Relationship of Effective Circulating Volume with Sublingual Red Blood Cell Velocity and Microvessel Pressure Difference: A Clinical Investigation and Computational Fluid Dynamics Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Objectives
2.3. Patient Eligibility
2.4. Clinical Management
2.5. Calculation of Mean Circulatory Filling Pressure Analogue and Related Variables
2.6. Sublingual Microcirculation Analysis
2.7. Fluid Mechanics and Computational Fluid Dynamics
2.8. Statistical Analysis
3. Results
3.1. Baseline Systemic and Sublingual Microcirculation Variables
3.2. Correlation of Mean Circulatory Filling Pressure Analogue with Systemic and Sublingual Microcirculation Variables
3.3. Correlation of Mean Circulatory Filling Pressure Analogue with Sublingual Red Blood Cell Velocity and Microvessel Length
3.4. Correlation of Sublingual Red Blood Cell Velocity with Microvessel Diameter and Length
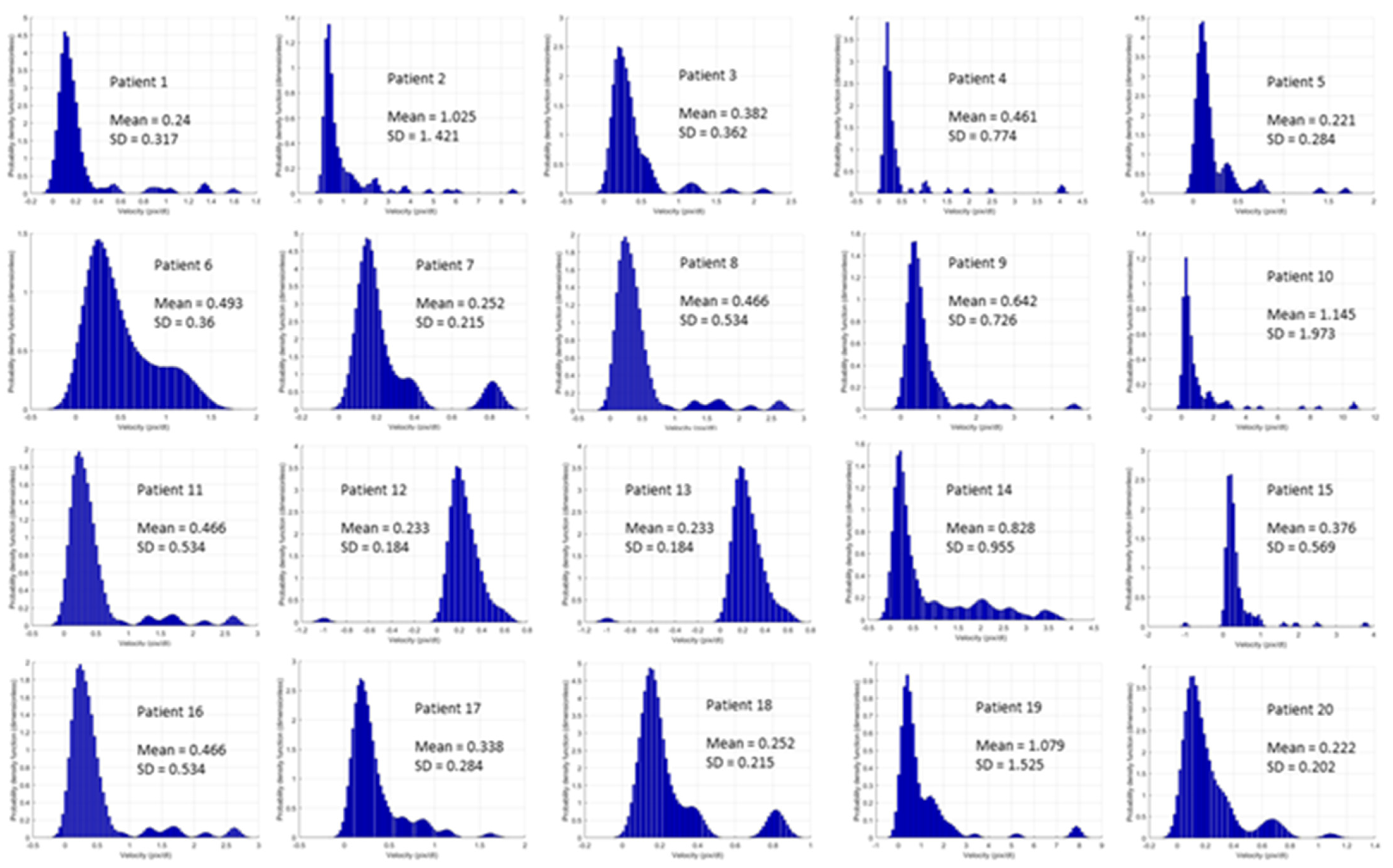
3.5. Correlation of Sublingual Red Blood Cell Velocity with Sublingual Density and Flow Variables and Oxygen Extraction Ratio
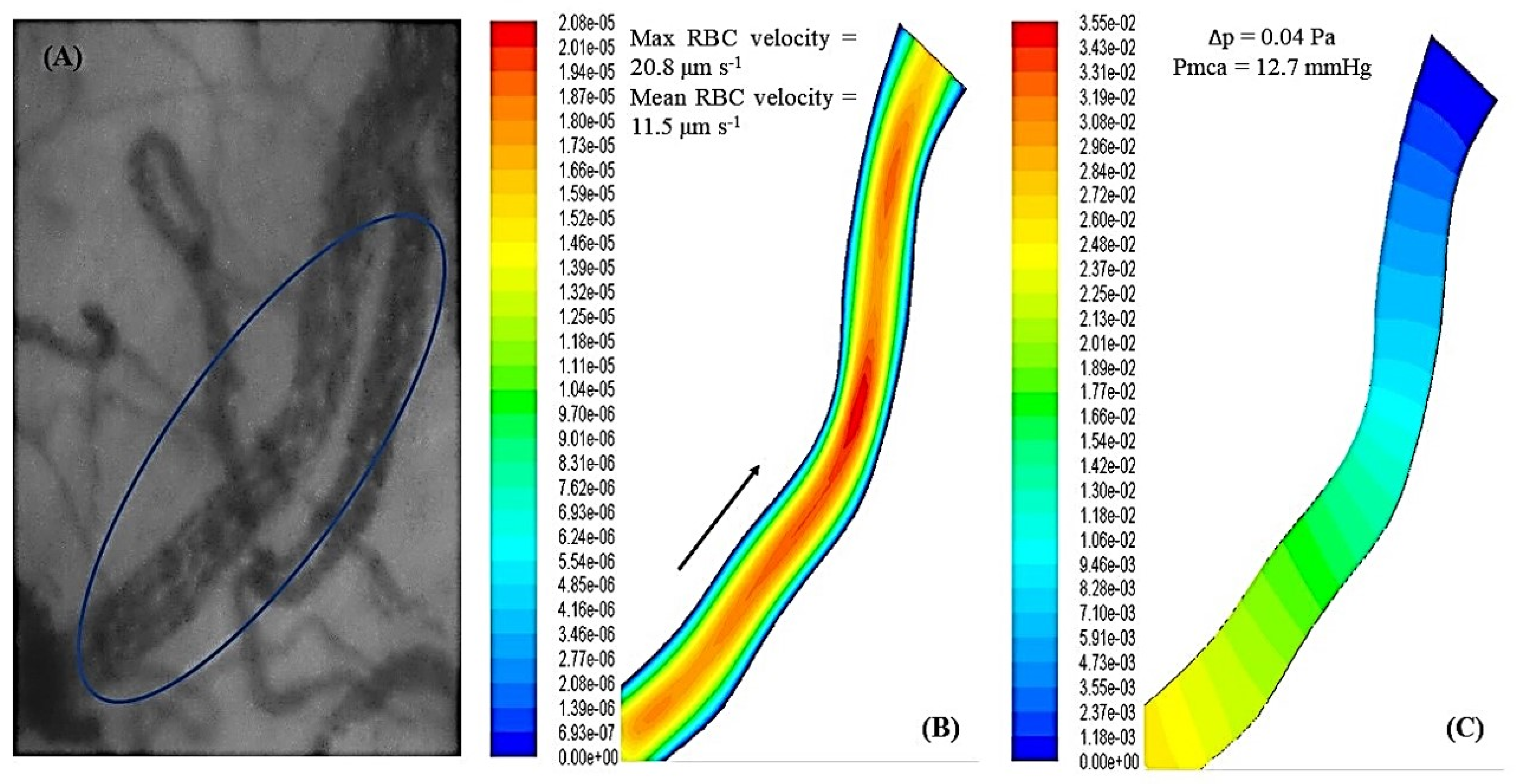
3.6. Fluid Mechanics and Computational Fluid Dynamics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carsetti, A.; Watson, X.; Cecconi, M. Haemodynamic coherence in perioperative setting. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Ince, C.; Ertmer, C. Hemodynamic coherence: Its meaning in perioperative and intensive care medicine. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.K.; Secomb, T.W. Effects of impaired microvascular flow regulation on metabolism-perfusion matching and organ function. Microcirculation 2021, 28, e12673. [Google Scholar] [CrossRef] [PubMed]
- Walley, K.R. Heterogeneity of oxygen delivery impairs oxygen extraction by peripheral tissues: Theory. J. Appl. Physiol. 1996, 81, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.C.; Secomb, T.W. Distinct roles of red-blood-cell-derived and wall-derived mechanisms in metabolic regulation of blood flow. Microcirculation 2021, 28, e12690. [Google Scholar] [CrossRef]
- Keeley, T.P.; Mann, G.E. Defining Physiological Normoxia for Improved Translation of Cell Physiology to Animal Models and Humans. Physiol. Rev. 2019, 99, 161–234. [Google Scholar] [CrossRef] [Green Version]
- Schödel, J.; Ratcliffe, P.J. Mechanisms of hypoxia signalling: New implications for nephrology. Nat. Rev. Nephrol. 2019, 15, 641–659. [Google Scholar] [CrossRef]
- Kanoore Edul, V.S.; Ince, C.; Estenssoro, E.; Ferrara, G.; Arzani, Y.; Salvatori, C.; Dubin, A. The Effects of Arterial Hypertension and Age on the Sublingual Microcirculation of Healthy Volunteers and Outpatients with Cardiovascular Risk Factors. Microcirculation 2015, 22, 485–492. [Google Scholar] [CrossRef]
