Novel Method for Early Prediction of Clinically Significant Drug–Drug Interactions with a Machine Learning Algorithm Based on Risk Matrix Analysis in the NICU
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Data Acquisition
2.3. Causal Probability, Severity, a Risk Matrix Development of DDIs
2.4. Establishment, Optimization, and Validation of Random Forest Model
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics
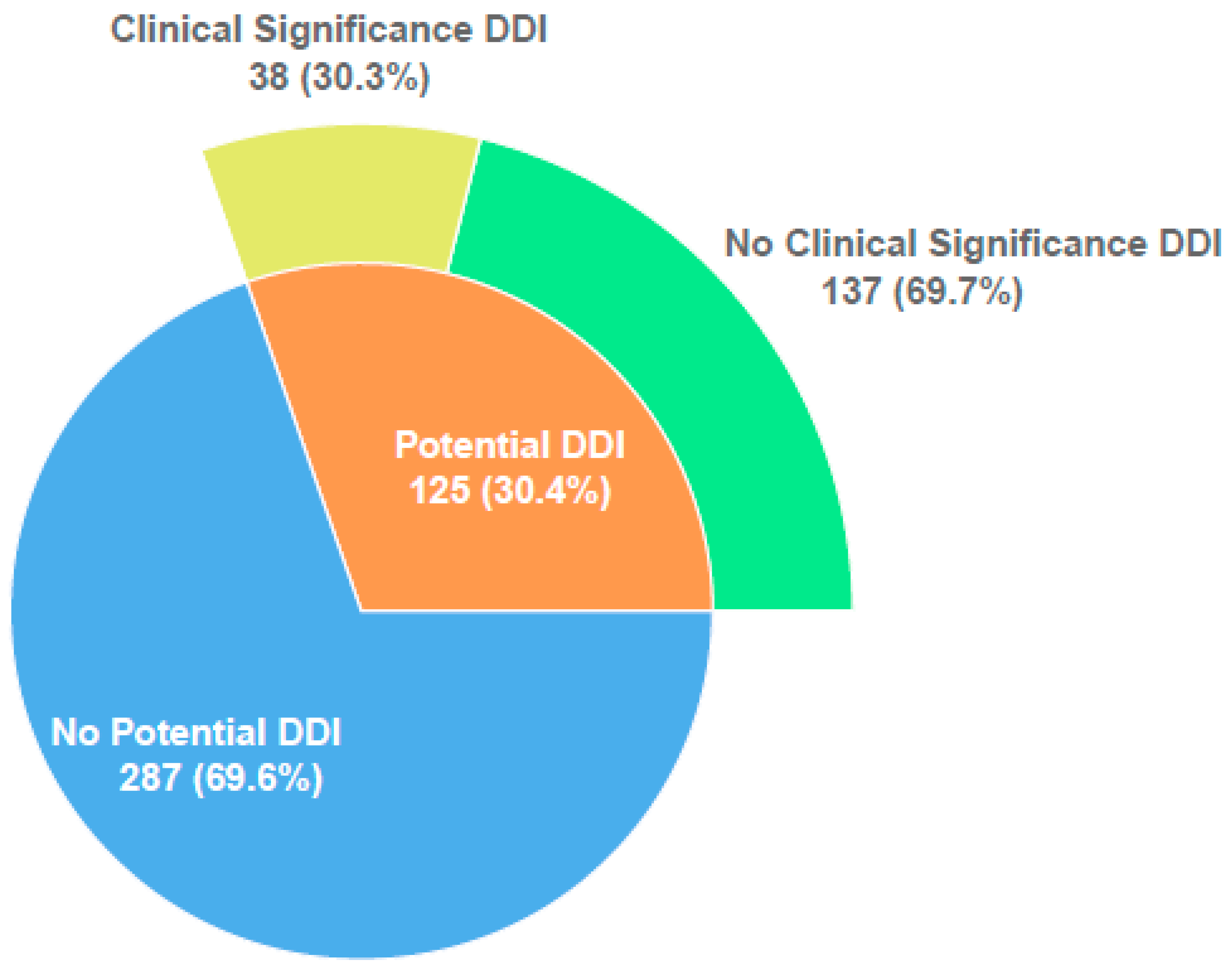
3.2. Characteristics of Potential and Observed DDIs: Incidence and Severity
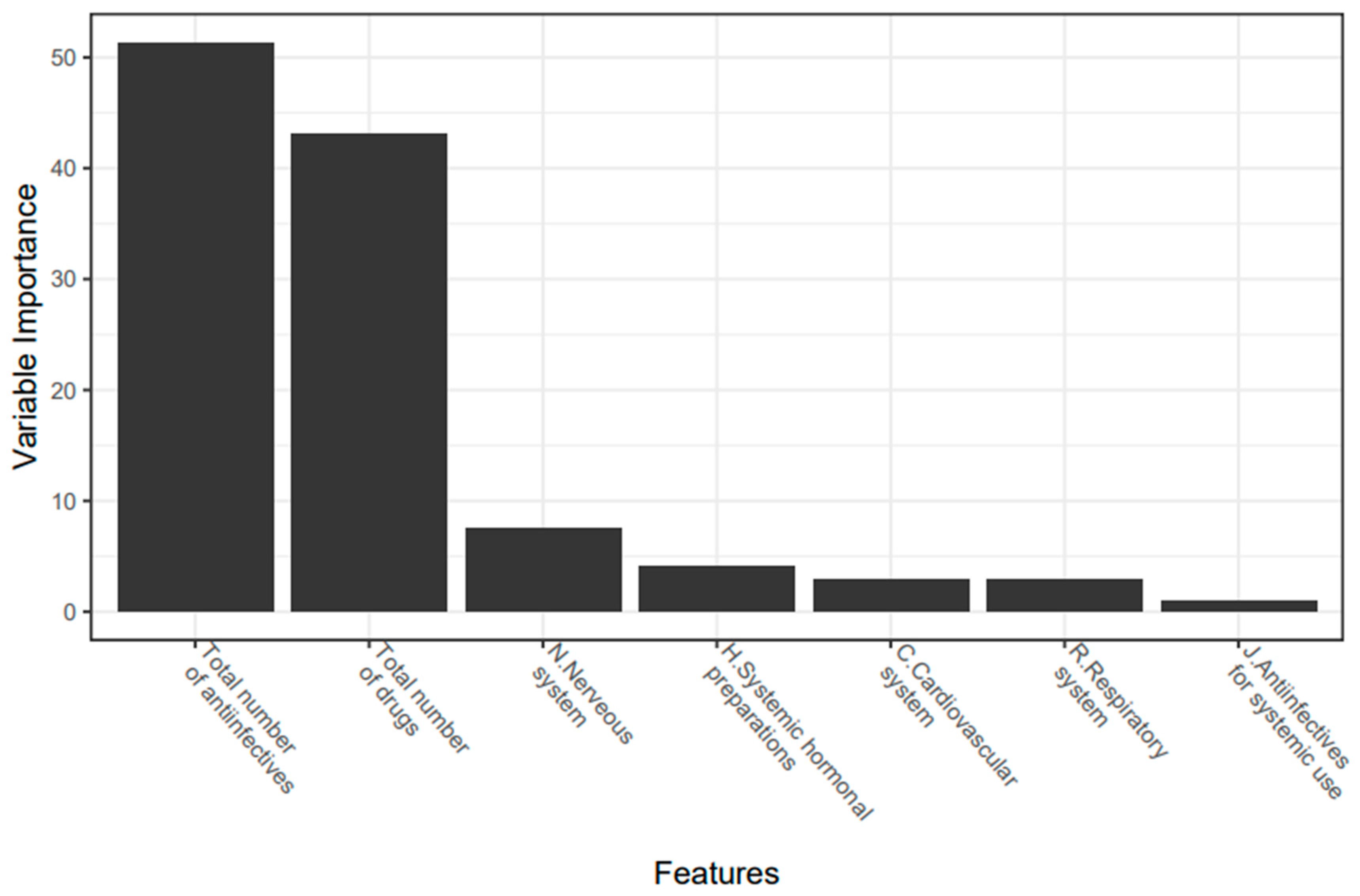
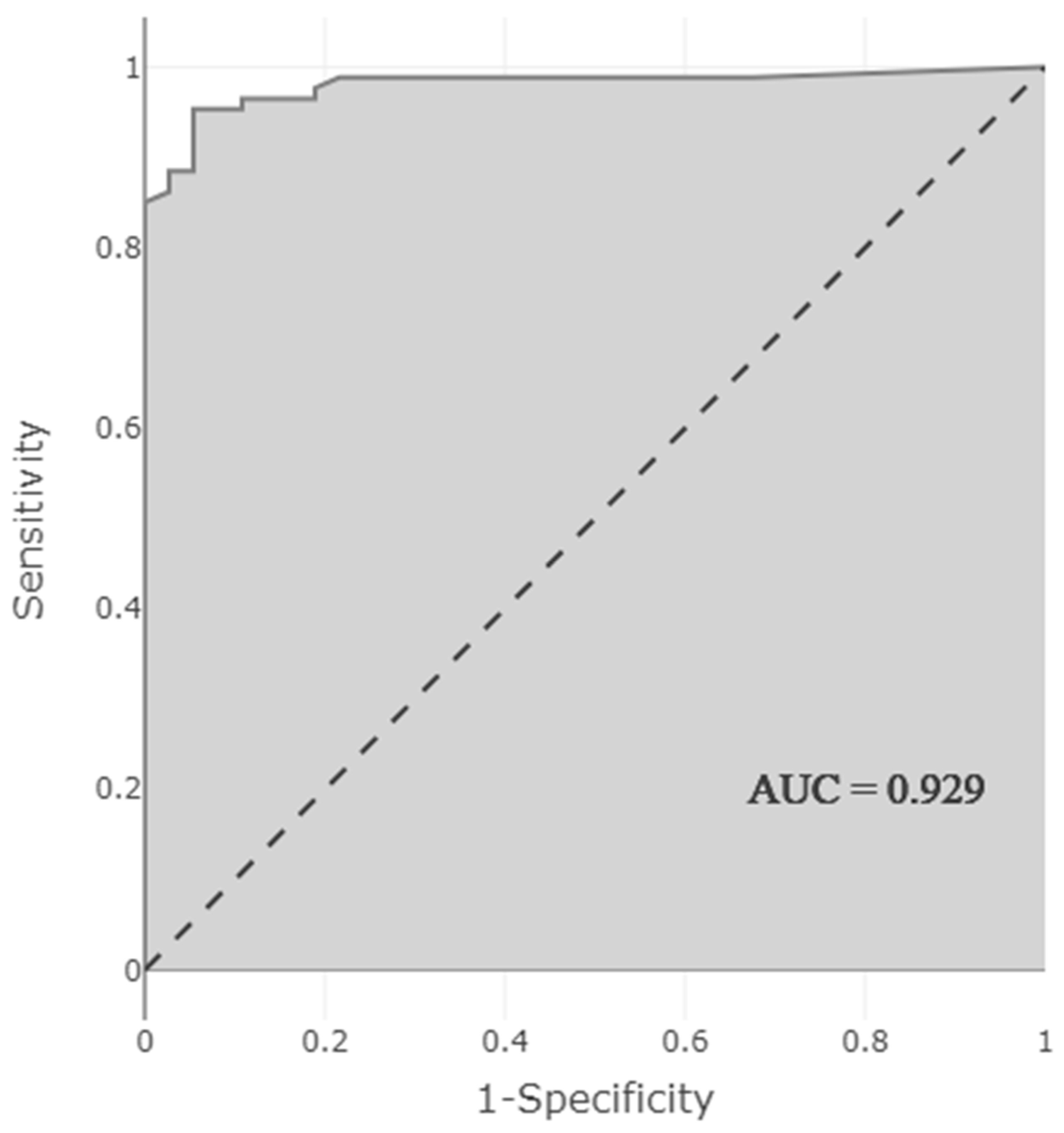
3.3. Development and Optimization of a Model to Predict the Presence of Clinically Relevant DDI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piekos, S.; Pope, C.; Ferrara, A.; Zhong, X.B. Impact of Drug Treatment at Neonatal Ages on Variability of Drug Metabolism and Drug-drug Interactions in Adult