Management of High-Risk Hypercholesterolemic Patients and PCSK9 Inhibitors Reimbursement Policies: Data from a Cohort of Italian Hypercholesterolemic Outpatients
Abstract
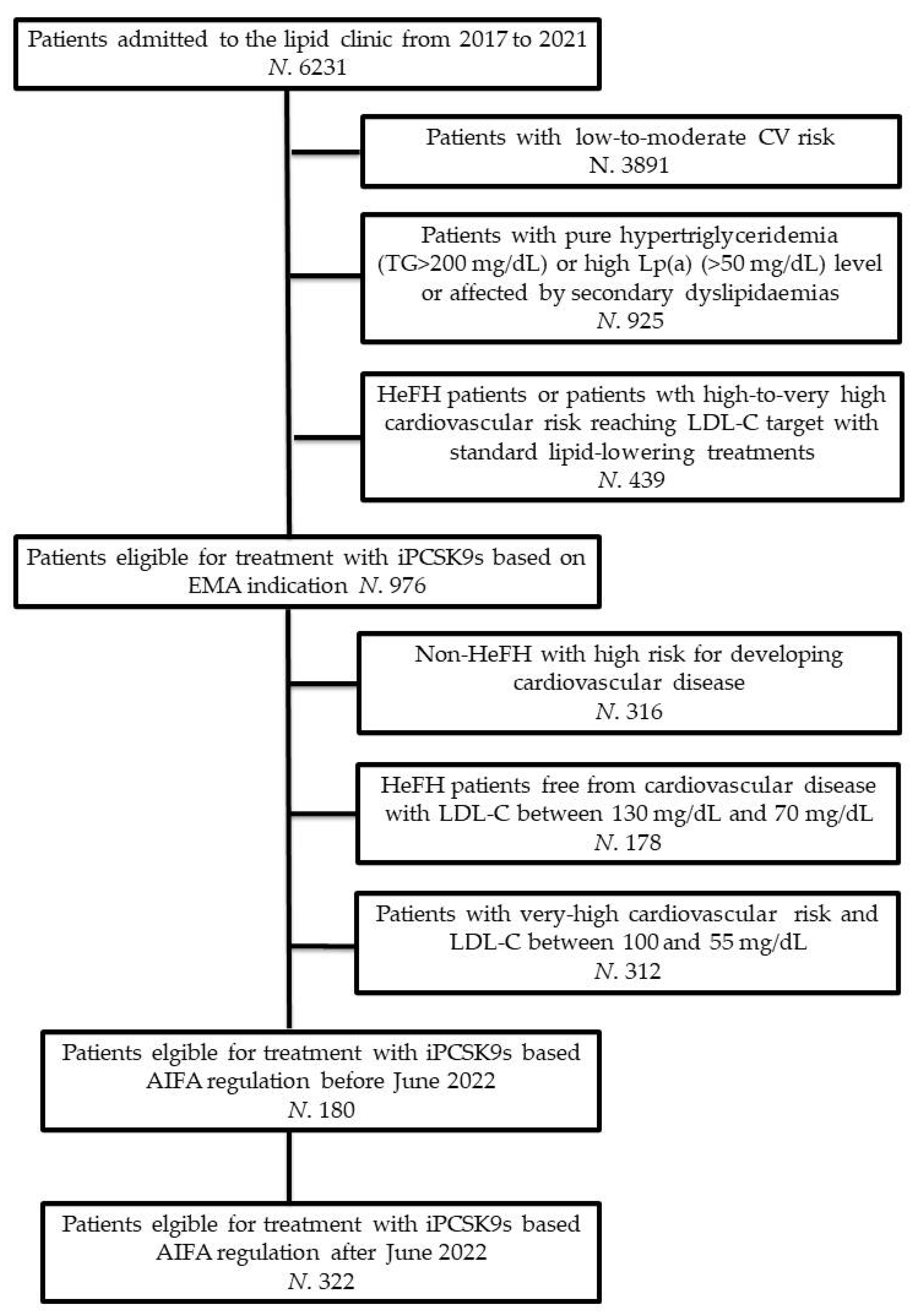
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Assessments
2.2.1. Clinical Data and Anthropometric Measurements
2.2.2. Laboratory Analyses
2.2.3. Statin Intolerance
2.2.4. Assessment of Safety and Tolerability
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, X.D.; Peng, Z.S.; Gu, H.M.; Wang, M.; Wang, G.Q.; Zhang, D.W. Regulation of PCSK9 Expression and Function: Mechanisms and Therapeutic Implications. Front. Cardiovasc. Med. 2021, 8, 764038. [Google Scholar] [CrossRef]
- Bove, M.; Cicero, A.F.G.; Borghi, C. Emerging drugs for the treatment of hypercholesterolemia. Expert Opin. Emerg. Drugs 2019, 24, 63–69. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Nault, P.; Giugliano, R.P.; Keech, A.C.; Pineda, A.L.; Kanevsky, E.; Kuder, J.; Murphy, S.A.; Jukema, J.W.; Lewis, B.S.; et al. Low-Density Lipoprotein Cholesterol Lowering with Evolocumab and Outcomes in Patients with Peripheral Artery Disease: Insights from the FOURIER Trial (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk). Circulation 2018, 137, 338–350. [Google Scholar] [CrossRef]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/documents/product-information/repatha-epar-product-information_en.pdf (accessed on 9 June 2022).