- Bond, O.; De Santis, P.; Iesu, E.; Franchi, F.; Vincent, J.L.; Creteur, J.; Taccone, F.S.; Scolletta, S. Relationship between Microcirculatory Perfusion and Arterial Elastance: A Pilot Study. Crit. Care. Res. Pract. 2019, 2019, 3256313. [Google Scholar] [CrossRef] [Green Version]
- Komori, M.; Takada, K.; Tomizawa, Y.; Nishiyama, K.; Kawamata, M.; Ozaki, M. Permissive range of hypercapnia for improved peripheral microcirculation and cardiac output in rabbits. Crit. Care Med. 2007, 35, 2171–2175. [Google Scholar] [CrossRef]
- Rothe, C.F.; Maass-Moreno, R.; Flanagan, A.D. Effects of hypercapnia and hypoxia on the cardiovascular system: Vascular capacitance and aortic chemoreceptors. Am. J. Physiol. 1990, 259, H932–H939. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A.; Laou, E.; Papagiannakis, N.; Varvarousi, G.; Ragias, D.; Koutsovasilis, A.; Makris, D.; Varvarousis, D.; Iacovidou, N.; Pantazopoulos, I.; et al. Determinants of venous return in steady-state physiology and asphyxia-induced circulatory shock and arrest: An experimental study. Intensiv. Care Med. Exp. 2022, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.P.; Joyner, M.J. Compensatory vasodilatation during hypoxic exercise: Mechanisms responsible for matching oxygen supply to demand. J. Physiol. 2012, 590, 6321–6326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ospina-Tascón, G.A.; Bautista, D.F.; Madriñán, H.J.; Valencia, J.D.; Bermúdez, W.F.; Quiñones, E.; Calderón-Tapia, L.E.; Hernandez, G.; Bruhn, A.; De Backer, D. Microcirculatory dysfunction and dead-space ventilation in early ARDS: A hypothesis-generating observational study. Ann. Intensiv. Care 2020, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Postnov, D.; Marsh, D.J.; Cupples, W.A.; Holstein-Rathlou, N.H.; Sosnovtseva, O. Synchronization in renal microcirculation unveiled with high-resolution blood flow imaging. Elife 2022, 11, e75284. [Google Scholar] [CrossRef]
- Chalkias, A.; Laou, E.; Mermiri, M.; Michou, A.; Ntalarizou, N.; Koutsona, S.; Chasiotis, G.; Garoufalis, G.; Agorogiannis, V.; Kyriakaki, A.; et al. Microcirculation-guided treatment improves tissue perfusion and hemodynamic coherence in surgical patients with septic shock. Eur. J. Trauma Emerg. Surg. 2022, 1–13, in press. [Google Scholar] [CrossRef]
- Chalkias, A. Increasing stress volume vs. increasing tissue perfusion in septic patients. Eur. J. Anaesthesiol. 2022, 39, 390–391. [Google Scholar] [CrossRef]
- Chalkias, A.; Laou, E.; Papagiannakis, N.; Spyropoulos, V.; Kouskouni, E.; Theodoraki, K.; Xanthos, T. Assessment of Dynamic Changes in Stressed Volume and Venous Return during Hyperdynamic Septic Shock. J. Pers. Med. 2022, 12, 724. [Google Scholar] [CrossRef]