Life. Curr. Pharmacol. Rep. 2017, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bhatt-Mehta, V. “Potential” Drug-Drug Interactions and the PICU: Should We Worry About ICU Polypharmacy? Pediatr. Crit. Care Med. 2016, 17, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.D.C.; Camarinha, B.D.; Ferreira Bezerra, N.C.; Panisset, A.G.; de Souza, R.B.; Silva, M.T.; Lopes, L.C. Severe Potential Drug-Drug Interactions and the Increased Length of Stay of Children in Intensive Care Unit. Front. Pharmacol. 2020, 11, 555407. [Google Scholar] [CrossRef] [PubMed]
- Lebowitz, M.B.; Olson, K.L.; Burns, M.; Harper, M.B.; Bourgeois, F. Drug-Drug Interactions Among Hospitalized Children Receiving Chronic Antiepileptic Drug Therapy. Hosp. Pediatr. 2016, 6, 282–289. [Google Scholar] [CrossRef]
- Yeh, M.L.; Chang, Y.J.; Yeh, S.J.; Huang, L.J.; Yen, Y.T.; Wang, P.Y.; Li, Y.C.; Hsu, C.Y. Potential drug-drug interactions in pediatric outpatient prescriptions for newborns and infants. Comput. Methods Programs Biomed. 2014, 113, 15–22. [Google Scholar] [CrossRef]
