Mechanical Circulatory Support in Delayed Surgery of Post-Infarction Ventricular Septal Rupture in Patients in Cardiogenic Shock—A Review
Abstract
:1. Introduction
1.1. Epidemiology
1.2. Pathophysiology
1.3. Surgical Treatment
1.4. The Role of Mechanical Circulatory Support
- An intra-aortic balloon pump is an easily accessible percutaneous implantable device that reduces the afterload (LV unloading only) and improves flow in coronary vessels. IABP could potentially help to unburden the left ventricle and reduce the left–right shunt, although with a poor effect, especially in unstable patients [21,22]. However, it is used in combination with other types of mechanical circulatory supports.
- LV-based Impella (LV to Ao; LV support and unloading) is a hemodynamically effective microaxial device that pumps blood from the left ventricle to the ascending aorta, generating flow over 5 L/min, which leads to significant direct unloading of the LV and increased cardiac output [23]. Reducing left–right shunt Impella decreases the right ventricle overload and pulmonary congestion, simultaneously posing a risk of shunt inversion and hypoxia of the central nervous system and myocardium; therefore, intensive monitoring is recommended [24]. The presence of VSR is considered a contraindication to implantation of this type of support due to the risk of aspiration of necrotic tissues, thus, some authors suggest using Impella with posterior VSR to reduce the possibility of embolization [24].
- Venoarterial ECMO (RA to femoral artery (FemA)—biventricular support with RV unloading)—supports systemic circulation (biventricular support), increases the level of arterial blood oxygen saturation, and ensures proper tissue oxygenation, which is essential in the setting of large left to right shunts, especially in a non-opening aortic valve [22,25]. This MCS unloads the right ventricle; however, at the same time, ECMO raises the left ventricular end-diastolic pressure and the total blood flow, which may contribute to overload LV and enlargement of the rupture [25,26]. ECMO with LV unloading is the simultaneous use of IABP or LV-located Impella with ECMO (i.e., Ecpella) [23,27]. Ecpella unloads LV, prevents the VSD enlargement, reduces afterload, and drains the right atrium, supporting the right ventricle. An interesting option is the applicability of left atrial venoarterial membrane oxygenation (LAVA ECMO biatrial) with transeptal located cannula, which at the same time drains the left and right atria [23]. Another effective method for indirect unloading of the left ventricle is the use of the pulmonary artery draining cannula.
- Tandem Heart (LA to FemA; LV support and unloading) is a percutaneous system with a continuous-flow centrifugal pump that generates a flow of about 5 L/min, which indirectly decompresses the left ventricle [28]. Placing the inflow cannula through the femoral vein in the left atrium requires a transseptal puncture, which may be a certain limitation in the use of this system [28]. Tandem Heart reduces LV end-diastolic pressure, although, similar to ECMO, it increases afterload due to return of blood through the outflow cannula to the femoral artery [28]. In the setting of VSR, there is a risk of shunt inversion due to intensive LV unloading as well as affecting the opening of the aortic valve [24].
- Left ventricular assist device (LVAD) (LV to aorta) is also a support in some centers for patients with post-infarction VSD. However, LVAD is used less frequently for short-term pre-operative stabilization of the patient. Post-infarction fragility of tissues may impede adequate implantation of the support and aspiration of necrotic tissues, which in some cases may lead to improper function of the pump [24]. With the more favorable location of the post-infarction area and the possibility of using LVAD, there is a risk of reversing the leak, which can be solved by using BiVAD [24].
