Evaluation of Baseline Characteristics and Prognostic Factors in Multisystemic Inflammatory Syndrome in Children: Is It Possible to Foresee the Prognosis in the First Step?
Abstract
:1. Introduction
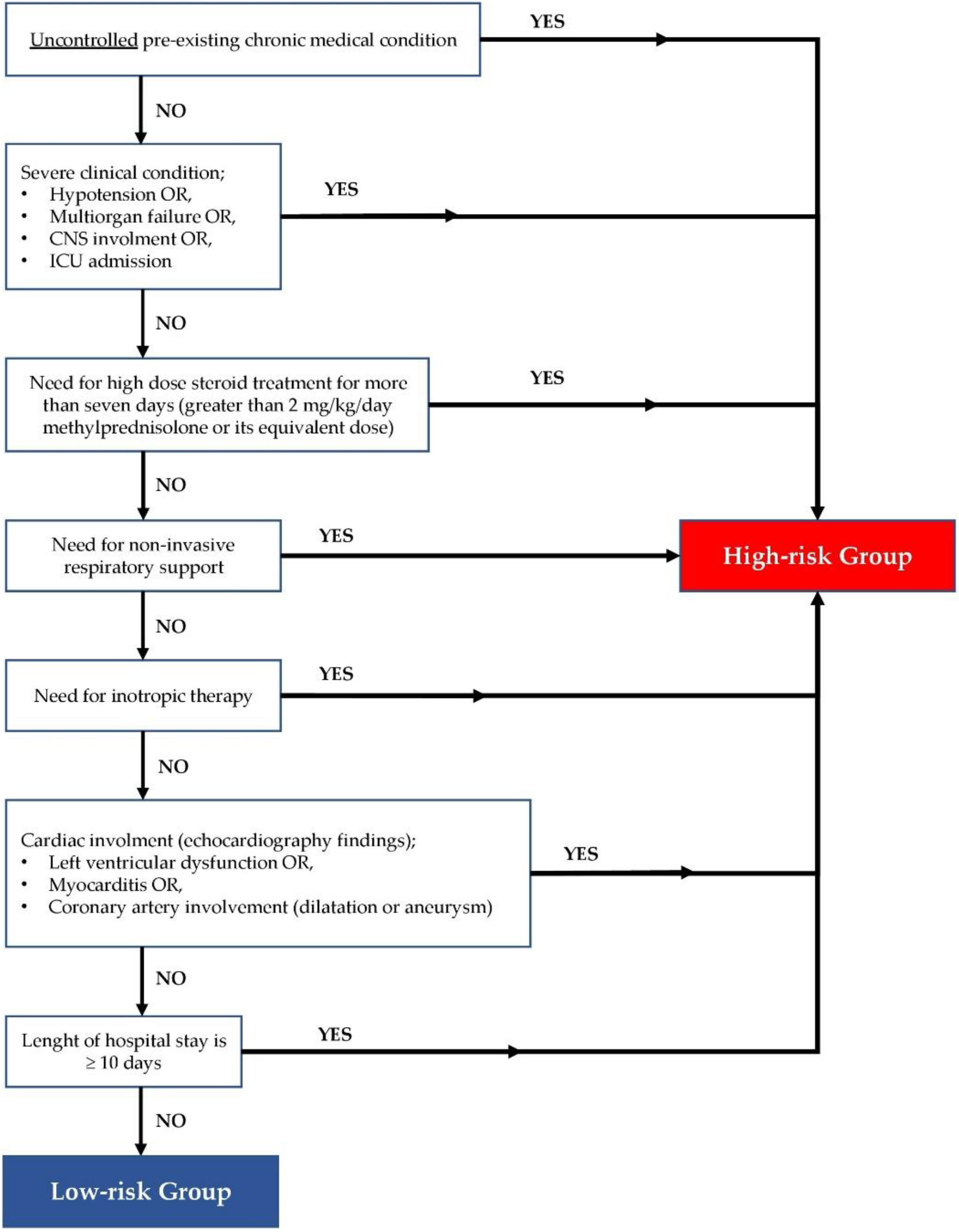
2. Materials and Methods
Statistical Analyses
3. Results
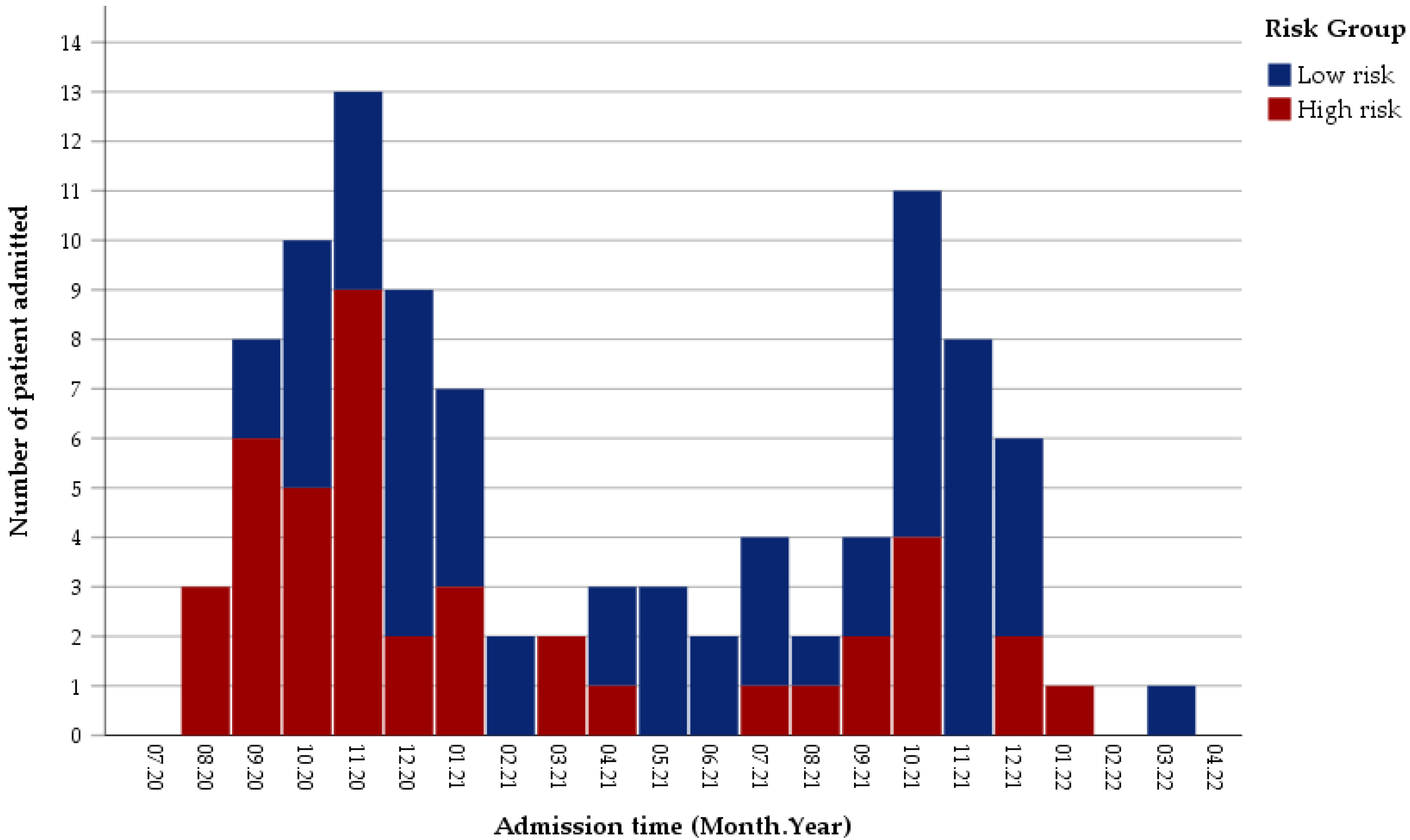
3.1. Distribution of Patients in the Study Period
3.2. Clinical Characteristics and COVID-19 Related Data of Patients
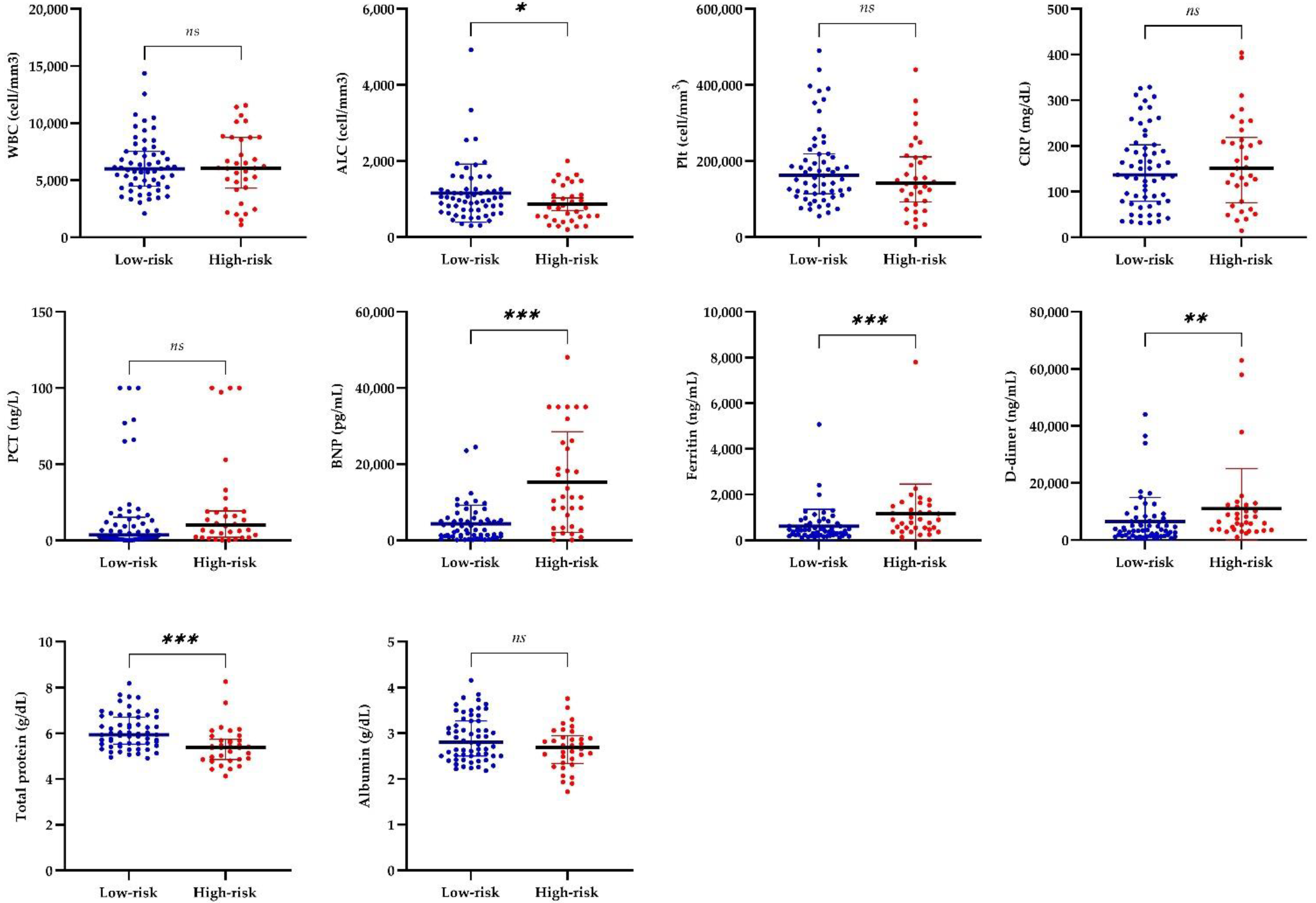
3.3. Laboratory and Cardiac Findings of Patients
3.4. Treatments of Patients with MIS-C
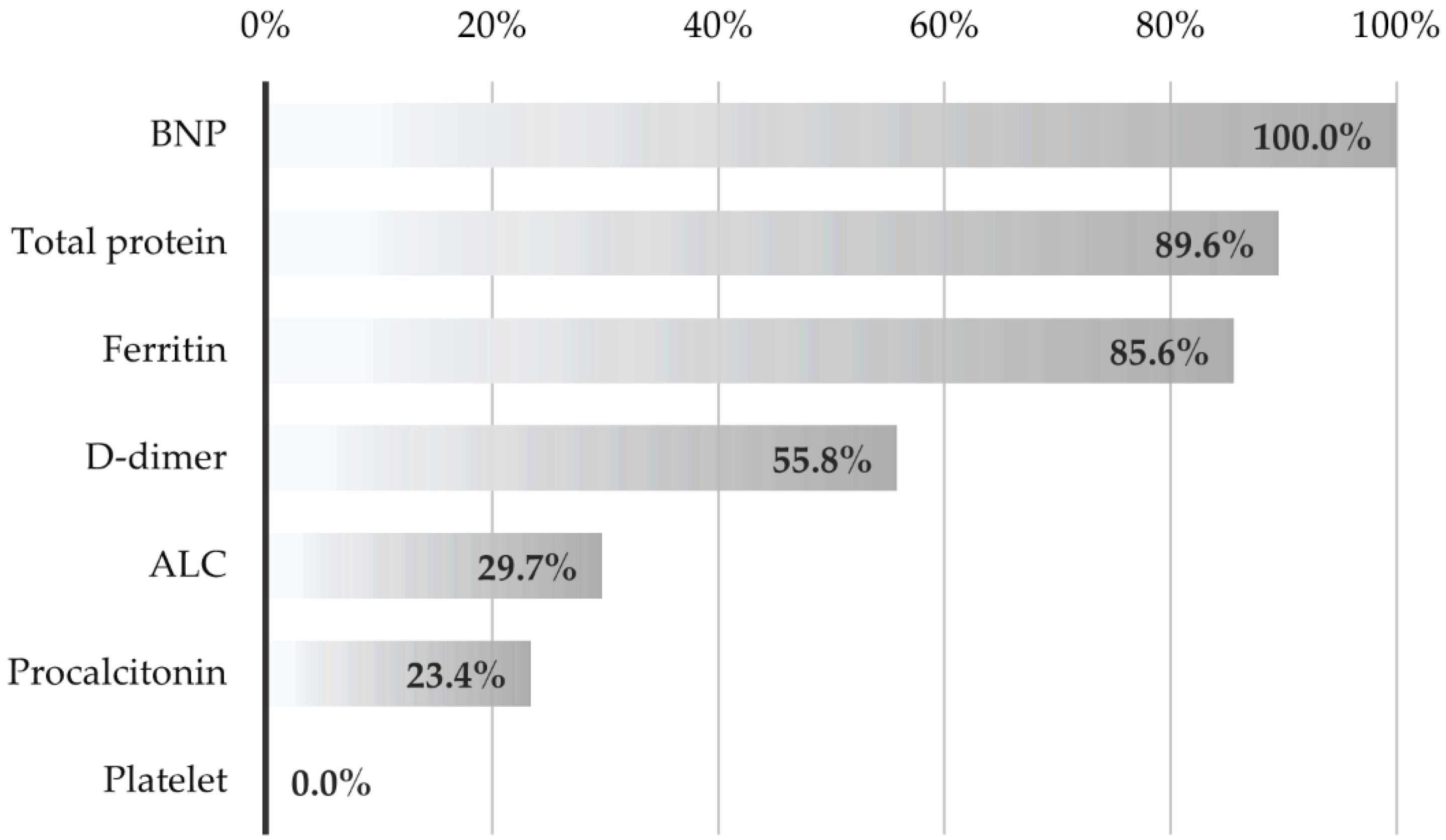