- Warden, B.A.; Fazio, S.; Shapiro, M.D. The PCSK9 revolution: Current status, controversies, and future directions. Trends Cardiovasc. Med. 2020, 30, 179–185. [Google Scholar] [CrossRef]
- Feng, X.; Berklein, F.; Rane, P.B.; Habib, M.; Lin, P.J. Patient Characteristics and Treatment Patterns among Medicare Beneficiaries Initiating PCSK9 Inhibitor Therapy. Cardiovasc. Drugs Ther. 2021, 35, 965–973. [Google Scholar] [CrossRef]
- Doshi, J.A.; Li, P.; Puckett, J.T.; Pettit, A.R.; Raman, S.; Parmacek, M.S.; Rader, D.J. Trends and Factors Associated with Insurer Approval of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor Prescriptions. Value Health 2020, 23, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef]
- Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_002317_044317_RCP.pdf&retry=0&sys=m0b1l3 (accessed on 9 June 2022).
- Available online: https://www.aifa.gov.it/documents/20142/1184740/PRALUENT_14494_INNOV._v.1.0.pdf (accessed on 9 June 2022).
- Available online: https://www.aifa.gov.it/-/modifica-registri-di-monitoraggio-repatha-e-praluent-inibitori-di-pcsk-9- (accessed on 9 June 2022).
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Cicero, A.; Morbini, M.; Bove, M.; D’Addato, S.; Fogacci, F.; Rosticci, M.; Borghi, C. Additional therapy for cholesterol lowering in ezetimibe-treated, statin-intolerant patients in clinical practice: Results from an internal audit of a university lipid clinic. Curr. Med. Res. Opin. 2016, 32, 1633–1638. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Impact of a short-term synbiotic supplementation on metabolic syndrome and systemic inflammation in elderly patients: A randomized placebo-controlled clinical trial. Eur. J. Nutr. 2021, 60, 655–663. [Google Scholar] [CrossRef]
- Piani, F.; Cicero, A.F.; Ventura, F.; Dormi, A.; Fogacci, F.; Patrono, D.; Mancini, R.; Ramazzotti, E.; Borghi, C.; D’Addato, S.; et al. Evaluation of twelve formulas for LDL-C estimation in a large, blinded, random Italian population. Int. J. Cardiol. 2021, 330, 221–227. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular fltration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Banach, M.; Rizzo, M.; Toth, P.P.; Farnier, M.; Davidson, M.H.; Al-Rasadi, K.; Aronow, W.S.; Athyros, V.; Djuric, D.M.; Ezhov, M.V.; et al. Position paper Statin intolerance—An attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch. Med. Sci. 2015, 11, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. J. Prev. Cardiol. 2021, 29, 5–115. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Lin, C.J.; Lee, Y.S.; Wu, T.H.; Chien, K.L. Efficacy of more intensive lipid-lowering therapy on cardiovascular diseases: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2020, 20, 334. [Google Scholar] [CrossRef]
- Gitt, A.K.; Lautsch, D.; Ferrieres, J.; Kastelein, J.; Drexel, H.; Horack, M.; Brudi, P.; Vanneste, B.; Bramlage, P.; Chazelle, F.; et al. Contemporary data on low-density lipoprotein cholesterol target value attainment and distance to target in a cohort of 57,885 statin-treated patients by country and region across the world. Data Brief. 2016, 9, 616–620. [Google Scholar] [CrossRef]