1.5. An Ideal Mechanical Circulatory Device for a Patient with Post-Infarcted VSR
2. Materials and Methods
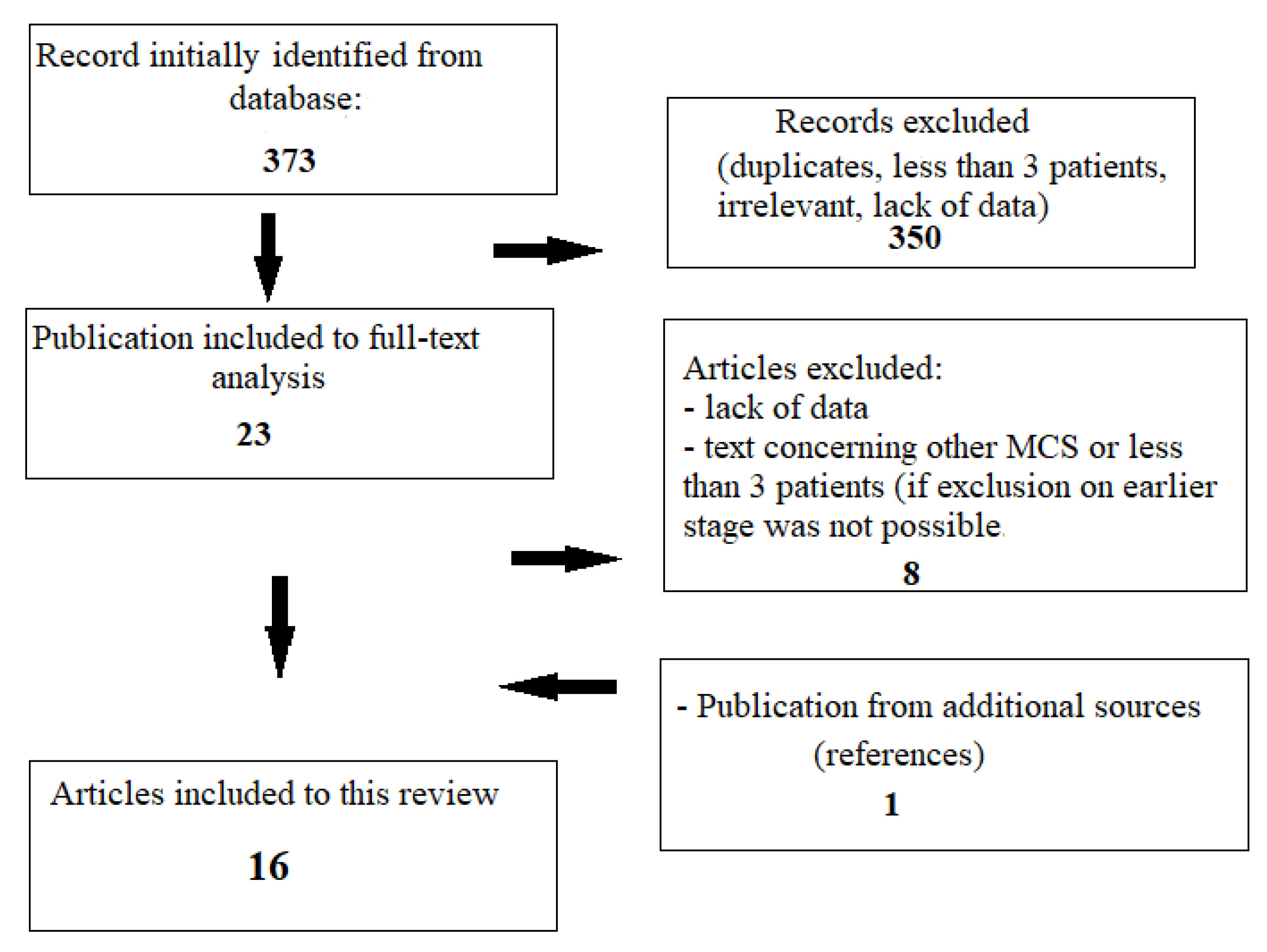
3. Results
3.1. Research Process
3.2. Mechanical Circulatory Support in Delayed Surgery of Post-Infarction VSR—Results
4. Discussion
- VSR is relatively rare, and it is impossible to conduct a randomized prospective scientific study in this field, therefore the analysis covers a limited number of publications, and the authors emphasize the need for further research.
