Efficacy and Safety of Dimethyl Fumarate in Patients with Moderate-to-Severe Plaque Psoriasis: DIMESKIN-2, a Multicentre Single-Arm Phase IIIb Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design and Treatment
2.3. Efficacy Measures
2.4. Quality of Life Measures
2.5. Safety
2.6. Statistical Analysis and Sample Size
3. Results
3.1. Baseline Clinical Characteristics of Patients
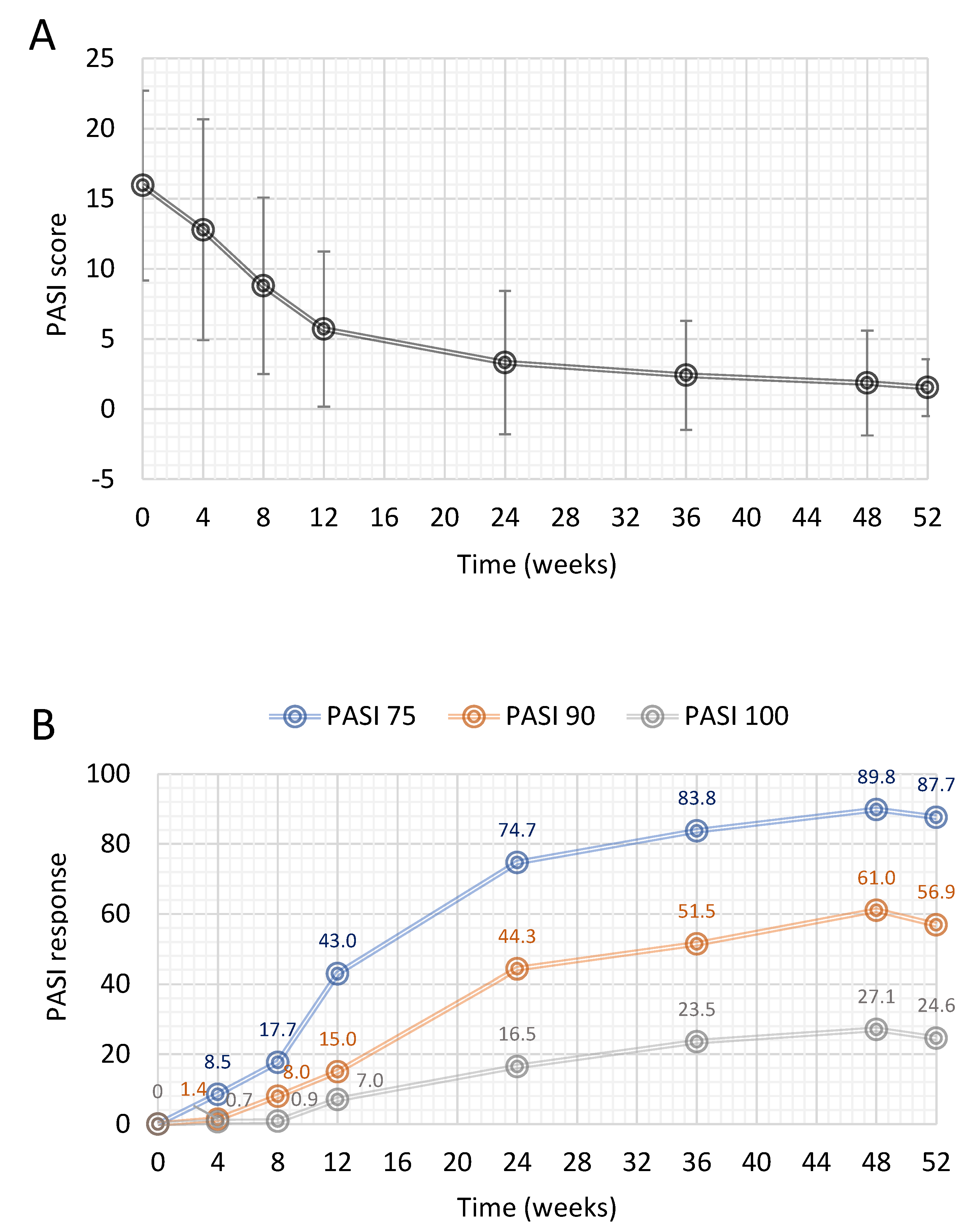
3.2. PASI Response
3.3. PGA and BSA
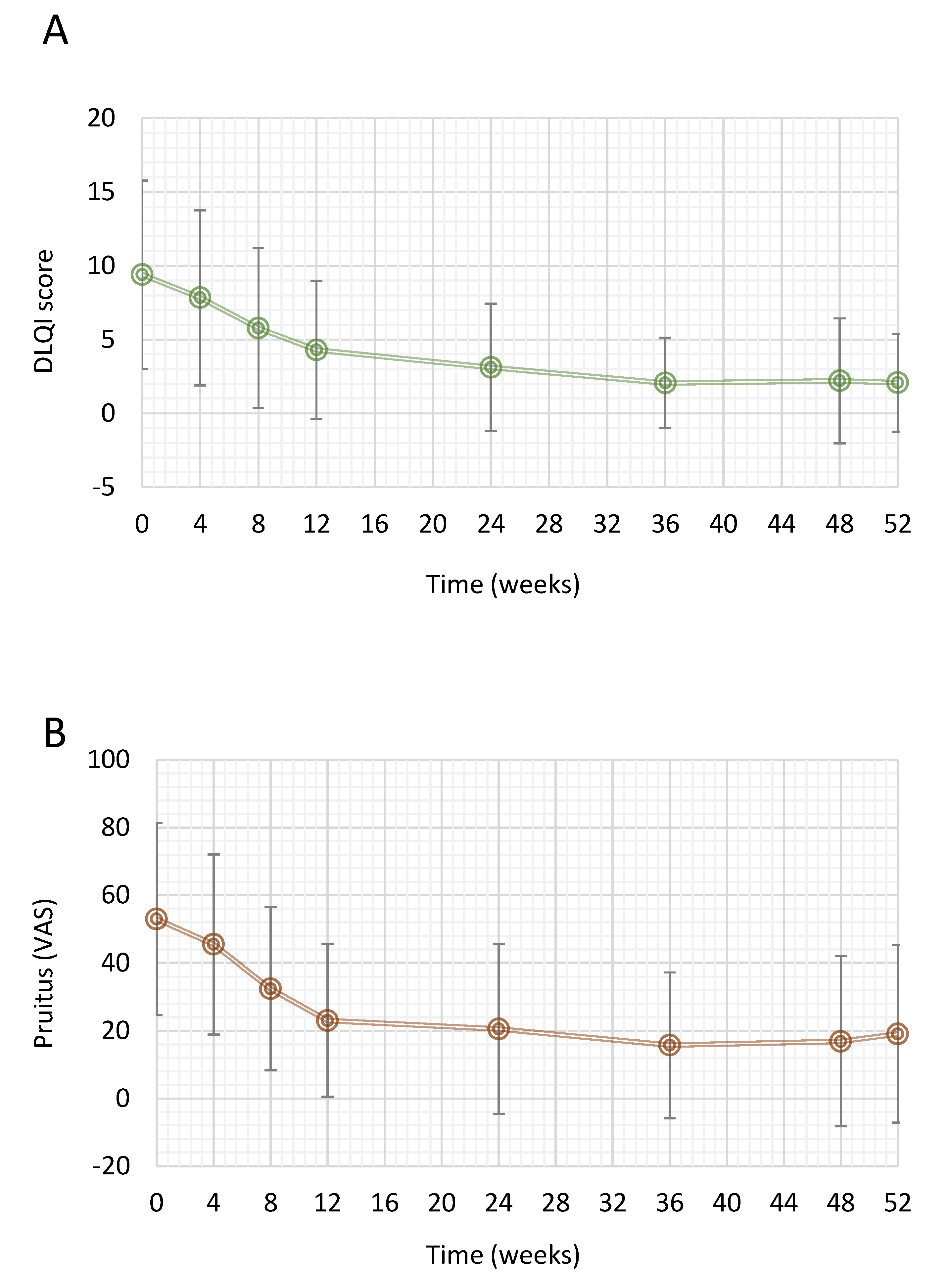
3.4. Quality of Life Measures
3.5. Safety
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Lewis-Beck, C.; Abouzaid, S.; Xie, L.; Baser, O.; Kim, E. Analysis of the Relationship between Psoriasis Symptom Severity and Quality of Life, Work Productivity, and Activity Impairment among Patients with Moderate-to-Severe Psoriasis Using Structural Equation Modeling. Patient Prefer. Adherence 2013, 7, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Altomare, G.; Ayala, F.; Bardazzi, F.; Bianchi, L.; Chiricozzi, A.; Costanzo, A.; Conti, A.; Dapavo, P.; de Simone, C.; et al. Italian Guidelines on the Systemic Treatments of Moderate-to-Severe Plaque Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 774–790. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; del Giglio, M.; Girolomoni, G. Treatment Approaches to Moderate to Severe Psoriasis. Int. J. Mol. Sci. 2017, 18, 2427. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Puig, L.; Joshi, A.; Skup, M.; Williams, D.; Li, J.; Betts, K.A.; Augustin, M. Comparison of Biologics and Oral Treatments for Plaque Psoriasis: A Meta-Analysis. JAMA Dermatol. 2020, 156, 258–269. [Google Scholar] [CrossRef]
- Kimball, A.B.; Jacobson, C.; Weiss, S.; Vreeland, M.G.; Wu, Y. The Psychosocial Burden of Psoriasis. Am. J. Clin. Dermatol. 2005, 6, 383–392. [Google Scholar] [CrossRef]
- Lockwood, S.J.; Prens, L.M.; Kimball, A.B. Adverse Reactions to Biologics in Psoriasis. Curr. Probl. Dermatol. 2018, 53, 1–14. [Google Scholar] [CrossRef]
- Kamata, M.; Tada, Y. Safety of Biologics in Psoriasis. J. Dermatol. 2018, 45, 279–286. [Google Scholar] [CrossRef]