- Leape, L.L.; Bates, D.W.; Cullen, D.J.; Cooper, J.; Demonaco, H.J.; Gallivan, T.; Hallisey, R.; Ives, J.; Laird, N.; Laffel, G.; et al. Systems analysis of adverse drug events. ADE Prevention Study Group. JAMA 1995, 274, 35–43. [Google Scholar] [CrossRef]
- Costa, H.T.; Leopoldino, R.W.D.; da Costa, T.X.; Oliveira, A.G.; Martins, R.R. Drug-drug interactions in neonatal intensive care: A prospective cohort study. Pediatr. Neonatol. 2021, 62, 151–157. [Google Scholar] [CrossRef]
- Kearns, G.L.; Abdel-Rahman, S.M.; Alander, S.W.; Blowey, D.L.; Leeder, J.S.; Kauffman, R.E. Developmental pharmacology-Drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 2003, 349, 1157–1167. [Google Scholar] [CrossRef]
- Salerno, S.N.; Edginton, A.; Gerhart, J.G.; Laughon, M.M.; Ambalavanan, N.; Sokol, G.M.; Hornik, C.D.; Stewart, D.; Mills, M.; Martz, K.; et al. Physiologically-Based Pharmacokinetic Modeling Characterizes the CYP3A-Mediated Drug-Drug Interaction between Fluconazole and Sildenafil in Infants. Clin. Pharmacol. Ther. 2021, 109, 253–262. [Google Scholar] [CrossRef]
- Percha, B.; Altman, R.B. Informatics confronts drug-drug interactions. Trends Pharmacol. Sci. 2013, 34, 178–184. [Google Scholar] [CrossRef]
- Horn, J.R.; Hansten, P.D.; Chan, L.N. Proposal for a new tool to evaluate drug interaction cases. Ann. Pharmacother. 2007, 41, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Lexicomp Online, Drug Interactions Online. Waltham, MA: UpToDate. Available online: https://online.lexi.com (accessed on 7 March 2022).
- Kuhn, M. Caret: Classification and Regression Training. R Package Version, 6, 0–85. Available online: https://cran.r-project.org/web/packages/caret/index.html (accessed on 14 March 2022).
