Application of Machine Learning in Hospitalized Patients with Severe COVID-19 Treated with Tocilizumab
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design and Population
2.3. Study Data
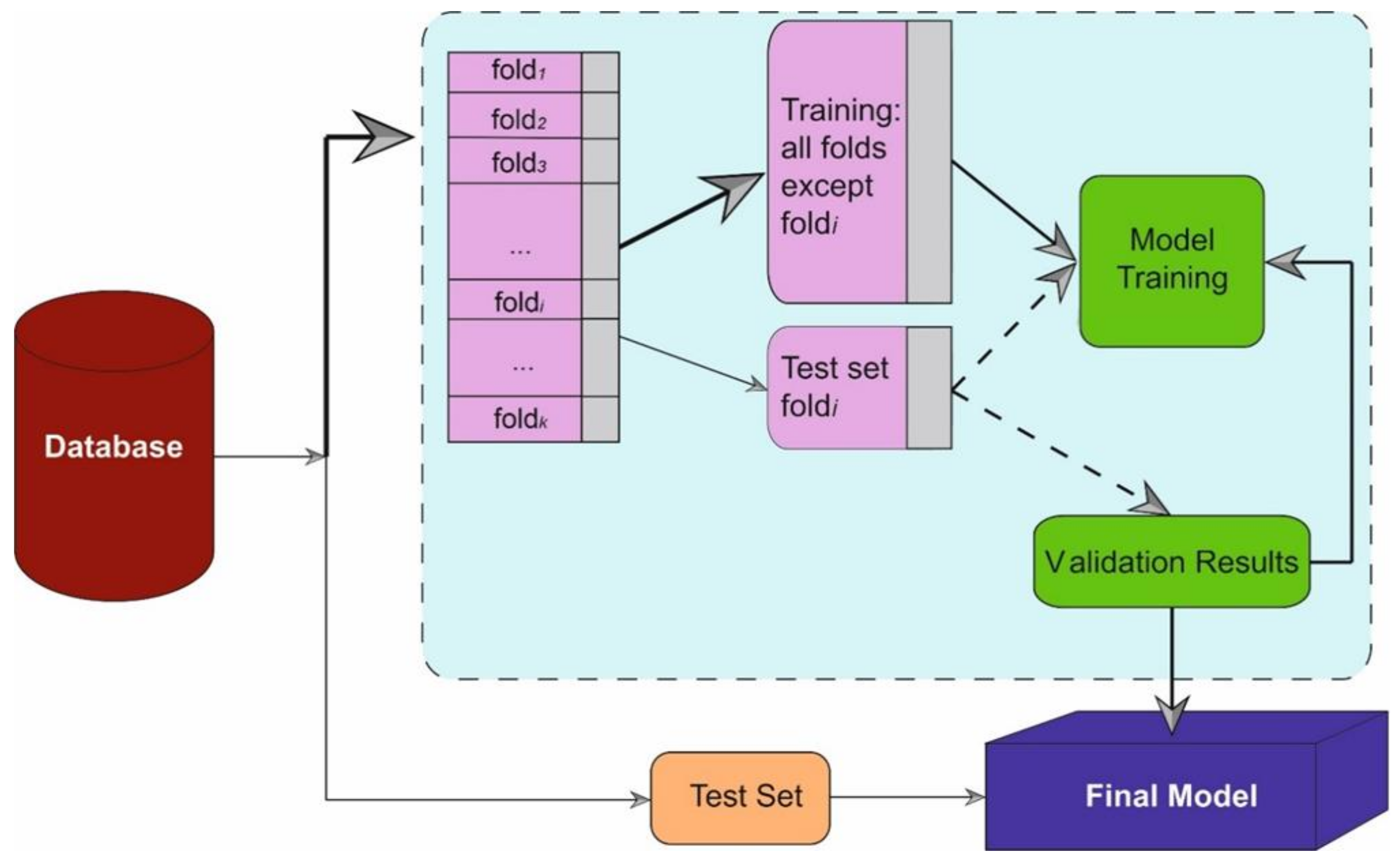
2.4. Method
2.4.1. Model Development
2.4.2. Performance Evaluation
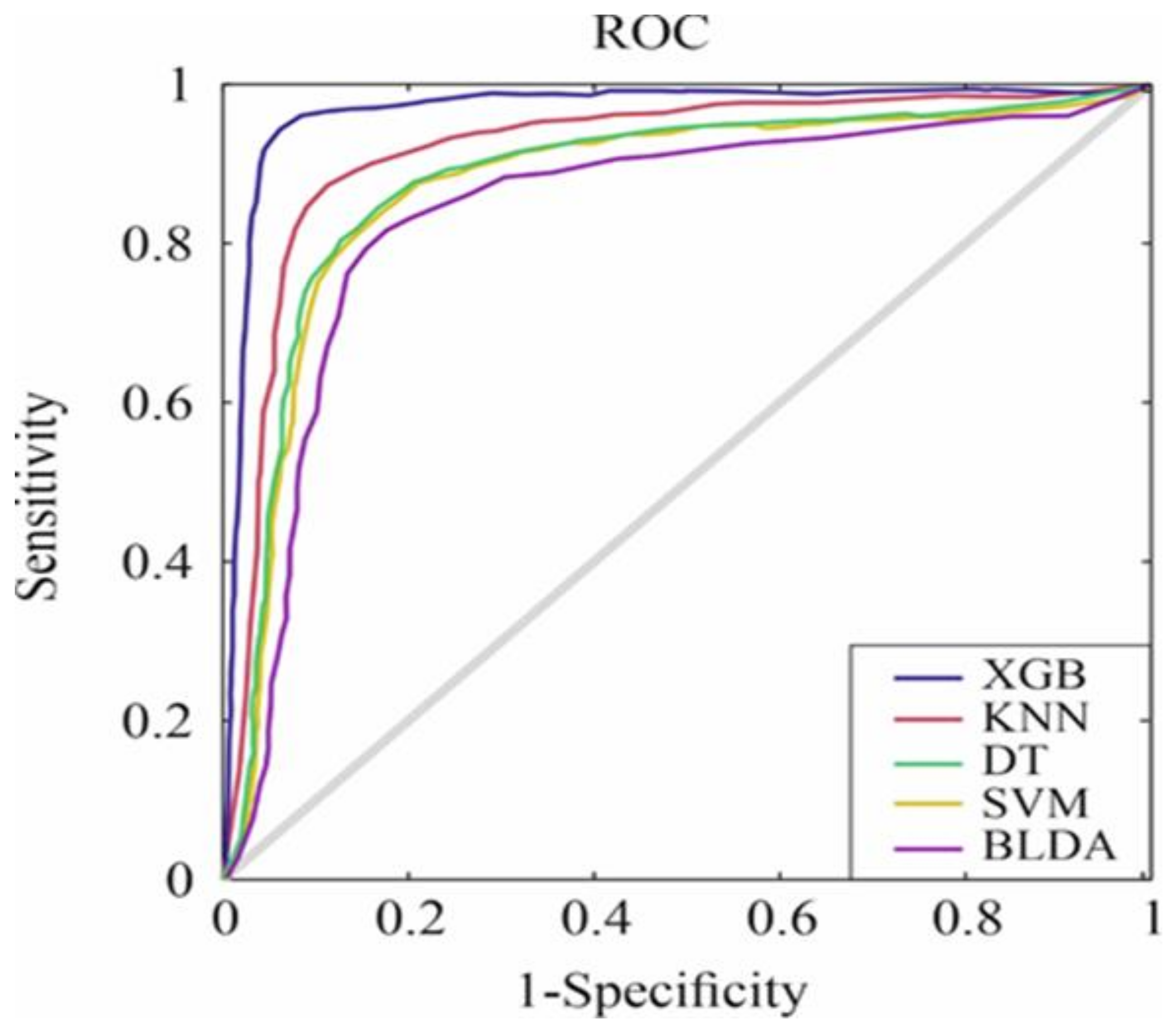
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Thakur, V.; Ratho, R.K.; Kumar, P.; Bhatia, S.K.; Bora, I.; Mohi, G.K.; Saxena, S.K.; Devi, M.; Yadav, D.; Mehariya, S. Multi-Organ Involvement in COVID-19: Beyond Pulmonary Manifestations. J. Clin. Med. 2021, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Verma, N.; Singh, T.G.; Grewal, A.K.; Kanojia, N.; Rani, L. COVID-19-Associated acute respiratory distress syndrome (CARDS): Mechanistic insights on therapeutic intervention and emerging trends. Int. Immunopharmacol. 2021, 101, 108328. [Google Scholar] [CrossRef] [PubMed]
- Elahi, R.; Karami, P.; Heidary, A.H.; Esmaeilzadeh, A. An updated overview of recent advances, challenges, and clinical considerations of IL-6 signaling blockade in severe coronavirus disease 2019 (COVID-19). Int. Immunopharmacol. 2022, 105, 108536. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, H.; Ou, C.; Liang, J.; Wang, Y.; Jiang, M.; Li, S. Clinical symptoms, comorbidities and complications in severe and non-severe patients with COVID-19: A systematic review and meta-analysis without cases duplication. Medicine 2020, 99, e23327. [Google Scholar] [CrossRef]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Azkur, A.K.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Melo, A.; Milby, K.M.; Caparroz, A.; Pinto, A.; Santos, R.; Rocha, A.P.; Ferreira, G.A.; Souza, V.A.; Valadares, L.; Vieira, R.; et al. Biomarkers of cytokine storm as red flags for severe and fatal COVID-19 cases: A living systematic review and meta-analysis. PLoS ONE 2021, 16, e0253894. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Mao, Z.; Xiao, M.; Wang, L.; Qi, S.; Zhou, F. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: A systematic review and meta-analysis. Crit. Care 2020, 24, 647. [Google Scholar] [CrossRef]
- Gustine, J.N.; Jones, D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2021, 191, 4–17. [Google Scholar] [CrossRef]
- Dorward, D.A.; Russell, C.D.; Um, I.H.; Elshani, M.; Armstrong, S.D.; Penrice-Randal, R.; Millar, T.; Lerpiniere, C.; Tagliavini, G.; Hartley, C.S.; et al. Tissue-Specific Immunopathology in Fatal COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 192–201. [Google Scholar] [CrossRef]
