein, R.A.; Lee, N.Y. Outcomes and prognostic variables in adenoid cystic carcinoma of the head and neck: A recent experience. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.H.; Lee, M.A.; Hong, Y.S.; Lee, K.S.; Jung, C.K.; Kim, Y.S.; Sun, D.I.; Kim, B.S.; Kim, M.S.; Kang, J.H. Prognostic ractors affecting the clinical outcome of adenoid cystic carcinoma of the head and neck. Jpn. J. Clin. Oncol. 2007, 37, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, J.P.; Jain, S.; Gupta, T.; Tiwari, M.; Laskar, S.G.; Dinshaw, K.A.; Chaturvedi, P.; D’cruz, A.K.; Shrivastava, S.K. Intraoral adenoid cystic carcinoma: Prognostic factors and outcome. Oral Oncol. 2008, 44, 986–993. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).