- Yamada, I.; Miyasaka, N.; Kobayashi, D.; Wakana, K.; Oshima, N.; Wakabayashi, A.; Sakamoto, J.; Saida, Y.; Tateishi, U.; Eishi, Y. Endometrial Carcinoma: Texture Analysis of Apparent Diffusion Coefficient Maps and Its Correlation with Histopathologic Findings and Prognosis. Radiol. Imaging Cancer 2019, 1, e190054. [Google Scholar] [CrossRef] [PubMed]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Rehmsmeier, M. Precrec: Fast and accurate precision–recall and ROC curve calculations in R. Bioinformatics 2017, 33, 145–147. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. RStudio. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Stark, A.; Smith, P.B.; Hornik, C.P.; Zimmerman, K.O.; Hornik, C.D.; Pradeep, S.; Clark, R.H.; Benjamin, D.K., Jr.; Laughon, M.; Greenberg, R.G. Medication Use in the Neonatal Intensive Care Unit and Changes from 2010 to 2018. J. Pediatr. 2022, 240, 66–71.e4. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lee, I.H.; Yang, M.; Cho, Y.S.; Jo, Y.H.; Bae, H.J.; Kim, Y.S.; Park, J.D. Clinical significance of potential drug-drug interactions in a pediatric intensive care unit: A single-center retrospective study. PLoS ONE 2021, 16, e0246754. [Google Scholar] [CrossRef]
- Rosen, K.; Wiesen, M.H.; Oberthur, A.; Michels, G.; Roth, B.; Fietz, C.; Muller, C. Drug-drug interactions in Neonatal Intensive Care Units: How to overcome a challenge. Minerva Pediatr. 2021, 73, 188–197. [Google Scholar] [CrossRef]
- Nasrollahi, S.; Meera, N.K. Prevalence of Potential Drug-Drug Interactions in Neonatal Intensive Care Unit of a Tertiary Care Hospital: A Prospective Observational Study. Int. J. Life Sci. Pharma Res. 2020, 10, P40–P45. [Google Scholar] [CrossRef]
- Abouelkheir, M.; Alsubaie, S. Pediatric acute kidney injury induced by concomitant vancomycin and piperacillin-tazobactam. Pediatr. Int. 2018, 60, 136–141. [Google Scholar] [CrossRef]
- Spriet, I.; Goyens, J.; Meersseman, W.; Wilmer, A.; Willems, L.; Van Paesschen, W. Interaction between valproate and meropenem: A retrospective study. Ann. Pharmacother. 2007, 41, 1130–1136. [Google Scholar] [CrossRef]
- Antoon, J.W.; Hall, M.; Herndon, A.; Carroll, A.; Ngo, M.L.; Freundlich, K.L.; Stassun, J.C.; Frost, P.; Johnson, D.P.; Chokshi, S.B.; et al. Prevalence of Clinically Significant Drug-Drug Interactions Across US Children’s Hospitals. Pediatrics 2020, 146, e20200858. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Aziz, S.; Noor, S.; Haider, I.; Shams, F.; Haq, I.; Khadim, F.; Khan, Q.; Khan, F.; Asif, M. Potential drug-drug interactions in pediatric patients admitted to intensive care unit of Khyber Teaching Hospital, Peshawar, Pakistan: A cross-sectional study. J. Crit. Care 2017, 40, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Segal, G.; Segev, A.; Brom, A.; Lifshitz, Y.; Wasserstrum, Y.; Zimlichman, E. Reducing drug prescription errors and adverse drug events by application of a probabilistic, machine-learning based clinical decision support system in an inpatient setting. J. Am. Med. Inform. Assoc. 2019, 26, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Leng, J.; Liu, Y. Deep learning for drug-drug interaction extraction from the literature: A review. Brief Bioinform. 2020, 21, 1609–1627. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, X.; Qiu, Y.; Xia, J.; Zhang, W.; Liu, S. A multimodal deep learning framework for predicting drug-drug interaction events. Bioinformatics 2020, 36, 4316–4322. [Google Scholar] [CrossRef]
