Early Serum Creatinine Levels after Aneurysmal Subarachnoid Hemorrhage Predict Functional Neurological Outcome after 6 Months
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population and Clinical Management
2.2. Laboratory Examinations
2.3. Statistical Analysis
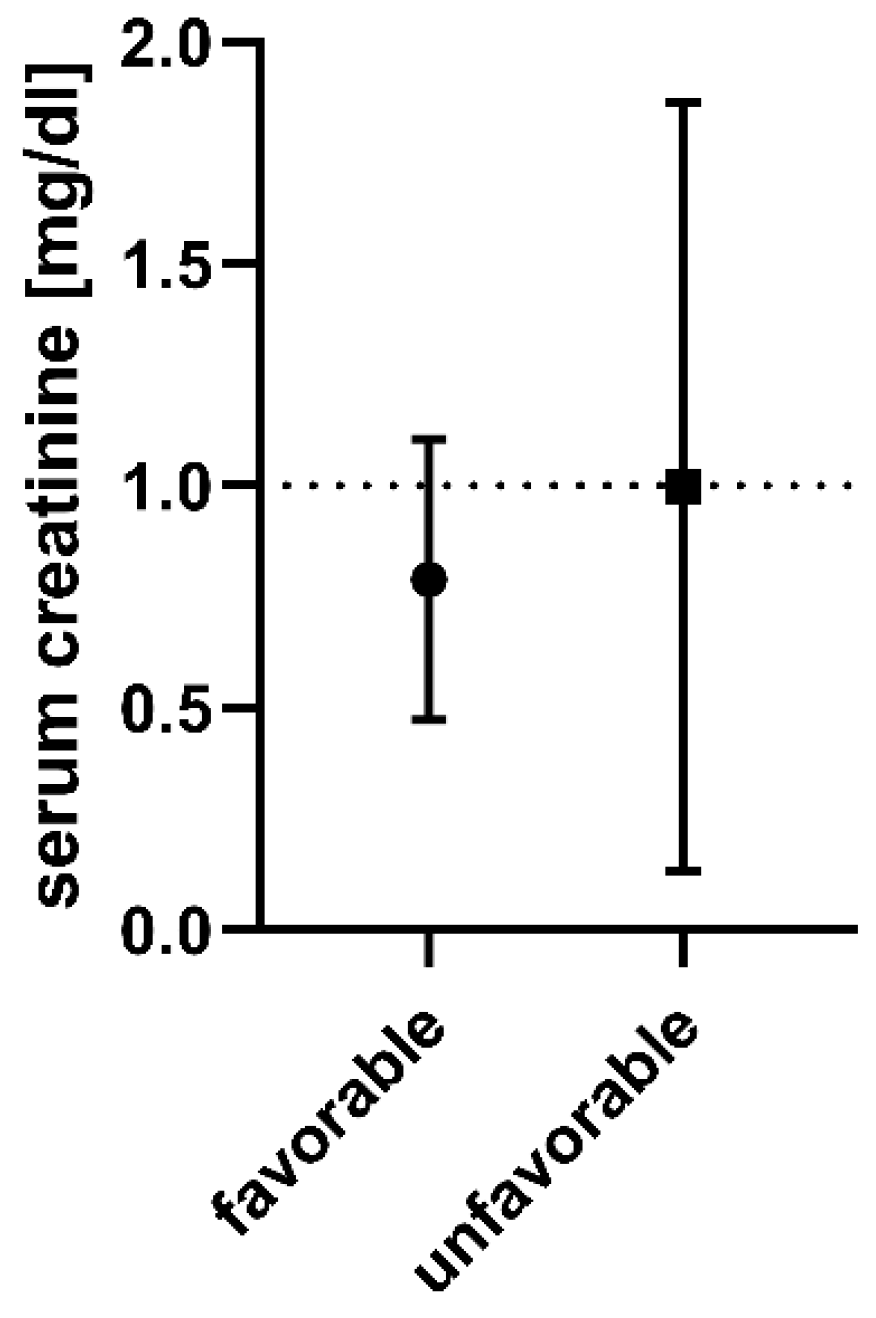
3. Results
3.1. Univariate Analysis
3.2. Renal Function Prior to SAH
3.3. Multivariable Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Connolly, E.S.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.C.; Selvin, S.; Gress, D.R. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 1998, 50, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Clermont, G.; Kersten, A.; Venkataraman, R.; Angus, D.C.; De Bacquer, D.; Kellum, J.A. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit. Care 2006, 10, R73. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef]
- Güresir, E.; Beck, J.; Vatter, H.; Setzer, M.; Gerlach, R.; Seifert, V.; Raabe, A. Subarachnoid hemorrhage and intracerebral hematoma: Incidence, prognostic factors, and outcome. Neurosurgery 2008, 63, 1088–1093, discussion 1093–4. [Google Scholar] [CrossRef] [PubMed]
- Güresir, E.; Schuss, P.; Berkefeld, J.; Vatter, H.; Seifert, V. Treatment results for complex middle cerebral artery aneurysms. A prospective single-center series. Acta Neurochir. 2011, 153, 1247–1252. [Google Scholar] [CrossRef]
- Raabe, A.; Beck, J.; Keller, M.; Vatter, H.; Zimmermann, M.; Seifert, V. Relative importance of hypertension compared with hypervolemia for increasing cerebral oxygenation in patients with cerebral vasospasm after subarachnoid hemorrhage. J. Neurosurg. 2005, 103, 974–981. [Google Scholar] [CrossRef]
- Bruder, M.; Schuss, P.; Berkefeld, J.; Wagner, M.; Vatter, H.; Seifert, V.; Güresir, E. Subarachnoid hemorrhage and intracerebral hematoma caused by aneurysms of the anterior circulation: Influence of hematoma localization on outcome. Neurosurg. Rev. 2014, 37, 653–659. [Google Scholar] [CrossRef]
- Schuss, P.; Konczalla, J.; Platz, J.; Vatter, H.; Seifert, V.; Güresir, E. Aneurysm-related subarachnoid hemorrhage and acute subdural hematoma: Single-center series and systematic review. J. Neurosurg. 2013, 118, 984–990. [Google Scholar] [CrossRef]
- Güresir, E.; Schuss, P.; Vatter, H.; Raabe, A.; Seifert, V.; Beck, J. Decompressive craniectomy in subarachnoid hemorrhage. Neurosurg. Focus 2009, 26, E4. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Lameire, N.H.; Vanholder, R.C.; Benoit, D.D.; Decruyenaere, J.M.A.; Colardyn, F.A. Acute renal failure in patients with sepsis in a surgical ICU: Predictive factors, incidence, comorbidity, and outcome. J. Am. Soc. Nephrol. 