- Due to the low incidence of mechanical complications of myocardial infarction, limited use of delayed surgery treatment and therapy with MCS, the most important investigated studies include a small number of patients. Analyzed groups cannot be directly compared; nevertheless, they emphasize the presence of certain trends.
- The retrospective nature of research is often related to the limited amount or lack of relevant data.
- Bad outcomes are underreported, which further limits the assessment of the effectiveness of MCS in this difficult patient cohort.
- The methodologies used and the inclusion and exclusion criteria for individual publications often differ significantly; therefore, the results have been summarized and expressed as percentages in order to make approximate comparisons of the obtained results of treatment. The results of the analysis and a summary of collected data can be found in Table 1.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnaoutakis, G.J.; Zhao, Y.; George, T.J.; Sciortino, C.M.; McCarthy, P.M.; Conte, J.V. Surgical repair of ventricular septal defect after myocardial infarction: Outcomes from the Society of Thoracic Surgeons National Database. Ann. Thorac. Surg. 2012, 94, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.M.; Kapadia, S.R.; Smedira, N.G.; Robich, M.; Tuzcu, E.M.; Menon, V.; Krishnaswamy, A. Ventricular septal rupture complicating acute myocardial infarction: A contemporary review. Eur. Heart J. 2014, 35, 2060–2068. [Google Scholar] [CrossRef]
- Novak, M.; Hlinomaz, O.; Groch, L.; Rezek, M.; Semenka, J.; Sikora, J.; Sitar, J. Ventricular Septal Rupture—A Critical Condition as a Complication of Acute Myocardial Infarction. J. Crit. Care Med. 2015, 1, 162–166. [Google Scholar] [CrossRef] [PubMed]
- French, J.K.; Hellkamp, A.S.; Armstrong, P.W.; Cohen, E.; Kleiman, N.S.; O’Connor, C.M.; Holmes, D.R.; Hochman, J.S.; Granger, C.B.; Mahaffey, K.W. Mechanical complications after percutaneous coronary intervention in ST-elevation myocardial infarction (from APEX-AMI). Am. J. Cardiol. 2010, 105, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, A.; Elgendy, I.Y.; Mahmoud, K.; Barakat, A.F.; Mentias, A.; Mohamed, A.H.; Ogunbayo, G.O.; Megaly, M.; Saad, M.; Omer, M.A.; et al. Temporal Trends and Outcomes of Mechanical Complications in Patients with Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2019, 12, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, I.; Jannati, F.; Jannati, M. Optimal Time Repair of Ventricular Septal Rupture Post Myocardial Infarction. J. Saudi Heart Assoc. 2020, 32, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, M.; Ronco, D.; Corazzari, C.; Fina, D.; Jiritano, F.; Meani, P.; Kowalewski, M.; Beghi, C.; Lorusso, R. Surgical Repair of Postinfarction Ventricular Septal Rupture: Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2021, 112, 326–337. [Google Scholar] [CrossRef]
- Menon, V.; Webb, J.G.; Hillis, L.D.; Sleeper, L.A.; Abboud, R.; Dzavik, V.; Slater, J.N.; Forman, R.; Monrad, E.S.; Talley, J.D.; et al. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: A report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK? J. Am. Coll. Cardiol. 2000, 36, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.S.; Granger, C.B.; Birnbaum, Y.; Pieper, K.S.; Morris, D.C.; Kleiman, N.S.; Vahanian, A.; Califf, R.M.; Topolet, E.J.; GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. Circulation 2000, 101, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Moreyra, A.E.; Huang, M.S.; Wilson, A.C.; Deng, Y.; Cosgrove, N.M.; Kostis, J.B.; MIDAS Study Group (MIDAS 13). Trends in incidence and mortality rates of ventricular septal rupture during acute myocardial infarction. Am. J. Cardiol. 2010, 106, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Menon, V. Contemporary management of post-MI ventricular septal rupture. J. Am. Coll. Cardiol. 2018, 720, 1964–2020. [Google Scholar]
