Therapeutic Potential of the Combination of Pentoxifylline and Vitamin-E in Inflammatory Bowel Disease: Inhibition of Intestinal Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Human Primary Intestinal Myofibroblasts and Cell Culture
2.2. Pharmacologic Treatment
2.3. Cytotoxicity Assay
2.4. Quantitative Real-Time PCR
2.5. Western Blot Analysis
2.6. Sirius Red/Fast Green Collagen Staining
2.7. Immunofluorescence Microscopy
2.8. DSS-Induced IBD Murine Models
2.9. Clinical Disease Scoring
2.10. Macroscopic Assessment and Histological Analysis
2.11. Quantification of Fibrosis
2.12. Statistical Analysis
3. Results
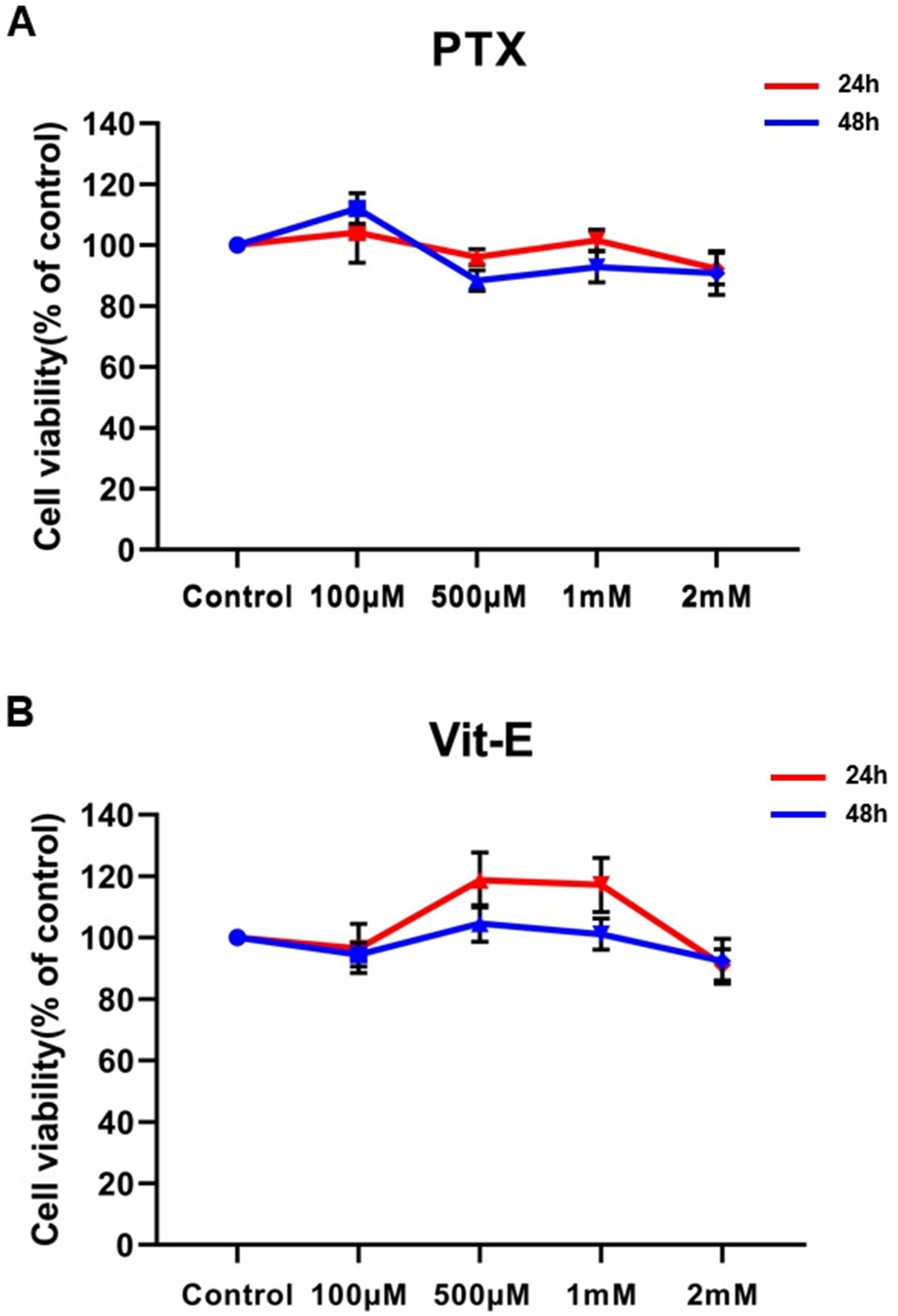
3.1. Given Concentrations of PTX or Vit-E Have No Significant Cytotoxicity against HIMFs
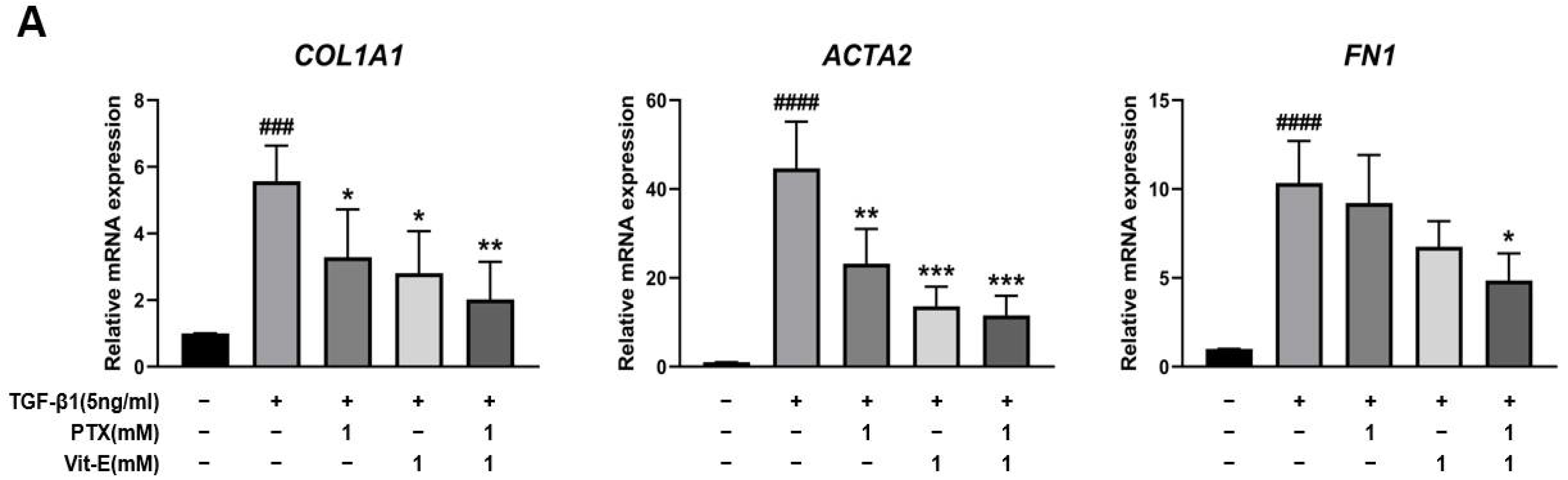
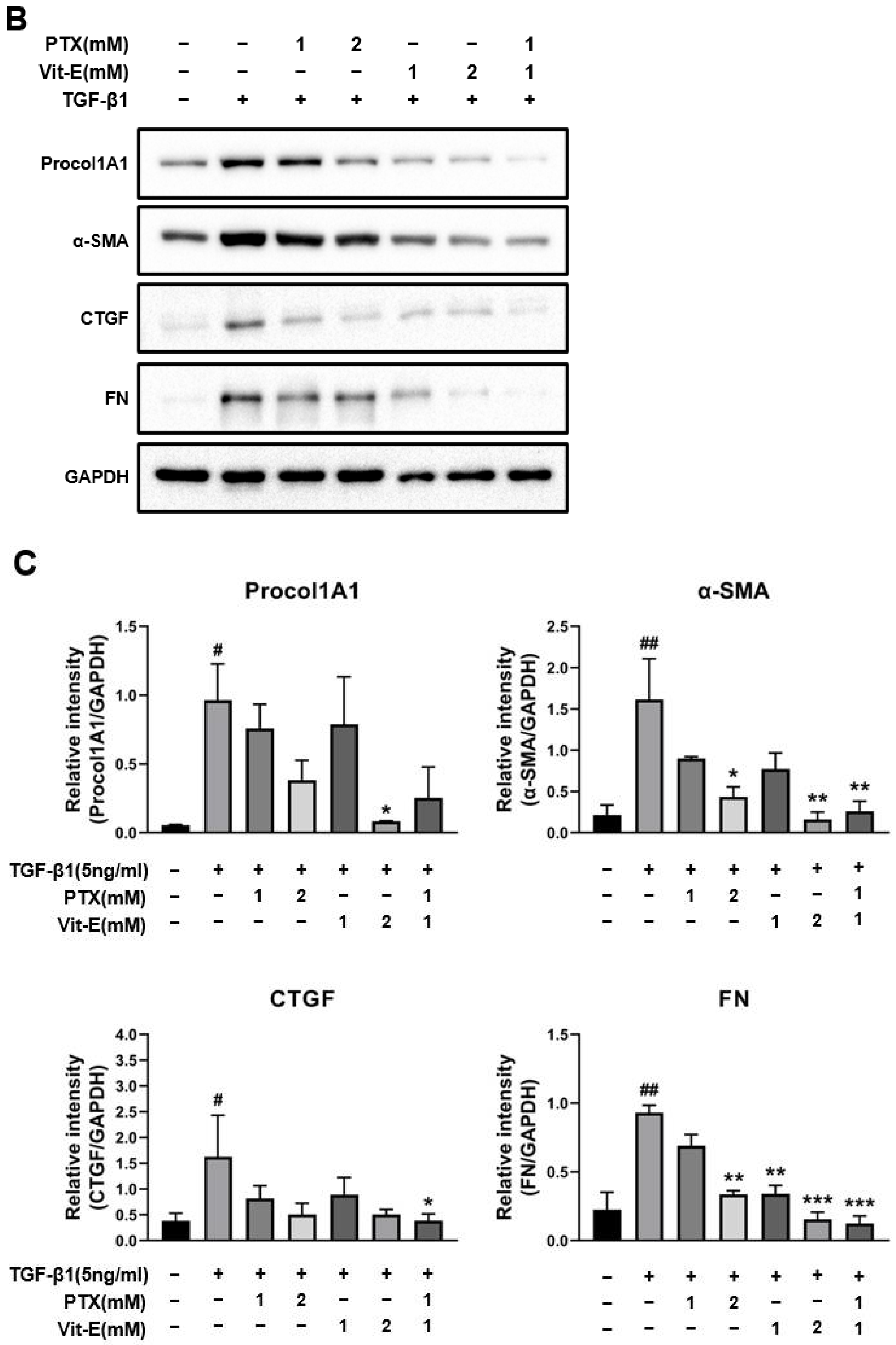
3.2. The Co-Treatment with PTX and Vit-E Suppresses TGF-β1-Induced Expression of Fibrogenic Markers More than Either Treatment Alone in HIMFs
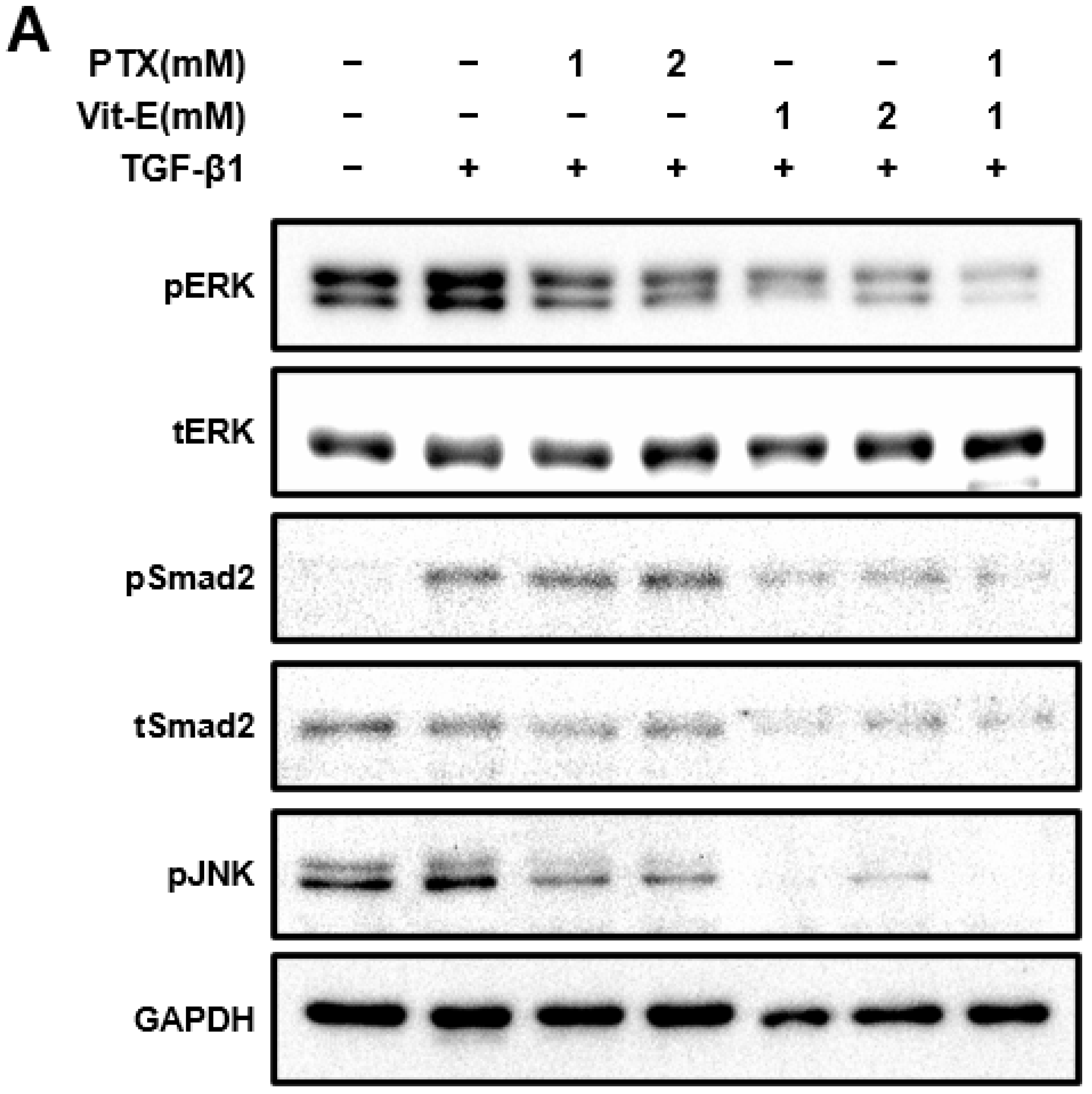
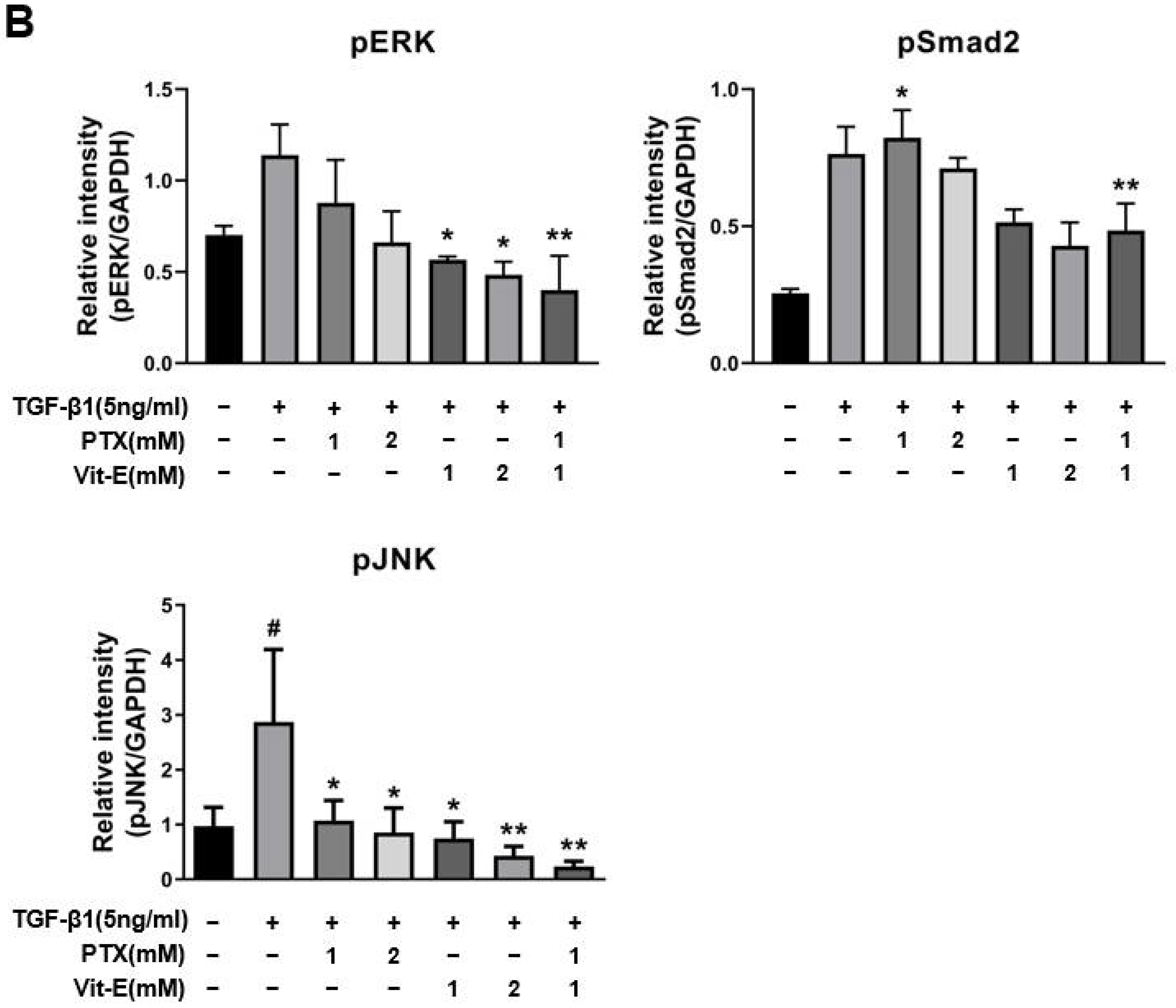
3.3. The Combination of PTX and Vit-E Exhibits Anti-Fibrotic Efects through Inhibition of TGF-β1-Mediated Downstream Signaling, including Both Smad-Dependent and Smad-Independent Pathways
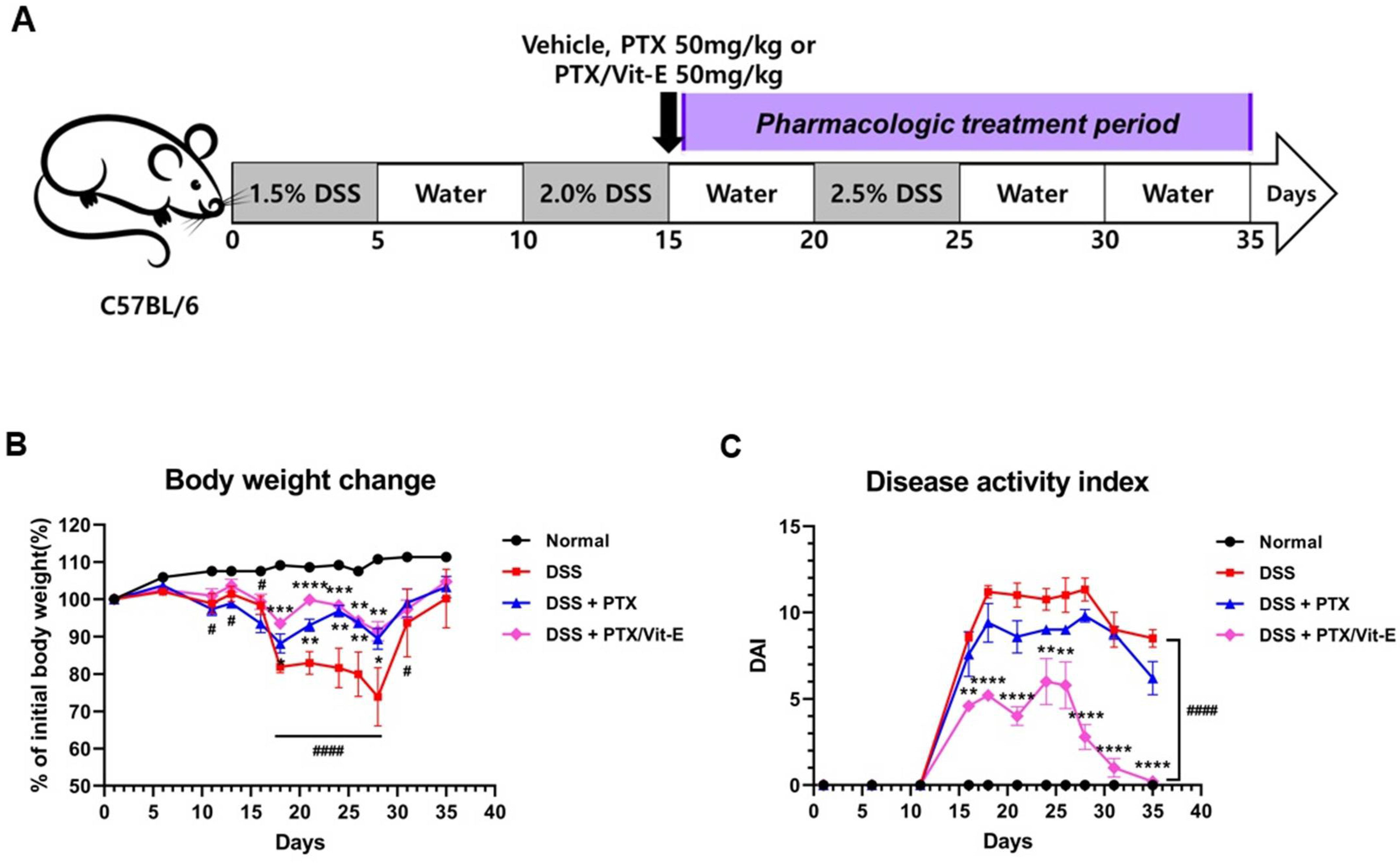
3.4. The Combination of PTX and Vit-E Improves In Vivo Clinical Indicators of IBD
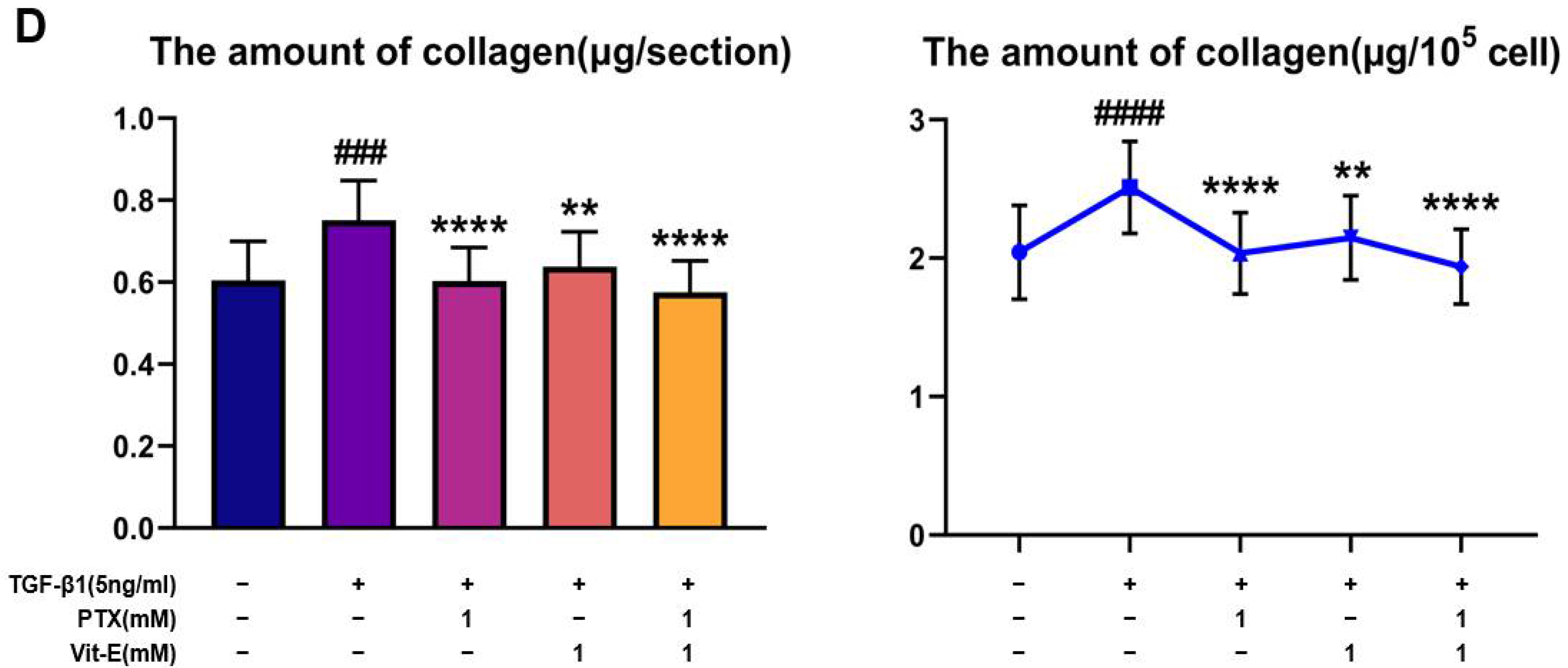
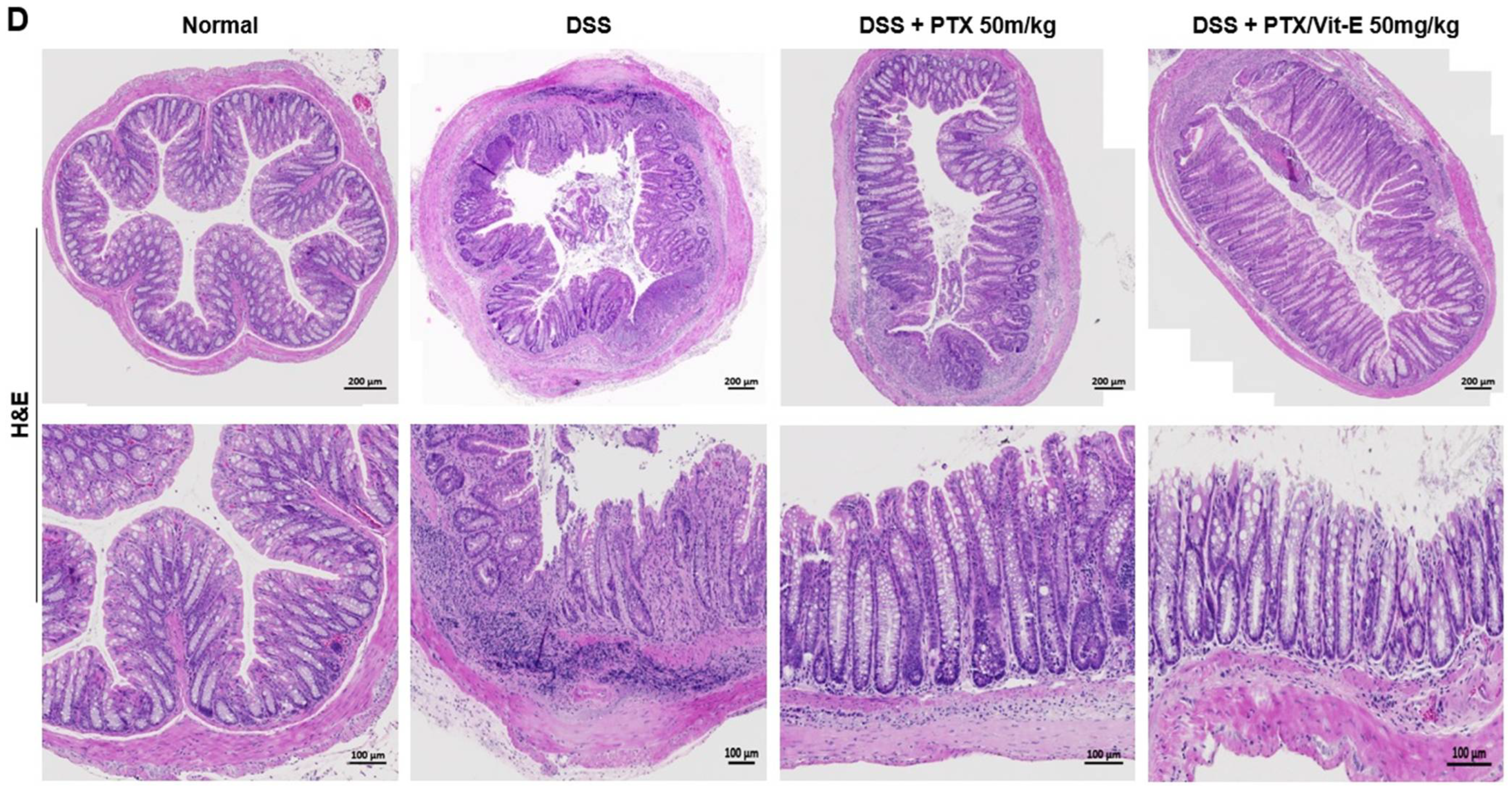
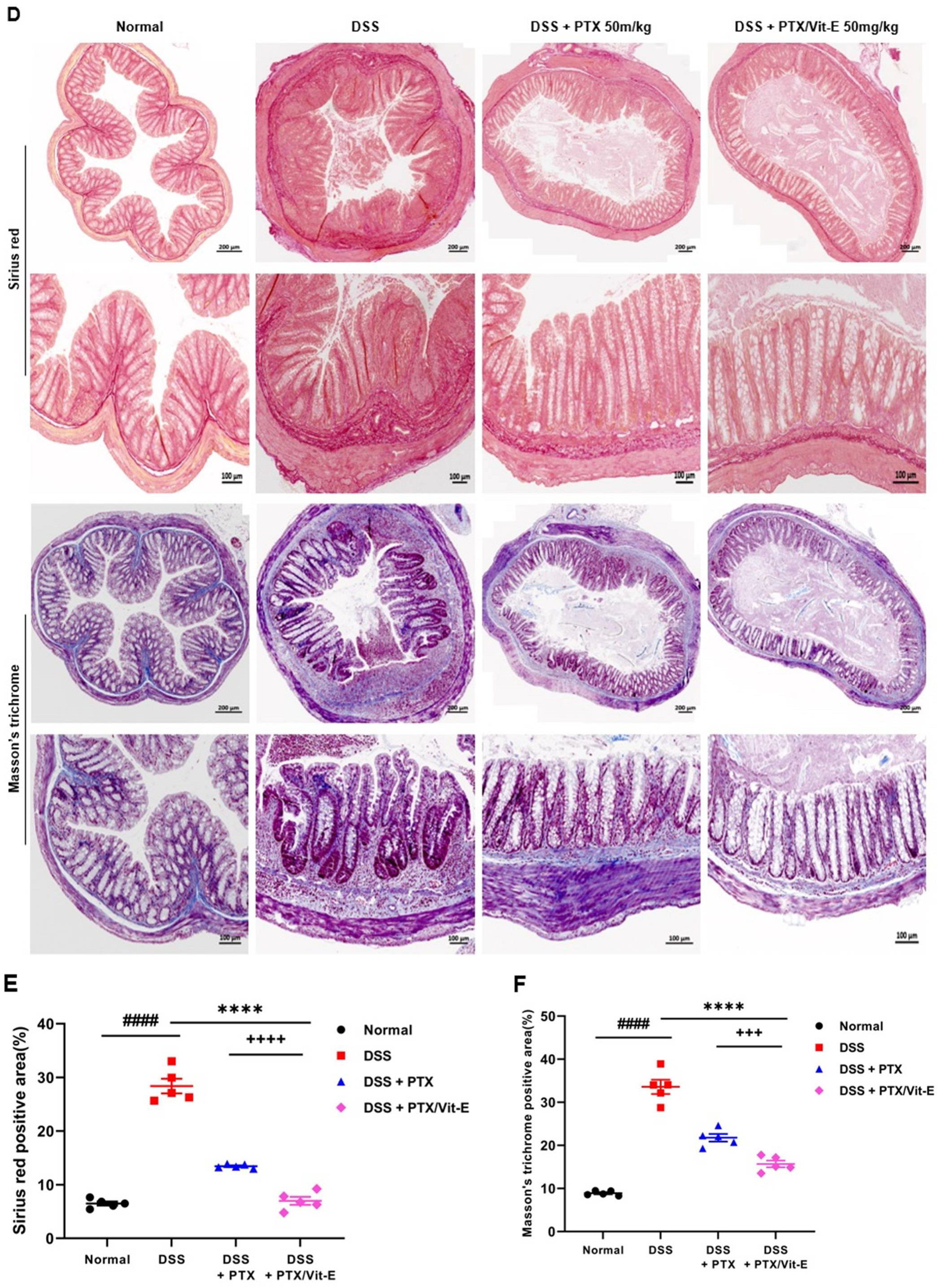
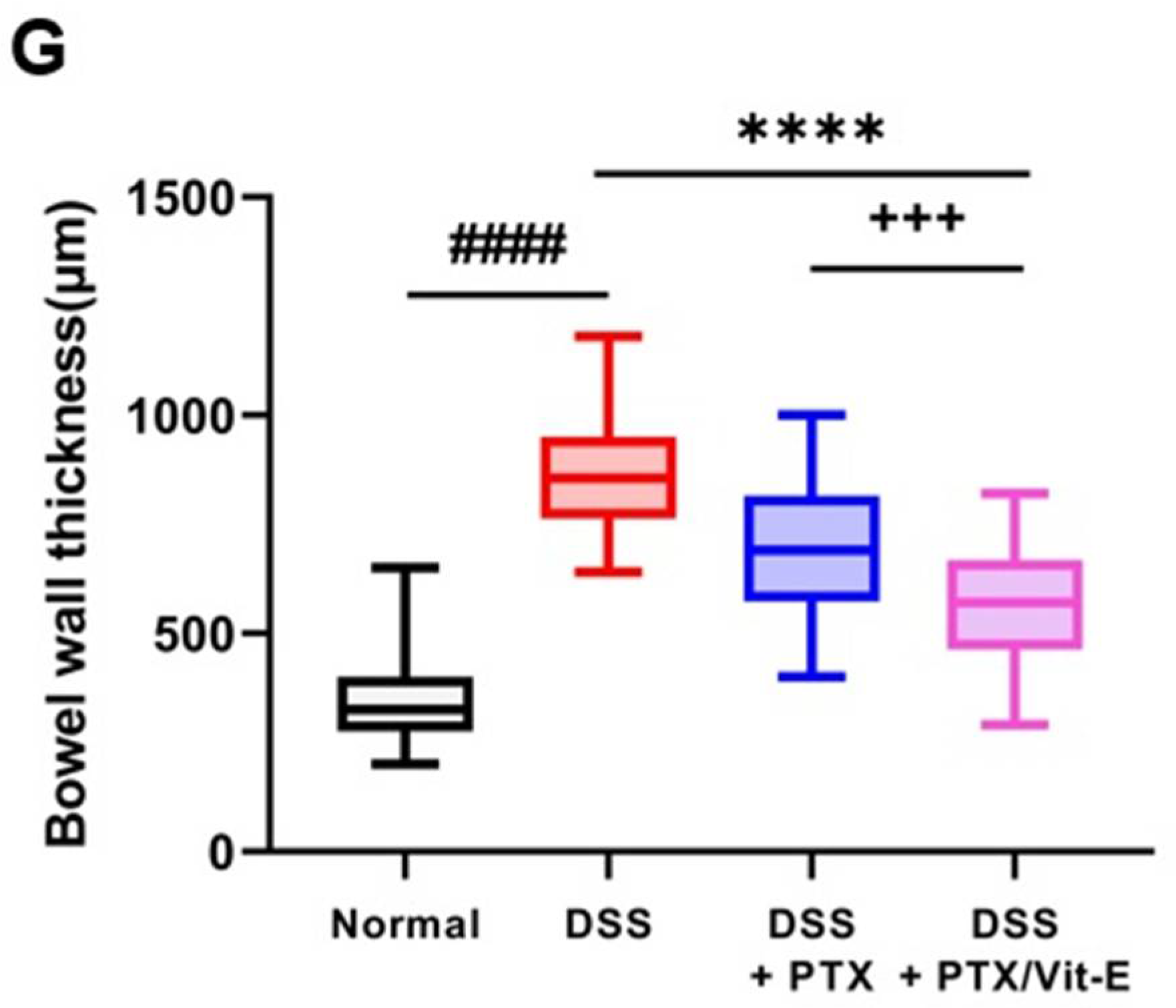
3.5. The Combination of PTX and Vit-E Ameliorates Intestinal Fibrosis in Animal Models of IBD
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Latella, G.; Sferra, R.; Speca, S.; Vetuschi, A.; Gaudio, E. Can we prevent, reduce or reverse intestinal fibrosis in IBD? Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1283–1304. [Google Scholar]
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Burke, J.P.; Mulsow, J.J.; O’keane, C.; Docherty, N.G.; Watson, R.W.; O’connell, P.R. Fibrogenesis in Crohn’s disease. Am. J. Gastroenterol. 2007, 102, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Fiocchi, C. Intestinal fibrosis in inflammatory bowel disease–Current knowledge and future perspectives. J. Crohn’s Colitis 2008, 2, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Fiocchi, C. Intestinal fibrosis in IBD-a dynamic, multifactorial process. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Thia, K.T.; Sandborn, W.J.; Harmsen, W.S.; Zinsmeister, A.R.; Loftus, E.V., Jr. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010, 139, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Lawrance, I.C.; Rogler, G.; Bamias, G.; Breynaert, C.; Florholmen, J.; Pellino, G.; Reif, S.; Speca, S.; Latella, G. Cellular and molecular mediators of intestinal fibrosis. J. Crohn’s Colitis 2017, 11, 1491–1503. [Google Scholar] [CrossRef]