2003, 14, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.; Reinprecht, A.; Illievich, U.M.; Fitzgerald, R.; Dietrich, W.; Czech, T.; Richling, B. Extracerebral organ dysfunction and neurologic outcome after aneurysmal subarachnoid hemorrhage. Crit. Care Med. 1999, 27, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Solenski, N.J.; Haley, E.C.; Kassell, N.F.; Kongable, G.; Germanson, T.; Truskowski, L.; Torner, J.C. Medical complications of aneurysmal subarachnoid hemorrhage: A report of the multicenter, cooperative aneurysm study. Participants of the Multicenter Cooperative Aneurysm Study. Crit. Care Med. 1995, 23, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Zygun, D.A.; Doig, C.J.; Gupta, A.K.; Whiting, G.; Nicholas, C.; Shepherd, E.; Conway-Smith, C.; Menon, D.K. Non-neurological organ dysfunction in neurocritical care. J. Crit. Care 2003, 18, 238–244. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; Wu, H.; Krafft, P.R.; Wang, Z.; Zhang, J.H. The harmful effects of subarachnoid hemorrhage on extracerebral organs. BioMed Res. Int. 2014, 2014, 858496. [Google Scholar] [CrossRef]
- Tujjar, O.; Belloni, I.; Hougardy, J.-M.; Scolletta, S.; Vincent, J.-L.; Creteur, J.; Taccone, F.S. Acute Kidney Injury After Subarachnoid Hemorrhage. J. Neurosurg. Anesthesiol. 2017, 29, 140–149. [Google Scholar] [CrossRef]
- Zacharia, B.E.; Ducruet, A.F.; Hickman, Z.L.; Grobelny, B.T.; Fernandez, L.; Schmidt, J.M.; Narula, R.; Ko, L.N.; Cohen, M.E.; Mayer, S.A.; et al. Renal dysfunction as an independent predictor of outcome after aneurysmal subarachnoid hemorrhage: A single-center cohort study. Stroke 2009, 40, 2375–2381. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef]
- Levin, A.; Warnock, D.G.; Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C. Improving outcomes from acute kidney injury: Report of an initiative. Am. J. Kidney Dis. 2007, 22, 1655–1658. [Google Scholar] [CrossRef]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed]
- Molitoris, B.A.; Levin, A.; Warnock, D.G.; Joannidis, M.; Mehta, R.L.; Kellum, J.A.; Ronco, C.; Shah, S. Improving outcomes from acute kidney injury. J. Am. Soc. Nephrol. 2007, 18, 1992–1994. [Google Scholar] [CrossRef]
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef]
- Eagles, M.E.; Powell, M.F.; Ayling, O.G.S.; Tso, M.K.; Macdonald, R.L. Acute kidney injury after aneurysmal subarachnoid hemorrhage and its effect on patient outcome: An exploratory analysis. J. Neurosurg. 2019, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Libório, A.B.; Leite, T.T.; Neves, F.M.d.O.; Teles, F.; Bezerra, C.T.d.M. AKI complications in critically ill patients: Association with mortality rates and RRT. Clin. J. Am. Soc. Nephrol. 