- de Keijzer, I.N.; Scheeren, T.W.L. Perioperative Hemodynamic Monitoring: An Overview of Current Methods. Anesthesiol. Clin. 2021, 39, 441–456. [Google Scholar] [CrossRef]
- Vos, J.J.; Kalmar, A.F.; Scheeren, T.W.L. Bedside assessment and clinical utility of mean systemic filling pressure in acute care. J. Emerg. Crit. Care Med. 2020, 4, 25. [Google Scholar] [CrossRef]
- Wijnberge, M.; Sindhunata, D.P.; Pinsky, M.R.; Vlaar, A.P.; Ouweneel, E.; Jansen, J.R.; Veelo, D.P.; Geerts, B.F. Estimating mean circulatory filling pressure in clinical practice: A systematic review comparing three bedside methods in the critically ill. Ann. Intensiv. Care 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkin, G.; Wright, C.; Bellomo, R.; Boyce, N. Use of a mean systemic filling pressure analogue during the closed-loop control of fluid replacement in continuous hemodiafiltration. J. Crit. Care 1994, 9, 124–133. [Google Scholar] [CrossRef]
- Henderson, W.R.; Griesdale, D.E.; Walley, K.R.; Sheel, A.W. Clinical review: Guyton—the role of mean circulatory filling pressure and right atrial pressure in controlling cardiac output. Crit. Care 2010, 14, 243. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, G. The rate of oxygen release and its effect on capillary O2 tension: A mathematical analysis. Respir. Physiol. 1986, 63, 79–96. [Google Scholar] [CrossRef]
- Dubin, A.; Kanoore Edul, V.S.; Caminos Eguillor, J.F.; Ferrara, G. Monitoring Microcirculation: Utility and Barriers—A Point-of-View Review. Vasc. Heal. Risk Manag. 2020, 16, 577–589. [Google Scholar] [CrossRef]
- Edul, V.S.; Enrico, C.; Laviolle, B.; Vazquez, A.R.; Ince, C.; Dubin, A. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit. Care Med. 2012, 40, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Edul, V.S.; Ince, C.; Navarro, N.; Previgliano, L.; Risso-Vazquez, A.; Rubatto, P.N.; Dubin, A. Dissociation between sublingual and gut microcirculation in the response to a fluid challenge in postoperative patients with abdominal sepsis. Ann. Intensiv. Care 2014, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Chalkias, A.; Laou, E.; Kolonia, K.; Ragias, D.; Angelopoulou, Z.; Mitsiouli, E.; Kallemose, T.; Smith-Hansen, L.; Eugen-Olsen, J.; Arnaoutoglou, E. Elevated preoperative suPAR is a strong and independent risk marker for postoperative complications in patients undergoing major noncardiac surgery (SPARSE). Surgery 2022, 171, 1619–1625. [Google Scholar] [CrossRef]
- Agha, R.; Abdall-Razak, A.; Crossley, E.; Dowlut, N.; Iosifidis, C.; Mathew, G.; STROCSS Group. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019, 72, 156–165. [Google Scholar] [CrossRef]