- Mubarik, A.; Iqbal, A.M. Ventricular Septal Rupture. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Becker, A.E.; Van Mantgem, J.P. Cardiac tamponade. A study of 50 hearts. Eur. J. Cardiol. 1975, 3, 349–358. [Google Scholar] [PubMed]
- Vondran, M.; Wehbe, M.S.; Etz, C.; Ghazy, T.; Rastan, A.J.; Borger, M.A.; Schroeter, T. Mechanical circulatory support for early surgical repair of postinfarction ventricular septal defect with cardiogenic shock. Artif. Organs 2021, 45, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 61, e78–e140. [Google Scholar] [CrossRef] [PubMed]
- Morimura, H.; Tabata, M. Delayed surgery after mechanical circulatory support for ventricular septal rupture with cardiogenic shock. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 868–873. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in: Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef]
- Daggett, W.M.; Buckley, M.J.; Akins, C.W.; Leinbach, R.C.; Gold, H.K.; Block, P.C.; Austen, W.G. Improved results of surgical management of postinfarction ventricular septal rupture. Ann. Surg. 1982, 196, 269–277. [Google Scholar] [CrossRef]
- David, T.E.; Dale, L.; Sun, Z. Postinfarction ventricular septal rupture: Repair by endocardial patch with infarct exclusion. J. Thorac. Cardiovasc. Surg. 1995, 110, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Zeymer, U.; Neumann, F.J.; Ferenc, M.; Olbrich, H.G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Pahuja, M.; Schrage, B.; Westermann, D.; Basir, M.B.; Garan, A.R.; Burkhoff, D. Hemodynamic Effects of Mechanical Circulatory Support Devices in Ventricular Septal Defect. Circ. Heart Fail. 2019, 12, e005981. [Google Scholar] [CrossRef] [PubMed]
- Villablanca, P.; Nona, P.; Lemor, A.; Qintar, M.; O’Neill, B.; Lee, J.; Frisoli, T.; Wang, D.D.; Eng, M.H.; O’Neill, W.W. Mechanical Circulatory Support in Cardiogenic Shock due to Structural Heart Disease. Interv. Cardiol. Clin. 2021, 10, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Ronco, D.; Matteucci, M.; Ravaux, J.M.; Marra, S.; Torchio, F.; Corazzari, C.; Massimi, G.; Beghi, C.; Maessen, J.; Lorusso, R. Mechanical Circulatory Support as a Bridge to Definitive Treatment in Post-Infarction Ventricular Septal Rupture. JACC Cardiovasc. Interv. 2021, 14, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, A.; Rosenberg, A.; Galiatsou, E.; Stock, U.A. Pros and Cons of Different Types of Mechanical Circulatory Support Device in Case of Postinfarction Ventricular Septal Defect. ASAIO J. 2021, 67, e110–e113. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Khalpey, Z.; Smith, R.; Burkhoff, D.; Kociol, R.D. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ. Heart Fail. 2018, 11, e004905. [Google Scholar] [CrossRef] [PubMed]
- Bréchot, N.; Demondion, P.; Santi, F.; Lebreton, G.; Pham, T.; Dalakidis, A.; Gambotti, L.; Luyt, C.E.; Schmidt, M.; Hekimian, G.; et al. Intra-aortic balloon pump protects against hydrostatic pulmonary oedema during peripheral venoarterial-extracorporeal membrane oxygenation. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 6–69. [Google Scholar] [CrossRef]