- Kastrin, A.; Ferk, P.; Leskosek, B. Predicting potential drug-drug interactions on topological and semantic similarity features using statistical learning. PLoS ONE 2018, 13, e0196865. [Google Scholar] [CrossRef]
- Han, K.; Cao, P.; Wang, Y.; Xie, F.; Ma, J.; Yu, M.; Wang, J.; Xu, Y.; Zhang, Y.; Wan, J. A Review of Approaches for Predicting Drug-Drug Interactions Based on Machine Learning. Front. Pharmacol. 2021, 12, 814858. [Google Scholar] [CrossRef]
- Xie, W.; Wang, L.; Cheng, Q.; Wang, X.; Wang, Y.; Bi, H.; He, B.; Feng, W. Integrated Random Negative Sampling and Uncertainty Sampling in Active Learning Improve Clinical Drug Safety Drug-Drug Interaction Information Retrieval. Front. Pharmacol. 2020, 11, 582470. [Google Scholar] [CrossRef]
- Hassanzad, M.; Tashayoie Nejad, S.; Mahboobipour, A.A.; Salem, F.; Baniasadi, S. Potential drug-drug interactions in hospitalized pediatric patients with respiratory disorders: A retrospective review of clinically important interactions. Drug Metab. Pers. Ther. 2020, 35. [Google Scholar] [CrossRef]
- Kovacevic, M.; Kovacevic, S.V.; Radovanovic, S.; Stevanovic, P.; Miljkovc, B. Adverse drug reactions caused by drug-drug interactions in cardiovascular disease patients: Introduction of a simple prediction tool using electronic screening database items. Curr. Med. Res. Opin. 2019, 35, 1873–1883. [Google Scholar] [CrossRef]
| Population Characteristics | |
|---|---|
| Sex, Male, n (%) | 232 (56.3%) |
| Sex ratio (male/female) | 1.29 |
| 5 min APGAR score, median (IQR) | 8 (2) |
| Gestational age (weeks), median (IQR) | 37 (4) |
| Extremely preterm (<28 weeks), n (%) | 7 (1.7%) |
| Very preterm (28 to 32 weeks), n (%) | 52 (12.6%) |
| Moderate preterm (32 to 34 weeks), n (%) | 16 (3.9%) |
| Late preterm (34 to 37 weeks), n (%) | 102 (24.8%) |
| Term (>37 weeks), n (%) | 235 (57%) |
| SGA at admission, n (%) | 88 (21.4%) |
| Birth weight (g), mean (SD) | 2631.1 (877.2) |
| Extremely low birth weight (<1000 g), n (%) | 26 (6.3%) |
| Very low birth weight (1000 to 1500 g), n (%) | 27 (6.6%) |
| Low birth weight (1500 to 2500 g), n (%) | 119 (28.9%) |
| Normal birth weight (>2500 g), n (%) | 240 (58.3%) |
| Multiple birth, n (%) | 53 (12.9%) |
| Caesarean section, n (%) | 337 (81.8%) |
| Diagnosis (ICD-10), n (%) | |
| Complications of labor and delivery, n (%) | 165 (40%) |
| Infectious diseases, n (%) | 46 (11.2%) |
| Diseases of the respiratory system, n (%) | 46 (11.2%) |
| Diseases of the circulatory system, n (%) | 37 (9%) |
| Other disorders of fluid, electrolyte, and acid-base balance, n (%) | 26 (6.3%) |
| Diseases of the digestive system, n (%) | 24 (5.8%) |
| Diseases of the nervous system, n (%) | 20 (4.9%) |
| Neonatal jaundice, n (%) | 19 (4.6%) |
| Congenital malformations, deformations and chromosomal abnormalities, n (%) | 15 (3.6%) |
| Metabolic disorders, n (%) | 9 (2.2%) |
| Neoplasms, n (%) | 6 (1.4%) |
| Drugs (ATC) (N = 2280), n (%) | |
| J. Anti-infectives for systemic use, n (%) | 905 (39.69%) |
| A. Alimentary tract and metabolism, n (%) | 591 (25.92%) |