- Saha, A.; Sharma, A.R.; Bhattacharya, M.; Sharma, G.; Lee, S.S.; Chakraborty, C. Tocilizumab: A Therapeutic Option for the Treatment of Cytokine Storm Syndrome in COVID-19. Arch. Med. Res. 2020, 51, 595–597. [Google Scholar] [CrossRef]
- Masotti, L.; Landini, G.; Panigada, G.; Grifoni, E.; Tarquini, R.; Cei, F.; Cimolato, B.; Vannucchi, V.; Di Pietro, M.; Piani, F.; et al. Predictors of poor outcome in tocilizumab treated patients with Sars-CoV-2 related severe respiratory failure: A multicentre real world study. Int. Immunopharmacol. 2022, 107, 108709. [Google Scholar] [CrossRef]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Shankar-Hari, M.; Vale, C.L.; Godolphin, P.J.; Fisher, D.; Higgins, J.; Spiga, F.; Savovic, J.; Tierney, J.; Baron, G.; et al. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA 2021, 326, 499–518. [Google Scholar] [CrossRef]
- Luo, L.; Luo, T.; Du, M.; Mei, H.; Hu, Y. Efficacy and safety of tocilizumab in hospitalized COVID-19 patients: A systematic review and meta-analysis. J. Infect. 2022, 84, 418–467. [Google Scholar] [CrossRef]
- Bartoletti, M.; Azap, O.; Barac, A.; Bussini, L.; Ergonul, O.; Krause, R.; Paño-Pardo, J.R.; Power, N.R.; Sibani, M.; Szabo, B.G.; et al. ESCMID COVID-19 living guidelines: Drug treatment and clinical management. Clin. Microbiol. Infect. 2022, 28, 222–238. [Google Scholar] [CrossRef]
- Vollmer, S.; Mateen, B.A.; Bohner, G.; Király, F.J.; Ghani, R.; Jonsson, P.; Cumbers, S.; Jonas, A.; McAllister, K.; Myles, P.; et al. Machine learning and artificial intelligence research for patient benefit: 20 critical questions on transparency, replicability, ethics, and effectiveness. BMJ 2020, 368, l6927. [Google Scholar] [CrossRef]
- Abd-Alrazaq, A.; Alajlani, M.; Alhuwail, D.; Schneider, J.; Al-Kuwari, S.; Shah, Z.; Hamdi, M.; Househ, M. Artificial Intelligence in the Fight Against COVID-19: Scoping Review. J. Med. Internet Res. 2020, 22, e20756. [Google Scholar] [CrossRef]
- Rasheed, J.; Jamil, A.; Hameed, A.A.; Al-Turjman, F.; Rasheed, A. COVID-19 in the Age of Artificial Intelligence: A Comprehensive Review. Interdiscip. Sci. 2021, 13, 153–175. [Google Scholar] [CrossRef]
- Syeda, H.B.; Syed, M.; Sexton, K.W.; Syed, S.; Begum, S.; Syed, F.; Prior, F.; Yu, F., Jr. Role of Machine Learning Techniques to Tackle the COVID-19 Crisis: Systematic Review. JMIR Med. Inform. 2021, 9, e23811. [Google Scholar] [CrossRef]