- Ergle, K.; Parto, P.; Krim, S.R. Percutaneous Ventricular Assist Devices: A Novel Approach in the Management of Patients with Acute Cardiogenic Shock. Ochsner J. 2016, 16, 243–249. [Google Scholar] [PubMed]
- Tycińska, A.; Grygier, M.; Biegus, J.; Czarnik, T.; Dąbrowski, M.; Depukat, R.; Gierlotka, M.; Gil, M.; Hawranek, M.; Hirnle, T.; et al. Mechanical circulatory support. An expert opinion of the Association of Intensive Cardiac Care and the Association of Cardiovascular Interventions of the Polish Cardiac Society. Kardiol. Pol. 2021, 79, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.; Younus, F.; Malik, A.; Farooq, M.U.; Kamal, A.; Shoaib, M.; Naeem, H.; Rana, G.; Rana, A.S.; Usman, M.; et al. One-year outcome and survival analysis of deferred ventricular septal repair in cardiogenic shock supported with mechanical circulatory support. PLoS ONE 2021, 16, e0256377. [Google Scholar] [CrossRef]
- Ariza-Solé, A.; Sánchez-Salado, J.C.; Sbraga, F.; Ortiz, D.; González-Costello, J.; Blasco-Lucas, A.; Alegre, O.; Toral, D.; Lorente, V.; Santafosta, E.; et al. The role of perioperative cardiorespiratory support in post infarction ventricular septal rupture-related cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Artemiou, P.; Gasparovic, I.; Bezak, B.; Hudec, V.; Glonek, I.; Hulman, M. Preoperative extracorporeal membrane oxygenation for postinfarction ventricular septal defect: Case series of three patients with a literature review. J. Card. Surg. 2020, 35, 3626–3630. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, A.; McGiffin, D.; Winearls, J.; Tesar, P.; Cole, C.; Vallely, M.; Clarke, A.; Fraser, J. Veno-Arterial ECMO in the Setting of Post-Infarct Ventricular Septal Defect: A Bridge to Surgical Repair. Heart Lung Circ. 2016, 25, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.; Korutla, V.; Suzuki, Y.; Acker, M.; Vallabhajosyula, P. Mechanical circulatory support as a bridge to definitive surgical repair after post-myocardial infarct ventricular septal defect. J. Card. Surg. 2015, 30, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Vega, J.D.; Alonso Salinas, G.L.; Viéitez Flórez, J.M.; Ariza Solé, A.; López de Sá, E.; Sanz Ruiz, R.; Burgos Palacios, V.; Raposeiras-Roubín, S.; Gómez Varela, S.; Sanchis, J.; et al. Temporal trends in postinfarction ventricular septal rupture: The CIVIAM Registry. Rev. Esp. Cardiol. Engl. Ed. 2021, 74, 757–764. [Google Scholar] [CrossRef] [PubMed]
- La Torre, M.W.; Centofanti, P.; Attisani, M.; Patanè, F.; Rinaldi, M. Posterior ventricular septal defect in presence of cardiogenic shock: Early implantation of the impella recover LP 5.0 as a bridge to surgery. Tex. Heart Inst. J. 2011, 38, 42–49. [Google Scholar]
- Gregoric, I.D.; Kar, B.; Mesar, T.; Nathan, S.; Radovancevic, R.; Patel, M.; Loyalka, P. Perioperative use of TandemHeart percutaneous ventricular assist device in surgical repair of postinfarction ventricular septal defect. ASAIO J. 2014, 60, 529–532. [Google Scholar] [CrossRef]
- Ronco, D.; Matteucci, M.; Kowalewski, M.; De Bonis, M.; Formica, F.; Jiritano, F.; Fina, D.; Folliguet, T.; Bonaros, N.; Russo, C.F.; et al. Surgical Treatment of Postinfarction Ventricular Septal Rupture. JAMA Netw. Open 2021, 4, e2128309. [Google Scholar] [CrossRef]