2015, 10, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Terao, Y.; Takada, M.; Tanabe, T.; Ando, Y.; Fukusaki, M.; Sumikawa, K. Microalbuminuria is a prognostic predictor in aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2007, 33, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Rau, C.-S.; Wu, S.-C.; Chien, P.-C.; Kuo, P.-J.; Chen, Y.-C.; Hsieh, H.-Y.; Hsieh, C.-H. Prediction of Mortality in Patients with Isolated Traumatic Subarachnoid Hemorrhage Using a Decision Tree Classifier: A Retrospective Analysis Based on a Trauma Registry System. Int. J. Environ. Res. Public Health 2017, 14, 1420. [Google Scholar] [CrossRef]
- Albanna, W.; Weiss, M.; Veldeman, M.; Conzen, C.; Schmidt, T.; Blume, C.; Zayat, R.; Clusmann, H.; Stoppe, C.; Schubert, G.A. Urea-Creatinine Ratio (UCR) After Aneurysmal Subarachnoid Hemorrhage: Association of Protein Catabolism with Complication Rate and Outcome. World Neurosurg. 2021, 151, e961–e971. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n = 369 | (%) |
|---|---|---|
| Mean age (±SD) [in years] | 56 | ±13.6 |
| Sex (Female) | 246 | (67) |
| Current smoking | 155 | (42) |
| Hypertension | 153 | (42) |
| Mean GCS (±SD) | 10.4 | ± 5 |
| Poor-grade SAH (WFNS grade IV + V) | 151 | (41) |
| Mean creatinine on admission (±SD) [in mg/dL] | 0.89 | ±0.67 |
| Early hydrocephalus | 248 | (67) |
| Aneurysm location | ||
| Acom/ACA | 152 | (41) |
| ICA | 86 | (23) |
| MCA | 73 | (20) |
| Posterior circulation | 58 | (16) |
| Mean aneurysm size (±SD) [in mm] | 7.7 | ±5 |
| Treatment | ||
| Surgical | 151 | (41) |
| Endovascular | 195 | (53) |
| No treatment | 23 | (6) |
| Vasospasm | 184 | (50) |
| DCI | 56 | (15) |
| Mean length of ICU stay (±SD) [in days] | 16.6 | ±15.4 |
| Favorable Outcome (mRS ≤ 2) | 185 | (50) |
| Low sCr | High sCr | p-Value | |||
|---|---|---|---|---|---|
| n = 297 | (%) | n = 72 | (%) | ||
| Mean age (± SD) [in years] | 55 | ±13 | 60 | ±15 | 0.005 |
| Sex (Female) | 220 | (74) | 26 | (36) | <0.001 |
| Current smoking | 124 | (42) | 31 | (44) | 0.77 |
| Hypertension | 116 | (39) | 37 | (51) | 0.06 |
| Mean GCS (±SD) | 10.7 | ±4.9 | 8.8 | ±5.4 | 0.003 |
| Poor-grade SAH (WFNS grade IV + V) | 111 | (37) | 40 | (56) | 0.007 |
| Mean creatinine on admission (± SD) [in mg/dL] | 0.72 | ±0.15 | 1.61 | ±1.25 | <0.001 |
| Early hydrocephalus | 198 | (67) | 50 | (69) | 0.68 |
| Aneurysm location | 0.24 | ||||
| Acom/ACA | 120 | (40) | 32 | (44) | |
| ICA | 70 | (24) | 16 | (22) | |
| MCA | 64 | (22) | 9 | (13) | |
| Posterior circulation | 43 | (14) | 15 | (21) | |
| Mean aneurysm size (± SD) [in mm] | 7.6 | ±4.9 | 8 | ±5.3 | 0.59 |
| Treatment | 0.017 | ||||
| Surgical | 126 | (35) | 25 | (42) | |
| Endovascular | 158 | (53) | 37 | (51) | |
| No treatment | 13 | (4) | 10 | (14) | |