- Chen, C.; Dong, D.; Qi, B.; Petersen, I.R.; Rabitz, H. Quantum Ensemble Classification: A Sampling-Based Learning Control Approach. IEEE Trans. Neural Netw. Learn. Syst. 2017, 28, 1345–1359. [Google Scholar] [CrossRef]
- Chang, W.; Liu, Y.; Wu, X.; Xiao, Y.; Zhou, S.; Cao, W. A New Hybrid XGBSVM Model: Application for Hypertensive Heart Disease. IEEE Access 2019, 7, 175248–175258. [Google Scholar] [CrossRef]
- Rivera-Lopez, R.; Canul-Reich, J. Construction of near-optimal axis-parallel decision trees using a differential-evolution-based approach. IEEE Access 2018, 6, 5548–5563. [Google Scholar] [CrossRef]
- Ma, D.; Yuan, S.; Shang, J.; Liu, J.; Dai, L.; Kong, X.; Xu, F. The Automatic Detection of Seizure Based on Tensor Distance and Bayesian Linear Discriminant Analysis. Int. J. Neural Syst. 2021, 31, 2150006. [Google Scholar] [CrossRef]
- Xing, W.; Bei, Y. Medical Health Big Data Classification Based on KNN Classification Algorithm. IEEE Access 2020, 8, 28808–28819. [Google Scholar] [CrossRef]
- Yu, S.; Li, X.; Zhang, X.; Wang, H. The OCS-SVM: An objective-cost-sensitive SVM with sample-based misclassification cost invariance. IEEE Access 2019, 7, 118931–118942. [Google Scholar] [CrossRef]
- Han, J.; Kamber, M.; Pei, J. Data Mining: Concepts and Techniques, 3rd ed.; Morgan Kaufmann Publishers: Burlington, MA, USA, 2012. [Google Scholar]
- Hariharan, A.; Hakeem, A.R.; Radhakrishnan, S.; Reddy, M.S.; Rela, M. The Role and Therapeutic Potential of NF-kappa-B Pathway in Severe COVID-19 Patients. Inflammopharmacology 2021, 29, 91–100. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.; Qiu, J.; Li, L.; Jia, X.; Kai, G. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020, 53, 66–70. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; Ní Choileáin, O.; Clarke, J.; O’Connor, E.; Hogan, G.; et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef]
- Ghanei, M.; Solaymani-Dodaran, M.; Qazvini, A.; Ghazale, A.H.; Setarehdan, S.A.; Saadat, S.H.; Ghobadi, H.; Hoseininia, S.; Elahikhah, M.; Samadi, A.H.; et al. The efficacy of corticosteroids therapy in patients with moderate to severe SARS-CoV-2 infection: A multicenter, randomized, open-label trial. Respir Res. 2021, 22, 245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shang, L.; Fan, G.; Gu, X.; Xu, J.; Wang, Y.; Huang, L.; Cao, B. The Efficacy and Safety of Janus Kinase Inhibitors for Patients with COVID-19: A Living Systematic Review and Meta-Analysis. Front. Med. 2022, 8, 800492. [Google Scholar] [CrossRef] [PubMed]