- De Luca, L.; Arca, M.; Temporelli, P.L.; Meessen, J.; Riccio, C.; Bonomo, P.; Colavita, A.R.; Gabrielli, D.; Gulizia, M.M.; Colivicchi, F.; et al. Current lipid lowering treatment and attainment of LDL targets recommended by ESC/EAS guidelines in very high-risk patients with established atherosclerotic cardiovascular disease: Insights from the START registry. Int. J. Cardiol. 2020, 316, 229–235. [Google Scholar] [CrossRef]
- Talasaz, A.H.; Ho, A.J.; Bhatty, F.; Koenig, R.A.; Dixon, D.L.; Baker, W.L.; Van Tassell, B. W Meta-analysis of clinical outcomes of PCSK9 modulators in patients with established ASCVD. Pharmacotherapy 2021, 41, 1009–1023. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Borghi, C. Successful treatment of a patient with mitochondrial myopathy with alirocumab. J. Clin. Lipidol. 2020, 14, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Dardano, A.; Daniele, G.; Penno, G.; Miccoli, R.; Del Prato, S. Breaking Therapeutic Inertia with Alirocumab in an 80-Year-Old Patient with Severe Hypercholesterolemia: A Case Report. Front. Med. 2021, 8, 699477. [Google Scholar] [CrossRef]
- Cicero, A.; Fogacci, F.; Cincione, I. Evaluating pharmacokinetics of bempedoic acid in the treatment of hypercholesterolemia. Exp. Opin. Drug Metab. Toxicol. 2021, 17, 1031–1038. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Hernandez, A.V.; Banach, M. Lipid and Blood Pressure Meta-Analysis Collaboration (LBPMC) Group and the International Lipid Expert Panel (ILEP). Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003121. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Landolfo, M.; Ventura, F.; Borghi, C. Current pharmacotherapeutic options for primary dyslipidemia in adults. Expert Opin. Pharmacother. 2019, 20, 1277–1288. [Google Scholar] [CrossRef]
- Marquina, C.; Zomer, E.; Vargas-Torres, S.; Zoungas, S.; Ofori-Asenso, R.; Liew, D.; Ademi, Z. Novel Treatment Strategies for Secondary Prevention of Cardiovascular Disease: A Systematic Review of Cost-Effectiveness. Pharmacoeconomics 2020, 38, 1095–1113. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, N.D.; De Gennaro, L.; Tricarico, L.; Caldarola, P. Budget impact analysis of PCSK9 inhibitors costs from a community payers’ perspective in Apulia, Italy. Open Heart 2019, 6, e001018. [Google Scholar] [CrossRef] [PubMed]
- Azari, S.; Rezapour, A.; Omidi, N.; Alipour, V.; Behzadifar, M.; Safari, H.; Tajdini, M.; Bragazzi, N.L. Cost-effectiveness analysis of PCSK9 inhibitors in cardiovascular diseases: A systematic review. Heart Fail. Rev. 2020, 25, 1077–1088. [Google Scholar] [CrossRef]
- Banerjee, Y.; Pantea Stoian, A.; Cicero, A.F.; Fogacci, F.; Nikolic, D.; Sachinidis, A.; Rizvi, A.A.; Janez, A.; Rizzo, M. Inclisiran: A small interfering RNA strategy targeting PCSK9 to treat hypercholesterolemia. Exp. Opin. Drug Saf. 2022, 21, 9–20. [Google Scholar] [CrossRef]
- Lee, E.; Yoon, K.H. How to Interpret Recent CV Outcome Trials and Future: PCSK9 Inhibitors. J. Lipid Atheroscler. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Singh, M.; McEvoy, J.W.; Khan, S.U.; Wood, D.A.; Graham, I.M.; Blumenthal, R.S.; Mishra, A.K.; Michos, E.D. Comparison of Transatlantic Approaches to Lipid Management: The AHA/ACC/Multisociety Guidelines vs. the ESC/EAS Guidelines. Mayo Clin. Proc. 2020, 95, 998–1014. [Google Scholar] [CrossRef]