- Longo, W.E.; Virgo, K.S.; Bahadursingh, A.N.; Johnson, F.E. Patterns of disease and surgical treatment among United States veterans more than 50 years of age with ulcerative colitis. Am. J. Surg. 2003, 186, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, G.; Geboes, K.; Rutgeerts, P. Medical therapy for Crohn’s disease strictures. Inflamm. Bowel Dis. 2004, 10, 55–60. [Google Scholar] [CrossRef]
- Rieder, F.; Bettenworth, D.; Imai, J.; Inagaki, Y. Intestinal fibrosis and liver fibrosis: Consequences of chronic inflammation or independent pathophysiology? Inflamm. Intest. Dis. 2016, 1, 41–49. [Google Scholar] [CrossRef]
- Vetuschi, A.; Pompili, S.; Gaudio, E.; Latella, G.; Sferra, R. PPAR-γ with its anti-inflammatory and anti-fibrotic action could be an effective therapeutic target in IBD. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8839–8848. [Google Scholar] [CrossRef]
- Johnson, L.A.; Luke, A.; Sauder, K.; Moons, D.S.; Horowitz, J.C.; Higgins, P.D. Intestinal fibrosis is reduced by early elimination of inflammation in a mouse model of IBD: Impact of a “top-down” approach to intestinal fibrosis in mice. Inflamm. Bowel Dis. 2012, 18, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, G.; Lenti, M.V.; Di Sabatino, A. Therapeutic Targeting of Intestinal Fibrosis in Crohn’s Disease. Cells 2022, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Border, W.A.; Noble, N.A. Transforming growth factor β in tissue fibrosis. N. Engl. J. Med. 1994, 331, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Gabbiani, G. Cell-matrix and cell-cell contacts of myofibroblasts: Role in connective tissue remodeling. Thromb. Haemost. 2003, 90, 993–1002. [Google Scholar] [CrossRef]
- Desmoulière, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef]
- Vallance, B.A.; Gunawan, M.I.; Hewlett, B.; Bercik, P.; Van Kampen, C.; Galeazzi, F.; Sime, P.J.; Gauldie, J.; Collins, S.M. TGF-β1 gene transfer to the mouse colon leads to intestinal fibrosis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 289, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.M.; Kim, S.H.; Kim, E.H. The Molecular Mechanism of Transforming Growth Factor-β Signaling for Intestinal Fibrosis: A Mini-Review. Front. Pharmacol. 2019, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Babyatsky, M.W.; Rossiter, G.; Podolsky, D.K. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology 1996, 110, 975–984. [Google Scholar] [CrossRef]