- Alunno, A.; Najm, A.; Machado, P.M.; Bertheussen, H.; Burmester, G.R.; Carubbi, F.; De Marco, G.; Giacomelli, R.; Hermine, O.; Isaacs, J.D.; et al. 2021 update of the EULAR points to consider on the use of immunomodulatory therapies in COVID-19. Ann. Rheum. Dis. 2022, 81, 34–40. [Google Scholar] [CrossRef]
- Duarte-Millán, M.A.; Mesa-Plaza, N.; Guerrero-Santillán, M.; Morales-Ortega, A.; Bernal-Bello, D.; Farfán-Sedano, A.I.; García de Viedma-García, V.; Velázquez-Ríos, L.; Frutos-Pérez, B.; De Ancos-Aracil, C.L.; et al. Prognostic factors and combined use of tocilizumab and corticosteroids in a Spanish cohort of elderly COVID-19 patients. J. Med. Virol. 2022, 94, 1540–1549. [Google Scholar] [CrossRef]
- Wardhani, S.O.; Fajar, J.K.; Soegiarto, G.; Wulandari, L.; Maliga, H.A.; Ilmawan, M.; Merysa, R.; Simamora, A.B.; Aini, Q.; Noviantari, K.; et al. The association between therapeutic plasma exchange and the risk of mortality among patients critically ill with COVID-19: A meta-analysis. F1000Research 2021, 10, 1280. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Narain, S.; Stefanov, D.G.; Chau, A.S.; Weber, A.G.; Marder, G.; Kaplan, B.; Malhotra, P.; Bloom, O.; Liu, A.; Lesser, M.L.; et al. Comparative Survival Analysis of Immunomodulatory Therapy for Coronavirus Disease 2019 Cytokine Storm. Chest 2021, 159, 933–948. [Google Scholar] [CrossRef]
- Chober, D.; Aksak-Wąs, B.; Bobrek-Lesiakowska, K.; Budny-Finster, A.; Hołda, E.; Mieżyńska-Kurtycz, J.; Jamro, G.; Parczewski, M. Effectiveness of Tocilizumab in Patients with Severe or Critical Lung Involvement in COVID-19: A Retrospective Study. J. Clin. Med. 2022, 11, 2286. [Google Scholar] [CrossRef]
- Campbell, C.; Andersson, M.I.; Ansari, M.A.; Moswela, O.; Misbah, S.A.; Klenerman, P.; Matthews, P.C. Risk of Reactivation of Hepatitis B Virus (HBV) and Tuberculosis (TB) and Complications of Hepatitis C Virus (HCV) Following Tocilizumab Therapy: A Systematic Review to Inform Risk Assessment in the COVID-19 Era. Front. Med. 2021, 8, 706482. [Google Scholar] [CrossRef]
- Adamidi, E.S.; Mitsis, K.; Nikita, K.S. Artificial intelligence in clinical care amidst COVID-19 pandemic: A systematic review. Comput. Struct. Biotechnol. J. 2021, 19, 2833–2850. [Google Scholar] [CrossRef]
- Bottino, F.; Tagliente, E.; Pasquini, L.; Napoli, A.D.; Lucignani, M.; Figà-Talamanca, L.; Napolitano, A. COVID Mortality Prediction with Machine Learning Methods: A Systematic Review and Critical Appraisal. J. Pers. Med. 2021, 11, 893. [Google Scholar] [CrossRef]