- Özarslan, N.G.; Ayhan, B.; Kanbak, M.; Çelebioğlu, B.; Demircin, M.; Ince, C.; Aypar, Ü. Comparison of the effects of sevoflurane, isoflurane, and desflurane on microcirculation in coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 2012, 26, 791–798. [Google Scholar] [CrossRef]
- Kertai, M.D.; White, W.D.; Gan, T.J. Cumulative duration of “triple low” state of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia is not associated with increased mortality. Anesthesiology 2014, 121, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willingham, M.; Ben Abdallah, A.; Gradwohl, S.; Helsten, D.; Lin, N.; Villafranca, A.; Jacobsohn, E.; Avidan, M.; Kaiser, H. Association between intraoperative electroencephalographic suppression and postoperative mortality. Br. J. Anaesth. 2014, 113, 1001–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milstein, D.M.; Helmers, R.; Hackmann, S.; Belterman, C.N.; van Hulst, R.A.; de Lange, J. Sublingual microvascular perfusion is altered during normobaric and hyperbaric hyperoxia. Microvasc. Res. 2016, 105, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Schwarte, L.A.; Schober, P.; Loer, S.A. Benefits and harms of increased inspiratory oxygen concentrations. Curr. Opin. Anaesthesiol. 2019, 32, 783–791. [Google Scholar] [CrossRef]
- Orbegozo Cortés, D.; Puflea, F.; Donadello, K.; Taccone, F.S.; Gottin, L.; Creteur, J.; Vincent, J.L.; De Backer, D. Normobaric hyperoxia alters the microcirculation in healthy volunteers. Microvasc. Res. 2015, 98, 23–28. [Google Scholar] [CrossRef]
- Parkin, W.G. Volume state control—a new approach. Crit. Care Resusc. 1999, 1, 311–321. [Google Scholar]
- Parkin, W.G.; Leaning, M.S. Therapeutic control of the circulation. J. Clin. Monit. Comput. 2008, 22, 391–400. [Google Scholar] [CrossRef]
- Pellegrino, V.A.; Mudaliar, Y.; Gopalakrishnan, M.; Horton, M.D.; Killick, C.J.; Parkin, W.G.; Playford, H.R.; Raper, R.F. Computer based haemodynamic guidance system is effective and safe in management of postoperative cardiac surgery patients. Anaesth. Intensiv. Care 2011, 39, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Meijs, L.P.B.; van Houte, J.; Conjaerts, B.C.M.; Bindels, A.J.G.H.; Bouwman, A.; Houterman, S.; Bakker, J. Clinical validation of a computerized algorithm to determine mean systemic filling pressure. J. Clin. Monit. Comput. 2022, 36, 191–198. [Google Scholar] [CrossRef]
- Berger, D.; Moller, P.W.; Takala, J. Reply to “Letter to the editor: Why persist in the fallacy that mean systemic pressure drives venous return?”. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1336–H1337. [Google Scholar] [CrossRef] [Green Version]