| AIFA (Old) | AIFA (Current) | EMA (ESC/EAS) | FDA (AHA/ACC) | |
|---|---|---|---|---|
| Age | ≤80 years old | ≤80 years old | No limit beyond patient fitness | No limit beyond patient fitness |
| Primary prevention | Patients with definitive diagnosis of HeFH (genetic test result or DLCNS ≥ 8) and LDL-C > 130 mg/dL despite maximally tolerated LLT | Patients with definitive diagnosis of HeFH (genetic test result or DLCNS ≥ 8) and LDL-C > 130 mg/dL despite maximally tolerated LLT | Patients with FH and another major cardiovascular risk factor (very-high risk FH patients) with LDL-C > 55 mg/dL despite maximally tolerated LLT Maybe considered in non-FH very high-risk patients with LDL-C > 55 mg/dL despite maximally tolerated LLT | Patients with severe primary hypercholesterolemia (LDL-C level ≥ 190 mg/dL) with LDL-C ≥ 100 mg/dL despite maximally tolerated LLT |
| LDL-C target | Not reported | Not reported | LDL-C reduction ≥50% from baseline and LDL-C < 55 mg/dL | LDL-C reduction ≥50% from baseline (No treat-to-target approach) |
| Secundary prevention | Patients with LDL-C > 100 mg/dL despite maximally tolerated LLT | Patients with LDL-C > 70 mg/dL despite maximally tolerated LLT | Patients with atherosclerosis-related cardiovascular disease and with LDL-C > 55 mg/dL despite maximally tolerated LLT | Patients with multiple major atherosclerosis-related cardiovascular events or 1 major ASCVD atherosclerosis-related cardiovascular event and multiple high-risk conditions with LDL-C ≥ 70 mg/dL despite maximally tolerated LLT |
| LDL-C target | Not reported | Not reported | LDL-C reduction ≥50% from baseline and LDL-C < 55 mg/dL If a second vascular events occur before 2 years, then consider LDL-C < 40 mg/dL | LDL-C reduction ≥50% from baseline (No treat-to-target approach) |
| Type 2 Diabetes | Patients with LDL-C > 100 mg/dL despite maximally tolerated LLT and type 2 diabetes with either at least one other CV risk factor or renal impairment and/or signs of retinopathy | Patients with LDL-C > 100 mg/dL despite maximally tolerated LLT and type 2 diabetes with either at least one other CV risk factor or renal impairment and/or signs of retinopathy | Patients at high-risk and with LDL-C > 70 mg/dL despite maximally tolerated LLT. Patients with type 2 diabetes at very high-risk and with LDL-C > 55 mg/dL despite maximally tolerated LLT. | Not considered if not included in the above listed categories |
| LDL-C target | Not reported | Not reported | LDL-C reduction <70 mg/dL If target organ damage, early-onset type 1 diabetes (>20 years), or more than 2 other cardiovascular risk factors, then consider <55 mg/dL | LDL-C reduction ≥50% from baseline if high-risk patients (No treat-to-target approach) |
| LDL-C (mg/dL) | Portion of Patients Who Achieved a Reduction in LDL-C > 50% (%) | Portion of Patients Who Achieved the LDL-C Target Level (%) | |||
|---|---|---|---|---|---|
| At Baseline; with Maximally Tolerated LLT | Therapeutic Efficacy of Alternative Interventions to PCSK9 Inhibitors | ||||
| Non-He-FH hypercholesterolemic individuals with high risk of developing CVD | Patients undergoing statin treatment | 118 ± 11 | 90 ± 9 * | 64 | 41 |