- Ma, B.; Meng, F.; Yan, G.; Yan, H.; Chai, B.; Song, F. Diagnostic classification of cancers using extreme gradient boosting algorithm and multi-omics data. Comput. Biol. Med. 2020, 121, 103761. [Google Scholar] [CrossRef]
- REMAP-CAP Investigators; Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; van Bentum-Puijk, W.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef]
- De Ardanaz, L.S.; Andreu-Ubero, J.M.; Navidad-Fuentes, M.; Ferrer-González, M.Á.; Del Valle, V.R.; Salcedo-Bellido, I.; Barrios-Rodríguez, R.; Cáliz-Cáliz, R.; Requena, P. Tocilizumab in COVID-19: Factors Associated with Mortality before and after Treatment. Front. Pharmacol. 2021, 12, 620187. [Google Scholar] [CrossRef]
- Eşkazan, A.E.; Balkan, İ.İ.; Demirbaş, K.C.; Ar, M.C.; Karaali, R.; Sekibağ, Y.; Mulamahmutoğlu, S.; Dumanlı, G.Y.; Çakmak, F.; Yurttaş, N.Ö.; et al. Tocilizumab in COVID-19: The Cerrahpaşa-PREDICT score. J. Infect. Chemother. 2021, 27, 1329–1335. [Google Scholar] [CrossRef]
- Heidari-Beni, F.; Vahedian-Azimi, A.; Shojaei, S.; Rahimi-Bashar, F.; Shahriary, A.; Johnston, T.P.; Sahebkar, A. The Level of Procalcitonin in Severe COVID-19 Patients: A Systematic Review and Meta-Analysis. Adv. Exp. Med. Biol. 2021, 1321, 277–286. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Rosas, I.O.; Diaz, G.; Gottlieb, R.L.; Lobo, S.M.; Robinson, P.; Hunter, B.D.; Cavalcante, A.W.; Overcash, J.S.; Hanania, N.A.; Skarbnik, A.; et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: A randomized clinical trial. Intensive Care Med. 2021, 47, 1258–1270. [Google Scholar] [CrossRef]
- Mutua, V.; Henry, B.M.; Csefalvay, C.V.; Cheruiyot, I.; Vikse, J.; Lippi, G.; Bundi, B.; Mong’are, N. Tocilizumab in addition to standard of care in the management of COVID-19: A meta-analysis of RCTs. Acta Biomed. 2022, 93, e2022014. [Google Scholar] [CrossRef]
- Moosazadeh, M.; Mousavi, T. Combination therapy of tocilizumab and steroid for COVID-19 patients: A meta-analysis. J. Med. Virol. 2022, 94, 1350–1356. [Google Scholar] [CrossRef]
- Albuquerque, A.M.; Tramujas, L.; Sewanan, L.R.; Williams, D.R.; Brophy, J.M. Mortality Rates Among Hospitalized Patients with COVID-19 Infection Treated with Tocilizumab and Corticosteroids: A Bayesian Reanalysis of a Previous Meta-analysis. JAMA Netw. Open 2022, 5, e220548. [Google Scholar] [CrossRef]
- Lohse, A.; Klopfenstein, T.; Balblanc, J.C.; Royer, P.Y.; Bossert, M.; Gendrin, V.; Charpentier, A.; Bozgan, A.M.; Badie, J.; Bourgoin, C.; et al. Predictive factors of mortality in patients treated with tocilizumab for acute respiratory distress syndrome related to coronavirus disease 2019 (COVID-19). Microbes Infect. 2020, 22, 500–503. [Google Scholar] [CrossRef]