- Rob, D.; Špunda, R.; Lindner, J.; Rohn, V.; Kunstýř, J.; Balík, M.; Rulíšek, J.; Kopecký, P.; Lipš, M.; Šmíd, O.; et al. A rationale for early extracorporeal membrane oxygenation in patients with postinfarction ventricular septal rupture complicated by cardiogenic shock. Eur. J. Heart Fail. 2017, 19 (Suppl. S2), 97–103. [Google Scholar] [CrossRef]
- Huang, S.M.; Huang, S.C.; Wang, C.H.; Wu, I.H.; Chi, N.H.; Yu, H.Y.; Hsu, R.B.; Chang, C.I.; Wang, S.S.; Chen, Y.S. Risk factors and outcome analysis after surgical management of ventricular septal rupture complicating acute myocardial infarction: A retrospective analysis. J. Cardiothorac. Surg. 2015, 10, 66. [Google Scholar] [CrossRef]
- Matteucci, M.; Fina, D.; Jiritano, F.; Meani, P.; Raffa, G.M.; Kowalewski, M.; Aldobayyan, I.; Turkistani, M.; Beghi, C.; Lorusso, R. The use of extracorporeal membrane oxygenation in the setting of postinfarction mechanical complications: Outcome analysis of the Extracorporeal Life Support Organization Registry. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 369–374. [Google Scholar] [CrossRef]
- Fujimoto, K.; Kawahito, K.; Yamaguchi, A.; Sakuragawa, H.; Tsuboi, J.; Yuri, K.; Tanaka, M.; Endo, H.; Adachi, H.; Ino, T. Percutaneous extracorporeal life support for treatment of fatal mechanical complications associated with acute myocardial infarction. Artif. Organs 2001, 25, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
| Study | Type of Treatment | Number of Patients Qualified for the Procedure | VSR Diagnosis to Surgery | MCS Duration before Surgery | Early Mortality | ||
|---|---|---|---|---|---|---|---|
| n | % | ||||||
| 1. Morimura H. et al., 2020 [17] | Delayed surgery with preoperative ECMO and IABP | 8 | 1.9 days | 36.9 h (ECMO) 43.2 h (IABP) | 1 | 12.5% | |
| 3 during 2 years | 37% during 2 years | ||||||
| 2. Malik J. et al., 2021 [30] | Delayed surgery with preoperative ECMO or/and IABP or LVAD | 27 | 18.8 days | 13.2 days | 3 | 11% (operative mortality) | |
| 9 (overall mortality after one year from any cause) | 33% (overall mortality after one year from any cause) | ||||||
| 3. Ariza-Sole A. et al., 2020 [31] | Delayed surgery with preoperative ECMO | 5 | 5.2 days | ~5 days | 0 | 0% | |
| 5 + 2 (+ECMO as a bridge to decision) | 2 | 28.5% (mortality including patients on ECMO as a bridge to decision) | |||||
| Urgent surgery without/with postoperative ECMO | 15 | 5 | 33% | ||||
| 4. Ronco D. et al., 2021 [24] | Delayed surgery with preoperative ECMO or ECMO in combinations with other MCS (+Impella; +PA/RV cannula; +IABP) | 100 | ̴ 6.3 days | 5.7 days (ECMO) | 29.2% | ||
| 5. Artemiou P. et al., 2020 [32] | Delayed surgery with preoperative ECMO | 3 | 1st patient: 13 days | 1st patient: 12 days | 1 (third patient) | 33.33% | |
| 2nd patient: 17 days | 2nd patient: 17 days | ||||||
| 3rd patient: 11 days | 3rd patient: 9 days | ||||||
| 6. McLaughlin A. et al., 2016 [33] | Delayed surgery with preoperative ECMO | 3 1 patient with VSR+PMR | 1st patient: 4 days | 1st patient: no data | 0 | 0% | |
| 2nd patient: no data | 2nd patient: 7 days | ||||||
| 3rd patient: 9 days | 3rd patient: 5 days | ||||||
| 7. Hobbs R et.al., 2015 [34] | Delayed surgery with preoperative ECMO (+IABP)/BIVAD | 3 | 1st patient: 2 days | 2 days | 1 (after conversion to BIVAD) | 33.3% | |
| 2nd patient: 11 days | 7 days | ||||||
| 3rd patient: 5 days | 4 days | ||||||
| 8. Sanchez Vega J.D. et al., 2020 [35] | Surgery performed from 4th day with preoperative use of ECMO | No data | an average of 5 days [1,2,3,4,5,6] in all 3 groups with ECMO | 4 days | 36% | ||