- Mrowietz, U.; Szepietowski, J.C.; Loewe, R.; van de Kerkhof, P.; Lamarca, R.; Ocker, W.G.; Tebbs, V.M.; Pau-Charles, I. Efficacy and Safety of LAS41008 (Dimethyl Fumarate) in Adults with Moderate-to-Severe Chronic Plaque Psoriasis: A Randomized, Double-Blind, Fumaderm®- and Placebo-Controlled Trial (BRIDGE). Br. J. Dermatol. 2017, 176, 615–623. [Google Scholar] [CrossRef]
- Inzinger, M.; Weger, W.; Heschl, B.; Salmhofer, W.; Quehenberger, F.; Wolf, P. Methotrexate vs. Fumaric Acid Esters in Moderate-to-Severe Chronic Plaque Psoriasis: Data Registry Report on the Efficacy under Daily Life Conditions. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.; Adamczyk, A.; Kellerer, C.; Belge, K.; Brück, J.; Berner, T.; Merten, K.; Núnez Gõmez, N.; Neureither, M.; Röcken, M.; et al. Fumaderm® in Daily Practice for Psoriasis: Dosing, Efficacy and Quality of Life. Br. J. Dermatol. 2014, 171, 1197–1205. [Google Scholar] [CrossRef]
- Moharregh-Khiabani, D.; Linker, R.; Gold, R.; Stangel, M. Fumaric Acid and Its Esters: An Emerging Treatment for Multiple Sclerosis. Curr. Neuropharmacol. 2009, 7, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Mrowietz, U.; Radtke, M.A.; Thaci, D.; Rustenbach, S.J.; Spehr, C.; Augustin, M. Drug Safety of Systemic Treatments for Psoriasis: Results from The German Psoriasis Registry PsoBest. Arch. Dermatol. Res. 2015, 307, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.W.; Griffiths, C.E.M. Novel Systemic Therapies for the Treatment of Psoriasis. Expert Opin. Pharmacother. 2016, 17, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Malara, G.; Fabbrocini, G.; Trifirò, C.; Burlando, M. Dimethyl Fumarate Titration for the Systemic Treatment of Moderate-to-Severe Plaque Psoriasis. Drugs Context 2021, 10, 2020-12-14. [Google Scholar] [CrossRef]
- Corazza, M.; Odorici, G.; Conti, A.; di Lernia, V.; Motolese, A.; Bardazzi, F.; di Nuzzo, S.; Monti, A.; Arginelli, F.; Filippi, F.; et al. Dimethyl Fumarate Treatment for Psoriasis in a Real-Life Setting: A Multicentric Retrospective Study. Dermatol. Ther. 2021, 34, e15066. [Google Scholar] [CrossRef]
- Mrowietz, U.; Kragballe, K.; Reich, K.; Spuls, P.; Griffiths, C.E.M.; Nast, A.; Franke, J.; Antoniou, C.; Arenberger, P.; Balieva, F.; et al. Definition of Treatment Goals for Moderate to Severe Psoriasis: A European Consensus. Arch. Dermatol. Res. 2011, 303, 1–10. [Google Scholar] [CrossRef]