- Chamorro-de-Vega, E.; Rodriguez-Gonzalez, C.G.; Manrique-Rodríguez, S.; Lobato-Matilla, E.; García-Moreno, F.; Olmedo, M.; Correa-Rocha, R.; Valerio, M.; Aldámiz-Echevarria, T.; Machado, M.; et al. Clinical course of severe patients with COVID-19 treated with tocilizumab: Report from a cohort study in Spain. Expert Rev. Clin. Pharmacol. 2021, 14, 249–260. [Google Scholar] [CrossRef]
- Lakatos, B.; Szabo, B.G.; Bobek, I.; Gopcsa, L.; Beko, G.; Kiss-Dala, N.; Petrik, B.; Gaspar, Z.; Farkas, B.F.; Sinko, J.; et al. Laboratory parameters predicting mortality of adult in-patients with COVID-19 associated cytokine release syndrome treated with high-dose tocilizumab. Acta Microbiol. Immunol. Hung. 2021, 68, 145–152. [Google Scholar] [CrossRef]
- Song, Y.; Ye, Y.; Su, S.H.; Stephens, A.; Cai, T.; Chung, M.T.; Han, M.K.; Newstead, M.W.; Yessayan, L.; Frame, D.; et al. A digital protein microarray for COVID-19 cytokine storm monitoring. Lab Chip 2021, 21, 331–343. [Google Scholar] [CrossRef]
- Sinha, P.; Jafarzadeh, S.R.; Assoumou, S.A.; Bielick, C.G.; Carpenter, B.; Garg, S.; Harleen, S.; Neogi, T.; Nishio, M.J.; Sagar, M.; et al. The Effect of IL-6 Inhibitors on Mortality Among Hospitalized COVID-19 Patients: A Multicenter Study. J. Infect. Dis. 2021, 223, 581–588. [Google Scholar] [CrossRef]
- Sinha, P.; Mostaghim, A.; Bielick, C.G.; McLaughlin, A.; Hamer, D.H.; Wetzler, L.M.; Bhadelia, N.; Fagan, M.A.; Linas, B.P.; Assoumou, S.A.; et al. Early administration of interleukin-6 inhibitors for patients with severe COVID-19 disease is associated with decreased intubation, reduced mortality, and increased discharge. Int. J. Infect. Dis. 2020, 99, 28–33. [Google Scholar] [CrossRef]
- Eimer, J.; Vesterbacka, J.; Svensson, A.K.; Stojanovic, B.; Wagrell, C.; Sönnerborg, A.; Nowak, P. Tocilizumab shortens time on mechanical ventilation and length of hospital stay in patients with severe COVID-19: A retrospective cohort study. J. Intern. Med. 2021, 289, 434–436. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, B.; Del Toro, M.D.; Borobia, A.M.; Carcas, A.; Jarrín, I.; Yllescas, M.; Ryan, P.; Pachón, J.; Carratalà, J.; Berenguer, J.; et al. Identification and validation of clinical phenotypes with prognostic implications in patients admitted to hospital with COVID-19: A multicentre cohort study. Lancet Infect. Dis. 2021, 21, 783–792. [Google Scholar] [CrossRef]