| Surgery performed within 1–3 day with preoperative ECMO | No data | 1–3 days | 50% | ||||
| Surgery performed within 24 h with preoperative ECMO | No data | within 24 h | 62.2% | ||||
| All types of treatment | 122 | 2.6 ± 3.5 days | 60% | ||||
| 9. La Torre et al., 2011 [36] | Delayed surgery with preoperative Impella Recover LP 5.0 | 5 | No data | 14 ± 6 days | 2 | 40% | |
| 10. Gregoric ID et al., 2014 [37] | Delayed surgery with preoperative Tandem Heart | 8 | No data | 7 ± 3 days | 0 | 0% within 30 days | |
| 11. Ronco D. et al., 2021 [38] | Surgery with preoperative ECMO | 35 | No data | No data | 19 | 54.28% | |
| Urgent surgery | 212 | No data | No data | 108 | 50.94% | ||
| 12. Rob D. et al., 2017 [39] | Delayed surgery with preoperative ECMO | 7 | No data | Mean duration of ECMO support was 12 (±6) days (no data if it includes only preoperative period) | 4 | 57.1% | |
| Patient in cardiogenic shock treated without preoperative ECMO | 7 | 6 | 85.71% | ||||
| 13. Huang S.M. et al., 2015 [40] | Surgery with preoperative ECMO | 6 | No data | No data | 2 | 33% | |
| Urgent surgery | 41 | 17 | 41.46% | ||||
| All the patients with VSR | 47 | No data (AMI to VSR repair 5.3 ± 10.4 days) | 17 | 36.2% | |||
| 14. Matteucci M. et al., 2020 [41] | Surgery with pre- or intraoperative ECMO (VSR+other AMI mechanical complications) | 25 | No data | No data | 15 | 60% | |
| Surgery with postoperative use of ECMO (VSR+other AMI mechanical complications) | 42 | No data | No data | 20 | 47.6% | ||
| VSR group with pre- and postoperative ECMO with and without surgery | 102 | No data | 208.2 ± 242.5 h | 66 | 64.70% | ||
| All the patients with AMI mechanical complications with or without surgery with pre- or postoperative MCS | 158 | No data | 5.9 days | 99 | 62.70% | ||
| 15. Fujimoto K. et al., 2001 [42] | Surgery with preoperative ECMO in patients with a critical general condition (VSR+ other AMI mechanical complication) | 9 | 76 ± 5.7 h | 5 | 55% | ||
| 16. Vondran M. et al., 2021 [14] | Surgery with preoperative ECMO | 4 | No data | No data | 1 | 25% | |
| Surgery with preoperative IABP | 36 | No data | No data | 20 | 55.55% | ||
| Urgent surgery | 32 | 23 | 71.87% | ||||
| All patients with VSR | 53 | No data (AMI to VSR repair 11.9 ± 10.6 days) | 23 | 56.6% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żbikowska, K.; Wróbel, K. Mechanical Circulatory Support in Delayed Surgery of Post-Infarction Ventricular Septal Rupture in Patients in Cardiogenic Shock—A Review. J. Clin. Med. 2022, 11, 4728. https://doi.org/10.3390/jcm11164728
Żbikowska K, Wróbel K. Mechanical Circulatory Support in Delayed Surgery of Post-Infarction Ventricular Septal Rupture in Patients in Cardiogenic Shock—A Review. Journal of Clinical Medicine. 2022; 11(16):4728. https://doi.org/10.3390/jcm11164728
Chicago/Turabian StyleŻbikowska, Karolina, and Krzysztof Wróbel. 2022. "Mechanical Circulatory Support in Delayed Surgery of Post-Infarction Ventricular Septal Rupture in Patients in Cardiogenic Shock—A Review" Journal of Clinical Medicine 11, no. 16: 4728. https://doi.org/10.3390/jcm11164728
APA StyleŻbikowska, K., & Wróbel, K. (2022). Mechanical Circulatory Support in Delayed Surgery of Post-Infarction Ventricular Septal Rupture in Patients in Cardiogenic Shock—A Review. Journal of Clinical Medicine, 11(16), 4728. https://doi.org/10.3390/jcm11164728