| N. Nervous system, n (%) | 229 (10.05%) |
| B. Blood and blood-forming organs, n (%) | 175 (7.67%) |
| C. Cardiovascular system, n (%) | 170 (7.46%) |
| R. Respiratory system, n (%) | 81 (3.55%) |
| H. Systemic hormonal preparations, n (%) | 70 (3.07%) |
| S. Sensory organs, n (%) | 31 (1.36%) |
| M. Musculo-skeletal system, n (%) | 11 (0.48%) |
| G. Genito-urinary system and sex hormones, n (%) | 10 (0.44%) |
| L. Antineoplastic and immunomodulating agents, n (%) | 7 (0.31%) |
| Affecting Drug (Inhibitor/Inductor) | Affected Drug (Victim) | Mechanism of DDIs | ADRs Observed as a Result of DDI * | Duration of Exposure (Mean Day) | DIPS (Probability) | Lexicomp® (Severity) | Risk Score | Risk Category |
|---|---|---|---|---|---|---|---|---|
| Vancomycin | Amikacin | Additive/synergistic | Increase in creatinine (13) | 16.76 | 2 | 3 | 6 | 2 |
| Dexmedetomidine | Fentanyl | Additive | Bradycardia (6) Hypotension (2) | 3.25 | 2 | 3 | 6 | 2 |
| Amikacin | Furosemide | Additive/synergistic | Increase in creatinine (4) | 2 | 2 | 3 | 6 | 2 |
| Dexmedetomidine | Furosemide | Additive | Hypotension (3) | 4.33 | 2 | 3 | 6 | 2 |
| Phenytoin | Phenobarbital | Metabolism | Decreased effect of phenytoin (2) | 11 | 3 | 3 | 9 | 2 |
| Hydrocortisone | Furosemide | Additive | Hypokalemia (2) | 4.50 | 2 | 3 | 6 | 2 |
| Phenobarbital | Furosemide | Unknown | Hypotension (2) | 7.50 | 2 | 3 | 6 | 2 |
| Salbutamol | Furosemide | Additive | Hypokalemia (2) | 6.50 | 2 | 3 | 6 | 2 |
| Amiodarone | Flecainide | Additive | QTc prolongation | 2 | 4 | 4 | 16 | 3 |
| Furosemide | Captopril | Volume depletion | Increase in creatinine Hypotension | 4 | 2 | 3 | 5 | 2 |
| Hydrocortisone | Furosemide | Additive | Hypokalemia | 3 | 2 | 3 | 6 | 2 |
| Hydrochlorothiazide | Diazoxide | Decrease in insulin secretion | Hyperglycemia | 8 | 3 | 3 | 9 | 2 |
| Nifedipine | Propranolol | Additive | Hypotension | 5 | 2 | 3 | 6 | 2 |
| Caffeine | Adenosine | Antagonism | Decreased effect of adenosine | 10 | 3 | 4 | 12 | 3 |
| Amiodarone | Fluconazole | Metabolism | QTc prolongation | 3 | 2 | 4 | 8 | 2 |
| Phenobarbital | Levetiracetam | Unknown | Decreased effect of levetiracetam | 26 | 2 | 3 | 6 | 2 |
| Ibuprofen | Amikacin | Unknown | Increase in creatinine | 3 | 3 | 3 | 9 | 2 |
| Spironolactone | Captopril | Increase in potassium retention due to aldosterone reduction | Hyperkalemia | 18 | 3 | 3 | 9 | 2 |
| Fluconazole | Midazolam | Metabolism | Prolonged sedation | 1 | 2 | 3 | 6 | 2 |
| Diazoxide | Dexmedetomidine | Additive | Hypotension | 3 | 2 | 3 | 6 | 2 |
| Dexamethasone | Hydrochlorothiazide | Additive | Hypokalemia | 2 | 2 | 3 | 6 | 2 |
| Fluconazole | Ibuprofen | Metabolism | Decrease in hemoglobin | 2 | 2 | 3 | 6 | 2 |
| Ciprofloxacin | Phenytoin | Unknown | Decreased phenytoin plasma concentration | 5 | 4 | 2 | 8 | 2 |
| Allopurinol | Phenytoin | Unknown | Increased phenytoin plasma concentration | 1 | 3 | 3 | 9 | 2 |
| Midazolam | Fentanyl | Additive | Chest rigidity | 3 | 4 | 4 | 16 | 3 |
| Adenosine | Dexmedetomidine | Additive | Bradycardia | 3 | 2 | 3 | 6 | 2 |
| Phenobarbital | Topiramate | Unknown | Decreased effect of topiramate | 7 | 2 | 3 | 6 | 2 |
| Phenobarbital | Dexmedetomidine | Catecholamine reduction | Hypotension | 3 | 2 | 3 | 6 | 2 |