- Rosenbloom, J.; Castro, S.V.; Jimenez, S.A. Narrative review: Fibrotic diseases: Cellular and molecular mechanisms and novel therapies. Ann. Intern. Med. 2010, 152, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Rodansky, E.S.; Haak, A.J.; Larsen, S.D.; Neubig, R.R.; Higgins, P.D. Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-beta-induced fibrogenesis in human colonic myofibroblasts. Inflamm. Bowel Dis. 2014, 20, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Biancheri, P.; Giuffrida, P.; Docena, G.H.; MacDonald, T.T.; Corazza, G.R.; Di Sabatino, A. The role of transforming growth factor(TGF)-beta in modulating the immune response and fibrogenesis in the gut. Cytokine Growth Factor Rev. 2014, 25, 45–55. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.; Santos-Martinez, M.J.; Santana, A.; Paz-Cabrera, M.C.; Johnston, M.J.; Mourelle, M.; Salas, A.; Guarner, F. Transforming growth factor-beta type 1 receptor (ALK5) and Smad proteins mediate TIMP-1 and collagen synthesis in experimental intestinal fibrosis. J. Pathol. 2011, 224, 461–472. [Google Scholar] [CrossRef]
- Massagué, J.; Wotton, D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000, 19, 1745–1754. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Mulsow, J.J.; Watson, R.W.; Fitzpatrick, J.M.; O’Connell, P.R. Transforming growth factor-beta promotes pro-fibrotic behavior by serosal fibroblasts via PKC and ERK1/2 mitogen activated protein kinase cell signaling. Ann. Surg. 2005, 242, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.A.; Thatcher, T.H.; Olsen, K.C.; Maggirwar, S.B.; Phipps, R.P.; Sime, P.J. PPAR-γ ligands repress TGF-β-induced myofibroblast differentiation by targeting the PI3K/Akt pathway: Implications for therapy of fibrosis. PLoS ONE 2011, 6, e15909. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Gudey, S.K.; Landström, M. Non-Smad signaling pathways. Cell Tissue Res. 2012, 347, 11–20. [Google Scholar] [CrossRef]
- Wengrower, D.; Zanninelli, G. Prevention of fibrosis in experimental colitis by captopril: The role of tgf-beta1. Inflamm. Bowel Dis. 2004, 10, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, T.; Devriese, S.; Castermans, K.; Boland, S.; Leysen, D.; Vandewynckel, Y.P.; Devisscher, L.; Van den Bossche, L.; Van Welden, S.; Dullaers, M.; et al. Treatment of Intestinal Fibrosis in Experimental Inflammatory Bowel Disease by the Pleiotropic Actions of a Local Rho Kinase Inhibitor. Gastroenterology 2017, 153, 1054–1067. [Google Scholar] [CrossRef]