- Chen, H.; Xie, J.; Su, N.; Wang, J.; Sun, Q.; Li, S.; Jin, J.; Zhou, J.; Mo, M.; Wei, Y.; et al. Corticosteroid Therapy Is Associated with Improved Outcome in Critically Ill Patients with COVID-19 with Hyperinflammatory Phenotype. Chest 2021, 159, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Siefkas, A.; Zelin, N.S.; Barnes, G.; Dellinger, R.P.; Vincent, J.L.; Braden, G.; Burdick, H.; Hoffman, J.; Calvert, J.; et al. Machine Learning as a Precision-Medicine Approach to Prescribing COVID-19 Pharmacotherapy with Remdesivir or Corticosteroids. Clin. Ther. 2021, 43, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Sahashi, Y.; Kawahito, S.; Takahashi, M.; Iwagami, M.; Egorova, N.N. Prediction of in-hospital mortality with machine learning for COVID-19 patients treated with steroid and remdesivir. J. Med. Virol. 2022, 94, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Delgado, M.; Cernadas, E.; Barro, S.; Amorim, D. Do we Need Hundreds of Classifiers to Solve Real World Classification Problems? J. Mach. Learn. Res. 2014, 15, 3133–3181. [Google Scholar]

| Methods | Balanced Accuracy | Recall | Precision | F1 Score |
|---|---|---|---|---|
| SVM | 82.77 ± 0.47 | 82.87 ± 0.53 | 82.18 ± 0.57 | 82.52 ± 0.62 |
| BLDA | 80.91 ± 0.81 | 81.02 ± 0.78 | 80.31 ± 0.75 | 80.66 ± 0.73 |
| DT | 83.54 ± 0.63 | 83.63 ± 0.68 | 83.01 ± 0.64 | 83.32 ± 0.62 |
| KNN | 86.86 ± 0.54 | 86.98 ± 0.51 | 86.59 ± 0.46 | 86.79 ± 0.47 |
| XGB | 93.16 ± 0.25 | 93.25 ± 0.31 | 92.49 ± 0.27 | 92.87 ± 0.29 |
| Methods | AUC | MCC | DYI | Kappa |
| SVM | 0.82 ± 0.02 | 73.45 ± 0.58 | 82.78 ± 0.61 | 72.91 ± 0.59 |
| BLDA | 0.80 ± 0.02 | 71.79 ± 0.73 | 80.92 ± 0.72 | 71.89 ± 0.71 |
| DT | 0.83 ± 0.02 | 74.18 ± 0.67 | 83.55 ± 0.65 | 73.71 ± 0.68 |
| KNN | 0.86 ± 0.02 | 76.97 ± 0.48 | 86.87 ± 0.45 | 77.15 ± 0.46 |
| XGB | 0.93 ± 0.02 | 84.41 ± 0.25 | 93.17 ± 0.26 | 83.91 ± 0.28 |
| Method | |
|---|---|
| SVM | C = 1.0 |
| sigma = 0.5 | |
| Numerical tolerance = 0.001 | |
| Iteration limit = 100 | |
| Kernel function: Linear kernel, Gaussian, Quadratic and Cubic | |
| BLDA | Kernel: Bayesian |
| DT | Minimum number of instances in leaves = 4 |
| Minimum number of instances in internal nodes = 6 | |
| Maximum depth = 100 | |
| KNN | Number of neighbours = 20 |
| Distance metric: Euclidean | |
| Weight: Uniform | |
| XGB | Base estimator: tree |
| Maximum number of splits = 20 | |
| Learning rate = 0.1 | |
| Number of learners = 50 |
| Variable | Cohort |
|---|---|
| Number of patients | 67 |
| Age (years) (IQR) | 65 (57–74.5) |
| Male (%) | 43 (64.2) |
| Exitus, n (yes %) | 24 (35.8) |