- Berger, D.; Moller, P.W.; Weber, A.; Bloch, A.; Bloechlinger, S.; Haenggi, M.; Sondergaard, S.; Jakob, S.M.; Magder, S.; Takala, J. Effect of PEEP, blood volume, and inspiratory hold maneuvers on venous return. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H794–H806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ince, C.; Boerma, E.C.; Cecconi, M.; De Backer, D.; Shapiro, N.I.; Duranteau, J.; Pinsky, M.R.; Artigas, A.; Teboul, J.L.; Reiss, I.K.M.; et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: Results from a task force of the European Society of Intensive Care Medicine. Intensiv. Care Med. 2018, 44, 281–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massey, M.J.; Larochelle, E.; Najarro, G.; Karmacharla, A.; Arnold, R.; Trzeciak, S.; Angus, D.C.; Shapiro, N.I. The microcirculation image quality score: Development and preliminary evaluation of a proposed approach to grading quality of image acquisition for bedside videomicroscopy. J. Crit. Care 2013, 28, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Dobbe, J.G.; Streekstra, G.J.; Atasever, B.; van Zijderveld, R.; Ince, C. Measurement of functional microcirculatory geometry and velocity distributions using automated image analysis. Med. Biol. Eng. Comput. 2008, 46, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Georgakarakos, E.; Ioannidis, G.; Raptis, A.; Xenos, M. Computational Fluid Dynamic Analysis Supports the Hemodynamic Stability of Hybrid Combinations With the AFX Bifurcate and Nitinol-Based Proximal Segments in Solutions of Failed Endovascular Aneurysm Repair. Vasc. Endovasc. Surg. 2021, 55, 907–909. [Google Scholar] [CrossRef]
- Patankar, S.V. Numerical Heat Transfer and Fluid Flow, 1st ed.; CRC Press: Boca Raton, FL, USA, 1980. [Google Scholar]
- Kendall, M.G. Rank Correlation Methods, 3rd ed.; Hafner Publishing Company: New York, NY, USA, 1962. [Google Scholar]
- Weil, M.H.; Tang, W. Welcoming a new era of hemodynamic monitoring: Expanding from the macro to the microcirculation. Crit. Care Med. 2007, 35, 1204–1205. [Google Scholar] [CrossRef]
- Behem, C.R.; Graessler, M.F.; Friedheim, T.; Kluttig, R.; Pinnschmidt, H.O.; Duprée, A. The use of pulse pressure variation for predicting impairment of microcirculatory blood flow. Sci. Rep. 2021, 11, 9215. [Google Scholar] [CrossRef]
- Hilty, M.P.; Ince, C. Automated quantification of tissue red blood cell perfusion as a new resuscitation target. Curr. Opin. Crit. Care 2020, 26, 273–280. [Google Scholar] [CrossRef]
- Bouattour, K.; Teboul, J.L.; Varin, L.; Vicaut, E.; Duranteau, J. Preload Dependence Is Associated with Reduced Sublingual Microcirculation during Major Abdominal Surgery. Anesthesiology 2019, 130, 541–549. [Google Scholar] [CrossRef]
- Gazmuri, R.J.; de Gomez, C.A. From a pressure-guided to a perfusion-centered resuscitation strategy in septic shock: Critical literature review and illustrative case. J. Crit. Care 2020, 56, 294–304. [Google Scholar] [CrossRef]