- Li, C.; Flynn, R.S. Increased activation of latent TGF-β1 by αVβ3 in human Crohn’s disease and fibrosis in TNBS colitis can be prevented by cilengitide. Inflamm. Bowel Dis. 2013, 19, 2829–2839. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Chi, P. Pirfenidone suppresses TGF-β1-induced human intestinal fibroblasts activities by regulating proliferation and apoptosis via the inhibition of the Smad and PI3K/AKT signaling pathway. Mol. Med. Rep. 2018, 18, 3907–3913. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ren, J.; Hu, Q.; Deng, Y.; Chen, G.; Guo, K.; Li, R.; Li, Y.; Wu, L.; Wang, G.; et al. Oral pirfenidone protects against fibrosis by inhibiting fibroblast proliferation and TGF-β signaling in a murine colitis model. Biochem. Pharmacol. 2016, 117, 57–67. [Google Scholar] [CrossRef]
- Peterson, T.C.; Peterson, M.R.; Raoul, J.L. The effect of pentoxifylline and its metabolite-1 on inflammation and fibrosis in the TNBS model of colitis. Eur. J. Pharmacol. 2011, 662, 47–54. [Google Scholar] [CrossRef]
- Karatay, E.; Gül Utku, Ö.; Erdal, H.; Arhan, M.; Önal, İ.K.; Ibiş, M.; Ekinci, Ö.; Yilmaz Demirtaş, C.; G Dumlu, Ş. Pentoxifylline attenuates mucosal damage in an experimental model of rat colitis by modulating tissue biomarkers of inflammation, oxidative stress, and fibrosis. Turk. J. Med. Sci. 2017, 47, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Okunieff, P.; Augustine, E.; Hicks, J.E.; Cornelison, T.L.; Altemus, R.M.; Naydich, B.G.; Ding, I.; Huser, A.K.; Abraham, E.H.; Smith, J.J.; et al. Pentoxifylline in the treatment of radiation-induced fibrosis. J. Clin. Oncol. 2004, 22, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Hille, A.; Christiansen, H.; Pradier, O.; Hermann, R.M.; Siekmeyer, B.; Weiss, E.; Hilgers, R.; Hess, C.F.; Schmidberger, H. Effect of pentoxifylline and tocopherol on radiation proctitis/enteritis. Strahlenther. Onkol. 2005, 181, 606–614. [Google Scholar] [CrossRef]
- Hamama, S.; Gilbert-Sirieix, M.; Vozenin, M.C.; Delanian, S. Radiation-induced enteropathy: Molecular basis of pentoxifylline-vitamin E anti-fibrotic effect involved TGF-β1 cascade inhibition. Radiother. Oncol. 2012, 105, 305–312. [Google Scholar] [CrossRef]
- Satapathy, S.K.; Sakhuja, P.; Malhotra, V.; Sharma, B.C.; Sarin, S.K. Beneficial effects of pentoxifylline on hepatic steatosis, fibrosis and necroinflammation in patients with non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2007, 22, 634–638. [Google Scholar] [CrossRef]