| Hospital admission (days) after tocilizumab administration (IQR) | 14 (10–29.5) |
| IMV, n (yes %) | 13 (19.4) |
| 7-day mortality, n (yes %) | 7 (10.4) |
| 21-day mortality, n (yes %) | 10 (14.9) |
| Antivirals drugs, n (yes %) | 30 (44.8) |
| Lopinavir/ritonavir, n (yes %) | 27 (40.3) |
| Remdesivir, n (yes %) | 3 (4.5) |
| Hydroxychloroquine, n (yes %) | 34 (50.7) |
| Interferon-beta, n (yes %) | 7 (10.4) |
| Anakinra, n (yes %) | 33 (49.2) |
| Baseline situation at the start of tocilizumab treatment requiring supplemental oxygen, n (yes %) | 60 (89.5) |
| Baseline situation at the start of tocilizumab treatment requiring IMV, n (yes %) | 7 (10.4) |
| Smoker/ex-smoker, n (yes %) | 14 (20.9) |
| Diabetes, n (yes %) | 18 (26.9) |
| COPD, n (yes %) | 4 (5.9) |
| Arterial hypertension, n (yes %) | 34 (50.7) |
| Dyslipemia, n (yes %) | 21 (31.3) |
| Obesity [BMI ≥ 30 kg/m2], n (yes %) | 4 (5.9) |
| Ischemic heart disease, n (yes %) | 5 (7.5) |
| Chronic kidney disease, n (yes %) | 2 (2.9) |
| Lymphocytes (10 × 9/L) (IQR) | 0.95 (0.5–1.3) |
| CRP (mg/L) (IQR) | 12.5 (5.8–21.1) |
| LDH (U/L) (IQR) | 759 (538–934) |
| Procalcitonin (ng/mL) (IQR) | 3.49 (0.3–10.9) |
| Ferritin (µg/L) (IQR) | 828 (412–1388) |
| FiO2 (%) (IQR) | 37.5 (28–50) |
| PaFi (IQR) | 198.5 (135–251.5) |
| GPT (U/L) (IQR) | 39 (26–66.5) |
| GOT(U/L) (IQR) | 45 (31–67) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramón, A.; Zaragozá, M.; Torres, A.M.; Cascón, J.; Blasco, P.; Milara, J.; Mateo, J. Application of Machine Learning in Hospitalized Patients with Severe COVID-19 Treated with Tocilizumab. J. Clin. Med. 2022, 11, 4729. https://doi.org/10.3390/jcm11164729
Ramón A, Zaragozá M, Torres AM, Cascón J, Blasco P, Milara J, Mateo J. Application of Machine Learning in Hospitalized Patients with Severe COVID-19 Treated with Tocilizumab. Journal of Clinical Medicine. 2022; 11(16):4729. https://doi.org/10.3390/jcm11164729
Chicago/Turabian StyleRamón, Antonio, Marta Zaragozá, Ana María Torres, Joaquín Cascón, Pilar Blasco, Javier Milara, and Jorge Mateo. 2022. "Application of Machine Learning in Hospitalized Patients with Severe COVID-19 Treated with Tocilizumab" Journal of Clinical Medicine 11, no. 16: 4729. https://doi.org/10.3390/jcm11164729
APA StyleRamón, A., Zaragozá, M., Torres, A. M., Cascón, J., Blasco, P., Milara, J., & Mateo, J. (2022). Application of Machine Learning in Hospitalized Patients with Severe COVID-19 Treated with Tocilizumab. Journal of Clinical Medicine, 11(16), 4729. https://doi.org/10.3390/jcm11164729