| Vasospasm | 156 | (53) | 28 | (39) | 0.048 |
| DCI | 51 | (17) | 5 | (7) | 0.029 |
| Mean length of ICU stay (± SD) [in days] | 17 | ±16 | 14.8 | ±12.7 | 0.29 |
| Favorable Outcome (mRS ≤ 2) | 163 | (55) | 22 | (31) | <0.001 |
| Low sCr | High sCr | p-Value | |||
|---|---|---|---|---|---|
| n = 284 | (%) | n = 62 | (%) | ||
| Mean age (±SD) [in years] | 55 | ±13 | 60 | ±15 | 0.003 |
| Sex (Female) | 209 | (74) | 23 | (37) | <0.001 |
| Current smoking | 120 | (42) | 26 | (42) | 1 |
| Hypertension | 112 | (39) | 32 | (51) | 0.09 |
| Mean GCS (± SD) | 11 | ±5.2 | 9.5 | ±5.3 | 0.038 |
| Poor-grade SAH (WFNS grade IV + V) | 99 | (35) | 31 | (50) | 0.03 |
| Mean creatinine on admission (±SD) [in mg/dL] | 0.71 | ±0.15 | 1.5 | ±1.03 | <0.001 |
| Early hydrocephalus | 188 | (66) | 44 | (71) | 0.55 |
| Aneurysm location | 0.2 | ||||
| Acom/ACA | 117 | (41) | 30 | (48) | |
| ICA | 70 | (25) | 13 | (21) | |
| MCA | 59 | (21) | 7 | (11) | |
| Posterior circulation | 13 | (13) | 12 | (20) | |
| Mean aneurysm size (±SD) [in mm] | 7.5 | ±4.8 | 7.9 | ±5.6 | 0.54 |
| Treatment | 0.58 | ||||
| Surgical | 126 | (44) | 25 | (40) | |
| Endovascular | 158 | (56) | 37 | (60) | |
| Vasospasm | 153 | (54) | 28 | (45) | 0.26 |
| DCI | 51 | (18) | 5 | (8) | 0.06 |
| Mean length of ICU stay (± SD) [in days] | 17.4 | ±16.1 | 16.7 | ±12.7 | 0.76 |
| Favorable Outcome (mRS ≤ 2) | 162 | (57) | 22 | (36) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampmann, T.; Hadjiathanasiou, A.; Asoglu, H.; Wach, J.; Kern, T.; Vatter, H.; Güresir, E. Early Serum Creatinine Levels after Aneurysmal Subarachnoid Hemorrhage Predict Functional Neurological Outcome after 6 Months. J. Clin. Med. 2022, 11, 4753. https://doi.org/10.3390/jcm11164753
Lampmann T, Hadjiathanasiou A, Asoglu H, Wach J, Kern T, Vatter H, Güresir E. Early Serum Creatinine Levels after Aneurysmal Subarachnoid Hemorrhage Predict Functional Neurological Outcome after 6 Months. Journal of Clinical Medicine. 2022; 11(16):4753. https://doi.org/10.3390/jcm11164753
Chicago/Turabian StyleLampmann, Tim, Alexis Hadjiathanasiou, Harun Asoglu, Johannes Wach, Tamara Kern, Hartmut Vatter, and Erdem Güresir. 2022. "Early Serum Creatinine Levels after Aneurysmal Subarachnoid Hemorrhage Predict Functional Neurological Outcome after 6 Months" Journal of Clinical Medicine 11, no. 16: 4753. https://doi.org/10.3390/jcm11164753
APA StyleLampmann, T., Hadjiathanasiou, A., Asoglu, H., Wach, J., Kern, T., Vatter, H., & Güresir, E. (2022). Early Serum Creatinine Levels after Aneurysmal Subarachnoid Hemorrhage Predict Functional Neurological Outcome after 6 Months. Journal of Clinical Medicine, 11(16), 4753. https://doi.org/10.3390/jcm11164753