3.5. Evaluation of the Performance of Laboratory Tests in Distinguishing Cases According to Risk Groups
- An increase of 1000 units in BNP values increases the risk of being high-risk by 12.5%.
- A one-unit increase in total protein values reduces the risk of being high-risk by 36.8%.
- Those with a troponin value increased by ≥10-fold have a 9.5 times higher risk of being high-risk than those with a lower value.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory Shock in Children during COVID-19 Pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Health Alert Network. Multisystem İnflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). Available online: https://emergency.cdc.gov/han/2020/han00432.asp (accessed on 1 May 2022).
- WHO. Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. Available online: https://apps.who.int/iris/handle/10665/332095 (accessed on 20 March 2022).
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Ferris, A.; Kernan, K.F.; Schulert, G.S.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS–CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 2. Arthritis Rheumatol. 2021, 73, e13–e29. [Google Scholar] [CrossRef] [PubMed]
- Çetin, B.Ş. SARS-CoV-2 Infection in Children; What Do We Know So Far? J. Pediatr. Acad. 2020, 1, 76–85. [Google Scholar] [CrossRef]
- McMurray, J.C.; May, J.W.; Cunningham, M.W.; Jones, O.Y. Multisystem Inflammatory Syndrome in Children (MIS-C), a Post-viral Myocarditis and Systemic Vasculitis—A Critical Review of Its Pathogenesis and Treatment. Front. Pediatr. 2020, 8, 626182. [Google Scholar] [CrossRef] [PubMed]
- Cetin, B.S.; Kısaarslan, A.P.; Baykan, A.; Akyıldız, B.N.; Poyrazoglu, H. Erciyes Clinical Guideline For Multisystem Inflammatory Syndrome In Children (MIS-C) Associated With COVID-19. J. Pediatr. Acad. 2022, 2, e1–e8. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance (accessed on 1 May 2022).