- Lin, S.L.; Chen, R.H.; Chen, Y.M.; Chiang, W.C.; Lai, C.F.; Wu, K.D.; Tsai, T.J. Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4-activated transcription and profibrogenic effects of connective tissue growth factor. J. Am. Soc. Nephrol. 2005, 16, 2702–2713. [Google Scholar] [CrossRef]
- Hassan, I.; Dorjay, K.; Anwar, P. Pentoxifylline and its applications in dermatology. Indian Dermatol. Online J. 2014, 5, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Duncan, M.R. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J. Investig. Dermatol. 1989, 92, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Ricciarelli, R.; Maroni, P.; Ozer, N.; Zingg, J.M.; Azzi, A. Age-dependent increase of collagenase expression can be reduced by alpha-tocopherol via protein kinase C inhibition. Free Radic. Biol. Med. 1999, 27, 729–737. [Google Scholar] [CrossRef]
- Akeson, A.L.; Woods, C.W.; Mosher, L.B.; Thomas, C.E.; Jackson, R.L. Inhibition of IL-1 beta expression in THP-1 cells by probucol and tocopherol. Atherosclerosis 1991, 86, 261–270. [Google Scholar] [CrossRef]
- Chojkier, M.; Houglum, K.; Lee, K.S.; Buck, M. Long- and short-term D-alpha-tocopherol supplementation inhibits liver collagen alpha1(I) gene expression. Am. J. Physiol. 1998, 275, 1480–1485. [Google Scholar] [CrossRef]
- Soylu, A.R.; Aydogdu, N.; Basaran, U.N.; Altaner, S.; Tarcin, O.; Gedik, N.; Umit, H.; Tezel, A.; Dokmeci, G.; Baloglu, H.; et al. Antioxidants vitamin E and C attenuate hepatic fibrosis in biliary-obstructed rats. World J. Gastroenterol. 2006, 12, 6835–6841. [Google Scholar] [CrossRef] [PubMed]
- Zamin, I., Jr.; Mattos, A.A.; Mattos, A.Z.; Coral, G.; Santos, D.; Rhoden, C. The vitamin E reduces liver lipoperoxidation and fibrosis in a model of nonalcoholic steatohepatitis. Arq. Gastroenterol. 2010, 47, 86–92. [Google Scholar] [CrossRef]
- Bese, N.S.; Munzuroglu, F.; Uslu, B.; Arbak, S.; Yesiladali, G.; Sut, N.; Altug, T.; Ober, A. Vitamin E protects against the development of radiation-induced pulmonary fibrosis in rats. Clin. Oncol. 2007, 19, 260–264. [Google Scholar] [CrossRef]
- Tasanarong, A.; Kongkham, S.; Duangchana, S.; Thitiarchakul, S.; Eiam-Ong, S. Vitamin E ameliorates renal fibrosis by inhibition of TGF-beta/Smad2/3 signaling pathway in UUO mice. J. Med. Assoc. Thai. 2011, 94, S1–S9. [Google Scholar]
- Tasanarong, A.; Kongkham, S.; Thitiarchakul, S.; Eiam-Ong, S. Vitamin E ameliorates renal fibrosis in ureteral obstruction: Role of maintaining BMP-7 during epithelial-to-mesenchymal transition. J. Med. Assoc. Thai. 2011, 94, S10–S18. [Google Scholar]