- EMA. Summary of Product Characteristics: Skilarence (Dimethyl Fumarate); EMA: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Feldman, S.; Krueger, G. Psoriasis Assessment Tools in Clinical Trials. Ann. Rheum. Dis. 2005, 64, ii65–ii68. [Google Scholar] [CrossRef]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—A Simple Practical Measure for Routine Clinical Use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Friedman, L.M.; Furberg, C.D.; DeMets, D.; Reboussin, D.M.; Granger, C.B. Fundamentals of Clinical Trials, 5th ed.; Springer International Publishing: New York, NY, USA, 2015; ISBN 978-3-319-18538-5. [Google Scholar]
- Atwan, A.; Ingram, J.R.; Abbott, R.; Kelson, M.J.; Pickles, T.; Bauer, A.; Piguet, V. Oral Fumaric Acid Esters for Psoriasis. Cochrane Database Syst. Rev. 2015, 2015, CD010497. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on Clinial Investigation of Medicinal Products Indicated for the Treatment of Psoriasis; EMA: Amsterdam, The Netherlands, 2004; pp. 1–18. [Google Scholar]
- Reich, K.; Thaci, D.; Mrowietz, U.; Kamps, A.; Neureither, M.; Luger, T. Efficacy and Safety of Fumaric Acid Esters in the Long-Term Treatment of Psoriasis—A Retrospective Study (FUTURE). JDDG J. Ger. Soc. Dermatol. 2009, 7, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, R.; Otters, E.; Balak, D.; Thio, B. Long-Term Safety and Effectiveness of High-Dose Dimethylfumarate in the Treatment of Moderate to Severe Psoriasis: A Prospective Single-Blinded Follow-up Study. J. Dermatol. Treat. 2016, 27, 31–36. [Google Scholar] [CrossRef]
- Lundberg, L.; Johannesson, M.; Silverdahl, M.; Hermansson, C.; Lindberg, M. Health-Related Quality of Life in Patients with Psoriasis and Atopic Dermatitis Measured with SF-36, DLQI and a Subjective Measure of Disease Activity. Acta Derm. Venereol. 2000, 80, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Van de Kerkhof, P.C.M.; Loewe, R.; Mrowietz, U.; Falques, M.; Pau-Charles, I.; Szepietowski, J.C. Quality of Life Outcomes in Adults with Moderate-to-Severe Plaque Psoriasis Treated with Dimethylfumarate (DMF): A Post Hoc Analysis of the BRIDGE Study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Khilji, F.; Gonzalez, M.; Finlay, A. Clinical Meaning of Change in Dermatology Life Quality Index Scores. Br. J. Dermatol. 2002, 147, 50. [Google Scholar]