| Fentanyl | Furosemide | Unknown | Hypotension Decreased urine output | 5.50 | 2 | 3 | 6 | 2 |
| Salbutamol | Hydrochlorothiazide | Additive | Hypokalemia | 7 | 2 | 3 | 6 | 2 |
| Phenytoin | Topiramate | Metabolism | Decreased effect of topiramate | 5 | 2 | 3 | 6 | 2 |
| Ciprofloxacin | Midazolam | Metabolism | Prolonged sedation | 3 | 2 | 2 | 4 | 1 |
| Ferrous fumarate | Levothyroxine | Absorption | Decreased effect of levothyroxine | 13 | 2 | 4 | 8 | 2 |
| Cefuroxime | Amikacin | Additive/synergistic | Increase in creatinine | 1 | 2 | 3 | 6 | 2 |
| Nitroglycerine | Furosemide | Additive | Hypotension | 16 | 2 | 3 | 6 | 2 |
| Potassium chloride | Furosemide | Unknown | Hyponatremia | 1 | 2 | 2 | 4 | 1 |
| Methylprednisolone | Furosemide | Additive | Hypokalemia | 1 | 2 | 3 | 6 | 2 |
| Linezolid | Salbutamol | Metabolism | Hypertension | 10 | 3 | 4 | 12 | 3 |
| Nitroglycerine | Dexmedetomidine | Additive | Hypotension | 6 | 2 | 3 | 6 | 2 |
| Potassium chloride | Phenobarbital | Unknown | Hyponatremia | 6 | 2 | 3 | 6 | 2 |
| Dexmedetomidine | Salbutamol | Unknown | Hypokalemia | 1 | 2 | 2 | 4 | 1 |
| Furosemide | Levothyroxine | Unknown | Increase in free T4 | 7 | 2 | 3 | 6 | 2 |
| Furosemide | Levosimendan | Additive | Hypotension | 1 | 2 | 3 | 6 | 2 |
| Prednisolone | Furosemide | Additive | Hypokalemia | 3 | 3 | 3 | 9 | 2 |
| Adrenalin | Dopamine | Additive | Hypertension | 3 | 2 | 3 | 6 | 2 |
| P R O B A B I L I T Y | SEVERİTY | |||||
| A (1) n = 1 (0.30%) | B (2) n = 24 (7.32%) | C (3) n = 262 (79.88%) | D (4) n = 40 (12.20%) | X (5) n = 1 (0.30%) | ||
| Highly Probable (4) n = 3 (0.91%) | 4 | 8 | 12 | 16 | 20 | |
| Probable (3) n = 16 (4.88%) | 3 | 6 | 9 | 12 | 15 | |
| Possible (2) n = 55 (16.77%) | 2 | 4 | 6 | 8 | 10 | |
| Doubtful (1) n = 254 (77.44%) | 1 | 2 | 3 | 4 | 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalçın, N.; Kaşıkcı, M.; Çelik, H.T.; Allegaert, K.; Demirkan, K.; Yiğit, Ş.; Yurdakök, M. Novel Method for Early Prediction of Clinically Significant Drug–Drug Interactions with a Machine Learning Algorithm Based on Risk Matrix Analysis in the NICU. J. Clin. Med. 2022, 11, 4715. https://doi.org/10.3390/jcm11164715
Yalçın N, Kaşıkcı M, Çelik HT, Allegaert K, Demirkan K, Yiğit Ş, Yurdakök M. Novel Method for Early Prediction of Clinically Significant Drug–Drug Interactions with a Machine Learning Algorithm Based on Risk Matrix Analysis in the NICU. Journal of Clinical Medicine. 2022; 11(16):4715. https://doi.org/10.3390/jcm11164715
Chicago/Turabian StyleYalçın, Nadir, Merve Kaşıkcı, Hasan Tolga Çelik, Karel Allegaert, Kutay Demirkan, Şule Yiğit, and Murat Yurdakök. 2022. "Novel Method for Early Prediction of Clinically Significant Drug–Drug Interactions with a Machine Learning Algorithm Based on Risk Matrix Analysis in the NICU" Journal of Clinical Medicine 11, no. 16: 4715. https://doi.org/10.3390/jcm11164715
APA StyleYalçın, N., Kaşıkcı, M., Çelik, H. T., Allegaert, K., Demirkan, K., Yiğit, Ş., & Yurdakök, M. (2022). Novel Method for Early Prediction of Clinically Significant Drug–Drug Interactions with a Machine Learning Algorithm Based on Risk Matrix Analysis in the NICU. Journal of Clinical Medicine, 11(16), 4715. https://doi.org/10.3390/jcm11164715