- Kaya, V.; Yazkan, R.; Yıldırım, M.; Doguc, D.K.; Süren, D.; Bozkurt, K.K.; Yuksel, O.; Demirpence, O.; Sen, C.A.; Yalçın, A.Y. The relation of radiation-induced pulmonary fibrosis with stress and the efficiency of antioxidant treatment: An experimental study. Med. Sci. Monit. 2014, 20, 290–296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiao, T.B.; Lee, A.J. Role of Pentoxifylline and Vitamin E in Attenuation of Radiation-Induced Fibrosis. Ann. Pharmacother. 2005, 39, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.; Bhatia, S.; Smith, B.J.; Button, A.M.; Bodeker, K.; Buatti, J. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiong, M.; Xia, Y.F.; Cui, N.J.; Lu, R.B.; Deng, L.; Lin, Y.H.; Rong, T.H. Studies on pentoxifylline and tocopherol combination for radiation-induced heart disease in rats. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1552–1559. [Google Scholar] [CrossRef]
- Ng, Y.Y.; Chen, Y.M.; Tsai, T.J.; Lan, X.R.; Yang, W.C.; Lan, H.Y. Pentoxifylline inhibits transforming growth factor-beta signaling and renal fibrosis in experimental crescentic glomerulonephritis in rats. Am. J. Nephrol. 2009, 29, 43–53. [Google Scholar] [CrossRef]
- Hung, K.Y.; Huang, J.W.; Chiang, C.K.; Tsai, T.J. Preservation of peritoneal morphology and function by pentoxifylline in a rat model of peritoneal dialysis: Molecular studies. Nephrol. Dial. Transplant. 2008, 23, 3831–3840. [Google Scholar] [CrossRef]
- Speca, S.; Giusti, I.; Rieder, F.; Latella, G. Cellular and molecular mechanisms of intestinal fibrosis. World J. Gastroenterol. 2012, 18, 3635–3661. [Google Scholar] [CrossRef]
- Flier, S.N.; Tanjore, H.; Kokkotou, E.G.; Sugimoto, H.; Zeisberg, M.; Kalluri, R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J. Biol. Chem. 2010, 285, 20202–20212. [Google Scholar] [CrossRef]
- Li, C.; Kuemmerle, J.F. The fate of myofibroblasts during the development of fibrosis in Crohn’s disease. J. Dig. Dis. 2020, 21, 326–331. [Google Scholar] [CrossRef]
- Oliver, N.; Sternlicht, M.; Gerritsen, K.; Goldschmeding, R. Could aging human skin use a connective tissue growth factor boost to increase collagen content? J. Investig. Dermatol. 2010, 130, 338–341. [Google Scholar] [CrossRef]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 2012, 5, S24. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Huang, Y.; Chen, X.; Liu, J.; Lu, Y.; Bu, L.; Xia, L.; Xiao, W.; Chen, M.; Nie, Q.; et al. The role of CTGF in the diabetic rat retina and its relationship with VEGF and TGF-beta(2), elucidated by treatment with CTGFsiRNA. Acta Ophthalmol. 