- Basra, M.K.A.; Salek, M.S.; Camilleri, L.; Sturkey, R.; Finlay, A.Y. Determining the Minimal Clinically Important Difference and Responsiveness of the Dermatology Life Quality Index (DLQI): Further Data. Dermatology 2015, 230, 27–33. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Psoriasis: Assessment and Management; NICE: London, UK, 2017; pp. 1–56. [Google Scholar]
- Pathirana, D.; Ormerod, A.D.; Saiag, P.; Smith, C.; Spuls, P.I.; Nast, A.; Barker, J.; Bos, J.D.; Burmester, G.R.; Chimenti, S.; et al. European S3-Guidelines on the Systemic Treatment of Psoriasis Vulgaris. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1–70. [Google Scholar] [CrossRef]
- Mrowietz, U.; Barker, J.; Boehncke, W.H.; Iversen, L.; Kirby, B.; Naldi, L.; Reich, K.; Tanew, A.; van de Kerkhof, P.C.M.; Warren, R.B. Clinical Use of Dimethyl Fumarate in Moderate-to-Severe Plaque-Type Psoriasis: A European Expert Consensus. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 3–14. [Google Scholar] [CrossRef]
- Szepietowski, J.C.; Reich, A. Pruritus in Psoriasis: An Update. Eur. J. Pain 2016, 20, 41–46. [Google Scholar] [CrossRef]
- Théréné, C.; Brenaut, E.; Barnetche, T.; Misery, L. Efficacy of Systemic Treatments of Psoriasis on Pruritus: A Systemic Literature Review and Meta-Analysis. J. Investig. Dermatol. 2018, 138, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Shikiar, R.; Rentz, A.M. Satisfaction with Medication: An Overview of Conceptual, Methodologic, and Regulatory Issues. Value Health 2004, 7, 204–215. [Google Scholar] [CrossRef] [PubMed]
- ICH. ICH E1 Population Exposure: The Extent of Population Exposure to Assess Clinical Safety. Available online: https://database.ich.org/sites/default/files/E1_Guideline.pdf (accessed on 7 September 2021).
- Pezzolo, E.; Cazzaniga, S.; di Leo, S.; Naldi, L.; PsoReal Study Group. Efficacy and Safety of Dimethyl Fumarate in Comparison with Conventional Therapy for Psoriasis: An Italian Real-World Clinical Experience. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e534–e537. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Dimethyl Fumarate: A Review in Moderate to Severe Plaque Psoriasis. Drugs 2018, 78, 123–130. [Google Scholar] [CrossRef]
- European Medicines Agency. Summary of Product Characteristics: Skilarence 30 Mg Gastro-Resistant Tablets, Skilarence 120 Mg Gastro-Resistant Tablets. Available online: http://www.ema.europa.eu (accessed on 27 May 2021).