- De Backer, D.; Foulon, P. Minimizing catecholamines and optimizing perfusion. Crit. Care 2019, 23, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovas, A.; Sackarnd, J.; Rossaint, J.; Kampmeier, S.; Pavenstädt, H.; Vink, H. Identification of novel sublingual parameters to analyze and diagnose microvascular dysfunction in sepsis: The NOSTRADAMUS study. Crit. Care 2021, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Edul, V.S.; Ince, C.; Vazquez, A.R.; Rubatto, P.N.; Espinoza, E.D.; Welsh, S. Similar Microcirculatory Alterations in Patients with Normodynamic and Hyperdynamic Septic Shock. Ann. Am. Thorac. Soc. 2016, 13, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Bennett, V.A.; Vidouris, A.; Cecconi, M. Effects of Fluids on the Macro- and Microcirculations. Crit. Care 2018, 22, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalkias, A.; Papagiannakis, N.; Mavrovounis, G.; Kolonia, K.; Mermiri, M.; Pantazopoulos, I.; Laou, E.; Arnaoutoglou, E. Sublingual microcirculatory alterations during the immediate and early postoperative period: A systematic review and meta-analysis. Clin. Hemorheol. Microcirc. 2022, 80, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A.; Xanthos, T.; Papageorgiou, E.; Anania, A.; Beloukas, A.; Pavlopoulos, F. Intraoperative initiation of a modified ARDSNet protocol increases survival of septic patients with severe acute respiratory distress syndrome. Heart Lung 2018, 47, 616–621. [Google Scholar] [CrossRef]
- Maas, J.J.; Pinsky, M.R.; Geerts, B.F.; de Wilde, R.B.; Jansen, J.R. Estimation of mean systemic filling pressure in postoperative cardiac surgery patients with three methods. Intensiv. Care Med. 2012, 38, 1452–1460. [Google Scholar] [CrossRef] [Green Version]
- Werner-Moller, P.; Sondergaard, S.; Jakob, S.M.; Takala, J.; Berger, D. Effect of volume status on the estimation of mean systemic filling pressure. J. Appl. Physiol. 2019, 126, 1503–1513. [Google Scholar] [CrossRef]


| Heart rate (bmp) | 67.5 ± 7 |
| Systolic arterial pressure (mmHg) | 120 ± 7.4 |
| Diastolic arterial pressure (mmHg) | 71.3 ± 7.4 |
| Mean arterial pressure (mmHg) | 88.1 ± 7 |
| Cardiac output (L min−1) | 4.8 ± 1 |
| Cardiac index (L min−1 m−2) | 2.6 ± 0.3 |
| Stroke volume (mL beat−1) | 74.7 ± 9.6 |
| Stroke volume variation (%) | 5.9 ± 1.8 |
| Systemic vascular resistance (dynes sec cm−5) | 1306.3 ± 176.3 |
| Central venous pressure (mmHg) | 7.1 ± 0.7 |
| Analogue of mean circulatory filling pressure (mmHg) | 13.1 ± 0.9 |
| Pressure gradient for venous return (mmHg) | 5.9 ± 0.8 |
| Resistance to venous return (mmHg min−1 L−1) | 1.2 ± 0.2 |