2010, 88, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, G.; Elias, M.; Zhu, Y.; Wang, J. The role of cytokine and immune responses in intestinal fibrosis. J. Dig. Dis. 2020, 21, 308–314. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors. 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, W.; Wang, X.; Li, X.; Qi, S.; Zhang, Y.; Gao, M.Q. TGF-β1 Induces EMT in Bovine Mammary Epithelial Cells Through the TGFβ1/Smad Signaling Pathway. Cell. Physiol. Biochem. 2017, 43, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Kaimori, A.; Potter, J.; Kaimori, J.; Wang, C.; Mezey, E.; Koteish, A. Transforming Growth Factor-β1 Induces an Epithelial-to-Mesenchymal Transition State in Mouse Hepatocytes in vitro. J. Biol. Chem. 2007, 282, 22089–22101. [Google Scholar] [CrossRef]
- Kang, H.R.; Cho, S.J.; Lee, C.G.; Homer, R.J.; Elias, J.A. Transforming growth factor (TGF)-beta1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J. Biol. Chem. 2007, 282, 7723–7732. [Google Scholar] [CrossRef]
- Shen, K.; Johnson, D.W.; Gobe, G.C. The role of cGMP and its signaling pathways in kidney disease. Am. J. Physiol.-Renal Physiol. 2016, 311, F671–F681. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Geng, Y.; Xu, D.; Zhang, L.; Yang, Y.; Wei, Z.; Zhang, B.; Li, S.; Gao, X.; et al. Dibutyryl-cAMP attenuates pulmonary fibrosis by blocking myofibroblast differentiation via PKA/CREB/CBP signaling in rats with silicosis. Respir. Res. 2017, 18, 38. [Google Scholar] [CrossRef]
- Flores-Costa, R.; Duran-Güell, M.; Casulleras, M.; López-Vicario, C.; Alcaraz-Quiles, J.; Diaz, A.; Lozano, J.J.; Titos, E.; Hall, K.; Sarno, R.; et al. Stimulation of soluble guanylate cyclase exerts antiinflammatory actions in the liver through a VASP/NF-κB/NLRP3 inflammasome circuit. Proc. Natl. Acad. Sci. USA 2020, 117, 28263–28274. [Google Scholar] [CrossRef]
- Wójcik-Pszczoła, K.; Chłoń-Rzepa, G.; Jankowska, A.; Ślusarczyk, M.; Ferdek, P.E.; Kusiak, A.A.; Świerczek, A.; Pociecha, K.; Koczurkiewicz-Adamczyk, P.; Wyska, E.; et al. A Novel, Pan-PDE Inhibitor Exerts Anti-Fibrotic Effects in Human Lung Fibroblasts via Inhibition of TGF-β Signaling and Activation of cAMP/PKA Signaling. Int. J. Mol. Sci. 2020, 21, 4008. [Google Scholar] [CrossRef] [PubMed]
- Fehrholz, M.; Speer, C.P.; Kunzmann, S. Caffeine and rolipram affect Smad signalling and TGF-β1 stimulated CTGF and transgelin expression in lung epithelial cells. PLoS ONE 2014, 9, e97357. [Google Scholar] [CrossRef] [PubMed]