- Balak, D.M.W.; Fallah Arani, S.; Hajdarbegovic, E.; Hagemans, C.A.F.; Bramer, W.M.; Thio, H.B.; Neumann, H.A.M. Efficacy, Effectiveness and Safety of Fumaric Acid Esters in the Treatment of Psoriasis: A Systematic Review of Randomized and Observational Studies. Br. J. Dermatol. 2016, 175, 250–262. [Google Scholar] [CrossRef]
- Augustin, M.; Landeck, L.; Diemert, S.; Asadullah, K.; Hammann, U.; Ertner, K.; Hadshiew, I. Long-Term Treatment with Dimethyl Fumarate for Plaque Psoriasis in Routine Practice: Good Overall Effectiveness and Positive Effect on Impactful Areas. Dermatol. Ther. 2022, 12, 1121–1131. [Google Scholar] [CrossRef]
- Burlando, M.; Molle, M.F.; Cozzani, E.; Parodi, A. Dimethyl Fumarate as a Safe and Effective Therapy for Recalcitrant Psoriasis in Comorbid Patients. Dermatol. Rep. 2022, 14, 9091. [Google Scholar] [CrossRef]
- Roy, S.; Chen, N.; Cifaldi, M. PMS70 a comparison of non-responder imputation and last-observation-carried-forward analysis methods in rheumatoid arthritis clinical trials. Value Health 2011, 14, A136. [Google Scholar] [CrossRef][Green Version]
- Langley, R.G.B.; Reich, K.; Papavassilis, C.; Fox, T.; Gong, Y.; Gu Ttner, A. Methods for Imputing Missing Efficacy Data in Clinical Trials of Biologic Psoriasis Therapies: Implications for Interpretations of Trial Results. J. Drugs Dermatol. 2017, 16, 734–741. [Google Scholar]
- Kamata, M.; Tada, Y. Efficacy and Safety of Biologics for Psoriasis and Psoriatic Arthritis and Their Impact on Comorbidities: A Literature Review. Int. J. Mol. Sci. 2020, 21, 1690. [Google Scholar] [CrossRef]
- Kaushik, S.B.; Lebwohl, M.G. Psoriasis: Which Therapy for Which Patient: Psoriasis Comorbidities and Preferred Systemic Agents. J. Am. Acad. Dermatol. 2019, 80, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.B.; Lebwohl, M.G. Psoriasis: Which Therapy for Which Patient: Focus on Special Populations and Chronic Infections. J. Am. Acad. Dermatol. 2019, 80, 43–53. [Google Scholar] [CrossRef] [PubMed]

| Clinical Characteristic | n = 141 |
|---|---|
| General | |
| Male gender, n (%) | 94 (66.7) |
| Age (years) | 49.1 ± 14.7 |
| BMI (kg/m2) | 26.6 ± 5.2 |
| Current cigarette smoker, n (%) | 60 (43.8) |