| Statin-intolerant patients | 141 ± 12 | 122 ± 10 * | 42 | 29 | |
| HeFH individuals free from CVD with LDL-C between 130 mg/dL and 70 mg/dL | Patients undergoing statin treatment | 97 ± 7 | 89 ± 5 * | 62 | 44 |
| Statin-intolerant patients | 111 ± 10 | 92 ± 8 * | 39 | 28 | |
| Patients with very high CV risk with LDL-C between 100 mg/dL and 55 mg/dL | Patients undergoing statin treatment | 69 ± 5 | 59 ± 5 * | 68 | 49 |
| Statin-intolerant patients | 84 ± 6 | 71 ± 4 * | 43 | 27 | |
| Statin-Tolerant Patients (N. 128) | Statin-Intolerant Patients (N. 52) | |||
|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | Pre-Treatment | Post-Treatment | |
| Age (years) | 66 ± 8 | 62 ± 11 | ||
| Body mass index (kg/m2) | 26.6 ± 3.8 | 27.1 ± 3.9 | 27.6 ± 3.6 | 26.7 ± 3.2 * |
| Fasting plasma glucose (mg/dL) | 99 ± 14 | 99 ± 18 | 102 ± 22 | 101 ± 19 |
| Serum uric acid (mg/dL) | 5.7 ± 1.3 | 5.6 ± 1.3 | 5.1 ± 1.2 | 5.9 ± 1.1 * |
| eGFR (CKD-EPI, mL/min/1.73 m2) | 76.2 ± 21.1 | 75.3 ± 21.1 | 83 ± 15 | 85 ± 13 |
| Total cholesterol (mg/dL) | 201 ± 49 | 131 ± 45 * | 194 ± 48 | 125 ± 39 * |
| LDL-cholesterol (mg/dL) | 149 ± 47 | 52 ± 18 * | 143 ± 32 | 47 ± 12 * |
| HDL-cholesterol (mg/dL) | 51 ± 10 | 53 ± 11 | 53 ± 12 | 55 ± 12 |
| Triglycerides (mg/dL) | 162 ± 52 | 150 ± 42 | 143 ± 83 | 131 ± 31 |
| VLDL-cholesterol (mg/dL) | 32 ± 20 | 26 ± 16 * | 28 ± 17 | 21 ± 10 * |
| Apolipoprotein B (mg/dL) | 121 ± 41 | 59 ± 24 * | 101 ± 28 | 55 ± 15 * |
| Lipoprotein(a) (mg/dL) | 64 ± 21 | 56 ± 22 * | 77 ± 29 | 65 ± 23 * |
| AST (mg/dL) | 27 ± 11 | 27 ± 14 | 26 ± 6 | 29 ± 9 |
| ALT (mg/dL) | 27 ± 17 | 26 ± 14 | 26 ± 10 | 30 ± 12 |
| gamma-GT (mg/dL) | 37 ± 17 | 35 ± 20 | 33 ± 19 | 34 ± 15 |
| CPK (mg/dL) | 203 ± 57 | 253 ± 61 | 195 ± 59 | 196 ± 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogacci, F.; Giovannini, M.; Grandi, E.; Imbalzano, E.; Degli Esposti, D.; Borghi, C.; Cicero, A.F.G. Management of High-Risk Hypercholesterolemic Patients and PCSK9 Inhibitors Reimbursement Policies: Data from a Cohort of Italian Hypercholesterolemic Outpatients. J. Clin. Med. 2022, 11, 4701. https://doi.org/10.3390/jcm11164701
Fogacci F, Giovannini M, Grandi E, Imbalzano E, Degli Esposti D, Borghi C, Cicero AFG. Management of High-Risk Hypercholesterolemic Patients and PCSK9 Inhibitors Reimbursement Policies: Data from a Cohort of Italian Hypercholesterolemic Outpatients. Journal of Clinical Medicine. 2022; 11(16):4701. https://doi.org/10.3390/jcm11164701
Chicago/Turabian StyleFogacci, Federica, Marina Giovannini, Elisa Grandi, Egidio Imbalzano, Daniela Degli Esposti, Claudio Borghi, and Arrigo F. G. Cicero. 2022. "Management of High-Risk Hypercholesterolemic Patients and PCSK9 Inhibitors Reimbursement Policies: Data from a Cohort of Italian Hypercholesterolemic Outpatients" Journal of Clinical Medicine 11, no. 16: 4701. https://doi.org/10.3390/jcm11164701
APA StyleFogacci, F., Giovannini, M., Grandi, E., Imbalzano, E., Degli Esposti, D., Borghi, C., & Cicero, A. F. G. (2022). Management of High-Risk Hypercholesterolemic Patients and PCSK9 Inhibitors Reimbursement Policies: Data from a Cohort of Italian Hypercholesterolemic Outpatients. Journal of Clinical Medicine, 11(16), 4701. https://doi.org/10.3390/jcm11164701