- Yougbare, I. Alterations of cAMP/cGMP Signaling Pathways in Lupus Nephritis. J. Nephrol. Sci. 2021, 3, 8–12. [Google Scholar] [CrossRef]
- Hung, K.Y.; Huang, J.W.; Chen, C.T.; Lee, P.H.; Tsai, T.J. Pentoxifylline modulates intracellular signalling of TGF-beta in cultured human peritoneal mesothelial cells: Implications for prevention of encapsulating peritoneal sclerosis. Nephrol. Dial. Transplant. 2003, 18, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Shams, S.; Khan, S.; Ayaz, M.; Khan, H.A.; Hassan, H. Effect of stem cell and vitamin E for the reduction of liver fibrosis. J. Appl. Environ. Biol. Sci. 2018, 8, 111–117. [Google Scholar]
- Honzawa, Y.; Yamamoto, S.; Okabe, M.; Seno, H.; Nakase, H. Current Topics of the Mechanism of Intestinal Fibrosis in Crohn’s Disease. Immuno 2021, 1, 574–582. [Google Scholar] [CrossRef]
- Gordon, I.O.; Agrawal, N.; Willis, E.; Goldblum, J.R.; Lopez, R.; Allende, D.; Liu, X.; Patil, D.Y.; Yerian, L.; El-Khider, F.; et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment. Pharmacol. Ther. 2018, 47, 922–939. [Google Scholar] [CrossRef]
- de Bruyn, J.R.; Meijer, S.L.; Wildenberg, M.E.; Bemelman, W.A.; van den Brink, G.R.; D’Haens, G.R. Development of fibrosis in acute and longstanding ulcerative colitis. J. Crohn’s Colitis 2015, 9, 966–972. [Google Scholar] [CrossRef]
- Giuffrida, P.; Pinzani, M.; Corazza, G.R.; Di Sabatino, A. Biomarkers of intestinal fibrosis—One step towards clinical trials for stricturing inflammatory bowel disease. United Eur. Gastroenterol. J. 2016, 4, 523–530. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.J. Therapeutic Potential of the Combination of Pentoxifylline and Vitamin-E in Inflammatory Bowel Disease: Inhibition of Intestinal Fibrosis. J. Clin. Med. 2022, 11, 4713. https://doi.org/10.3390/jcm11164713
Lee HJ. Therapeutic Potential of the Combination of Pentoxifylline and Vitamin-E in Inflammatory Bowel Disease: Inhibition of Intestinal Fibrosis. Journal of Clinical Medicine. 2022; 11(16):4713. https://doi.org/10.3390/jcm11164713
Chicago/Turabian StyleLee, Hyun Joo. 2022. "Therapeutic Potential of the Combination of Pentoxifylline and Vitamin-E in Inflammatory Bowel Disease: Inhibition of Intestinal Fibrosis" Journal of Clinical Medicine 11, no. 16: 4713. https://doi.org/10.3390/jcm11164713
APA StyleLee, H. J. (2022). Therapeutic Potential of the Combination of Pentoxifylline and Vitamin-E in Inflammatory Bowel Disease: Inhibition of Intestinal Fibrosis. Journal of Clinical Medicine, 11(16), 4713. https://doi.org/10.3390/jcm11164713