| Disease characteristics | |
| Disease duration | 16 ± 12.1 |
| PASI at baseline | 15.9 ± 6.8 |
| BSA affected (%) | 26.5 ± 14.8 |
| PGA index * | 3.4 ± 0.65 |
| Moderate disease | 89 (63.1) |
| Severe disease | 41 (29.1) |
| Very severe disease | 9 (6.4) |
| DLQI score | 9.4 ± 6.4 |
| Pruritus (VAS) | 52 ± 28.4 |
| Previous treatment n, (%) * | 75 (53.2) |
| Topical | 55 (39) |
| Antipsoriatics | 35 (24.8) |
| Corticosteroids | 19 (13.5) |
| Emollients/protectives | 1 (0.71) |
| Systemic | 28 (19.9) |
| Antipsoriatics | 10 (7.1) |
| Corticosteroids | 2 (1.4) |
| Immunosuppressants | 14 (9.9) |
| Phototherapy | 2 (1.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellacani, G.; Bigi, L.; Parodi, A.; Burlando, M.; Lanna, C.; Campione, E.; Rongioletti, F.; Mugheddu, C.; Malara, G.; Moretti, G.; et al. Efficacy and Safety of Dimethyl Fumarate in Patients with Moderate-to-Severe Plaque Psoriasis: DIMESKIN-2, a Multicentre Single-Arm Phase IIIb Study. J. Clin. Med. 2022, 11, 4778. https://doi.org/10.3390/jcm11164778
Pellacani G, Bigi L, Parodi A, Burlando M, Lanna C, Campione E, Rongioletti F, Mugheddu C, Malara G, Moretti G, et al. Efficacy and Safety of Dimethyl Fumarate in Patients with Moderate-to-Severe Plaque Psoriasis: DIMESKIN-2, a Multicentre Single-Arm Phase IIIb Study. Journal of Clinical Medicine. 2022; 11(16):4778. https://doi.org/10.3390/jcm11164778
Chicago/Turabian StylePellacani, Giovanni, Laura Bigi, Aurora Parodi, Martina Burlando, Caterina Lanna, Elena Campione, Franco Rongioletti, Cristina Mugheddu, Giovanna Malara, Giovanna Moretti, and et al. 2022. "Efficacy and Safety of Dimethyl Fumarate in Patients with Moderate-to-Severe Plaque Psoriasis: DIMESKIN-2, a Multicentre Single-Arm Phase IIIb Study" Journal of Clinical Medicine 11, no. 16: 4778. https://doi.org/10.3390/jcm11164778
APA StylePellacani, G., Bigi, L., Parodi, A., Burlando, M., Lanna, C., Campione, E., Rongioletti, F., Mugheddu, C., Malara, G., Moretti, G., Stingeni, L., Hansel, K., Micali, G., Naldi, L., Pirro, F., & Peris, K. (2022). Efficacy and Safety of Dimethyl Fumarate in Patients with Moderate-to-Severe Plaque Psoriasis: DIMESKIN-2, a Multicentre Single-Arm Phase IIIb Study. Journal of Clinical Medicine, 11(16), 4778. https://doi.org/10.3390/jcm11164778