| Oxygen delivery (mL min−1) | 973.8 ± 116.2 |
| Oxygen consumption (mL min−1) | 247.4 ± 35.6 |
| Oxygen extraction ratio (%) | 25.8 ± 2.3 |
| Fraction of inspired oxygen (%) | 0.3 ± 0.03 |
| pH | 7.39 ± 0.02 |
| PaO2 (mmHg) | 92.5 ± 5.1 |
| PaCO2 (mmHg) | 39.2 ± 1.3 |
| HCO3 (mmol L−1) | 25.6 ± 1 |
| Base deficit (mmol L−1) | 2.08 ± 0.2 |
| Hemoglobin (g dL−1) | 14.1 ± 0.94 |
| Glucose (mg dL−1) | 113.6 ± 6.2 |
| Lactate (mmol L−1) | 0.8 ± 0.2 |
| SpO2 (%) | 99.6 ± 0.5 |
| SaO2 (%) | 100 ± 0.0 |
| ScvO2 (%) | 74.2 ± 2.3 |
| v-aPCO2 (mmHg) | 2.8 ± 0.9 |
| De Backer score (mm−1) | 3.7 ± 1.2 |
| De Backer score (small) (mm−1) | 2 ± 1.1 |
| Consensus PPV (%) | 94.2 ± 5.7 |
| Consensus PPV (small) (%) | 88.2 ± 10 |
| Vessel length (μm) | 137.3 ± 96.8 |
| Vessel diameter (μm) | 17.2 ± 4 |
| Velocity of red blood cells (μm s−1) | 15 ± 9 |
| Systemic Hemodynamic Variables | Spearman’s rho | p-Value |
| Cardiac output (L min−1) | 0.173 | 0.31 |
| Cardiac index (L min−1 m−2) | 0.145 | 0.41 |
| Mean arterial pressure (mmHg) | 0.398 | 0.012 |
| Systemic vascular resistance (dynes s cm−5) | 0.058 | 0.75 |
| Pressure gradient for venous return (mmHg) | 0.438 | 0.008 |
| Resistance of venous return (mmHg min−1 L−1) | 0.203 | 0.23 |
| Sublingual Microcirculation Variables | Spearman’s rho | p-Value |
| De Backer score (mm−1) | −0.189 | 0.27 |
| De Backer score (small) (mm−1) | 0.011 | 0.97 |
| Consensus PPV (%) | 0.017 | 0.95 |
| Consensus PPV (small) (%) | 0.06 | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalkias, A.; Xenos, M. Relationship of Effective Circulating Volume with Sublingual Red Blood Cell Velocity and Microvessel Pressure Difference: A Clinical Investigation and Computational Fluid Dynamics Modeling. J. Clin. Med. 2022, 11, 4885. https://doi.org/10.3390/jcm11164885
Chalkias A, Xenos M. Relationship of Effective Circulating Volume with Sublingual Red Blood Cell Velocity and Microvessel Pressure Difference: A Clinical Investigation and Computational Fluid Dynamics Modeling. Journal of Clinical Medicine. 2022; 11(16):4885. https://doi.org/10.3390/jcm11164885
Chicago/Turabian StyleChalkias, Athanasios, and Michalis Xenos. 2022. "Relationship of Effective Circulating Volume with Sublingual Red Blood Cell Velocity and Microvessel Pressure Difference: A Clinical Investigation and Computational Fluid Dynamics Modeling" Journal of Clinical Medicine 11, no. 16: 4885. https://doi.org/10.3390/jcm11164885
APA StyleChalkias, A., & Xenos, M. (2022). Relationship of Effective Circulating Volume with Sublingual Red Blood Cell Velocity and Microvessel Pressure Difference: A Clinical Investigation and Computational Fluid Dynamics Modeling. Journal of Clinical Medicine, 11(16), 4885. https://doi.org/10.3390/jcm11164885