- Cohen, J.M.; Carter, M.J.; Cheung, C.R.; Ladhani, S.; Group, E.P.-T.S. Lower Risk of Multisystem Inflammatory Syndrome in Children (MIS-C) with the Delta and Omicron Variants of SARS-CoV-2. MedrXiv 2022. [Google Scholar] [CrossRef]
- Merckx, J.; Cooke, S.; El Tal, T.; Bitnun, A.; Morris, S.K.; Yeh, E.A.; Yea, C.; Gill, P.; Papenburg, J.; Lefebvre, M.-A.; et al. Predictors of Severe Illness in Children with Multisystem Inflammatory Syndrome after SARS-CoV-2 Infection: A Multicentre Cohort Study. Can. Med. Assoc. J. 2022, 194, E513–E523. [Google Scholar] [CrossRef]
- Nayak, S.; Panda, P.C.; Biswal, B.; Agarwalla, S.K.; Satapathy, A.K.; Jena, P.K.; Gulla, K.M.; Rath, D.; Mahapatra, A.; Mishra, P.; et al. EICOMISC Study Group. Eastern India Collaboration on Multisystem Inflammatory Syndrome in Children (EICOMISC): A Multicenter Observational Study of 134 Cases. Front. Pediatr. 2022, 10, 834039. [Google Scholar] [CrossRef]
- Roberts, J.E.; Campbell, J.I.; Gauvreau, K.; Lamb, G.S.; Newburger, J.; Son, M.B.; Dionne, A. Differentiating Multisystem Inflammatory Syndrome in Children: A Single-Centre Retrospective Cohort Study. Arch. Dis. Child. 2022, 107, e3. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Overcoming COVID-19 Investigators. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.Y.; Oster, M.E.; Godfred-Cato, S.E.; Bryant, B.; Datta, S.D.; Campbell, A.P.; Leung, J.W.; Tsang, C.A.; Pierce, T.J.; Kennedy, J.L.; et al. Factors Linked to Severe Outcomes in Multisystem Inflammatory Syndrome in Children (MIS-C) in the USA: A Retrospective Surveillance Study. Lancet. Child Adolesc. Heal. 2021, 5, 323–331. [Google Scholar] [CrossRef]
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem Inflammatory Syndrome in Children Related to COVID-19: A Systematic Review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Gupta, S.; Sood, M.; Sharma, S.; Verma, S. A Systematic Review of Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 Infection. Pediatr. Infect. Dis. J. 2020, 39, e340–e346. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Ciftdogan, D.; Ekemen Keles, Y.; Cetin, B.S.; Dalgic Karabulut, N.; Emiroglu, M.; Bagci, Z.; Buyukcam, A.; Erdeniz, E.H.; Arga, G.; Yesil, E.; et al. COVID-19 Associated Multisystemic Inflammatory Syndrome in 614 Children with and without Overlap with Kawasaki Disease-Turk MIS-C Study Group. Eur. J. Pediatr. 2022, 181, 2031–2043. [Google Scholar] [CrossRef]
- Alsaied, T.; Tremoulet, A.H.; Burns, J.C.; Saidi, A.; Dionne, A.; Lang, S.M.; Newburger, J.W.; de Ferranti, S.; Friedman, K.G. Review of Cardiac Involvement in Multisystem Inflammatory Syndrome in Children. Circulation 2021, 143, 78–88. [Google Scholar] [CrossRef]
- Waseem, M.; Shariff, M.A.; Tay, E.T.; Mortel, D.; Savadkar, S.; Lee, H.; Kondamudi, N.; Liang, T. Multisystem Inflammatory Syndrome in Children. J. Emerg. Med. 2022, 62, 28–37. [Google Scholar] [CrossRef]
- Alkan, G.; Sert, A.; Oz, S.K.T.; Emiroglu, M.; Yılmaz, R. Clinical Features and Outcome of MIS-C Patients: An Experience from Central Anatolia. Clin. Rheumatol. 2021, 40, 4179–4189. [Google Scholar] [CrossRef]
- Williams, V.; Dash, N.; Suthar, R.; Mohandoss, V.; Jaiswal, N.; Kavitha, T.K.; Nallasamy, K.; Angurana, S.K. Clinicolaboratory Profile, Treatment, Intensive Care Needs, and Outcome of Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2: A Systematic Review and Meta-Analysis. J. Pediatr. Intensive Care 2022, 11, 001–012. [Google Scholar] [CrossRef]
- Clark, B.C.; Sanchez-de-Toledo, J.; Bautista-Rodriguez, C.; Choueiter, N.; Lara, D.; Kang, H.; Mohsin, S.; Fraisse, A.; Cesar, S.; Shaikh, A.S.; et al. Cardiac Abnormalities Seen in Pediatric Patients During the Severe Acute Respiratory Syndrome Coronavirus 2 Pandemic: An International Experience. J. Am. Heart Assoc. 2020, 9, 21. [Google Scholar] [CrossRef]
- Hejazi, O.I.; Loke, Y.-H.; Harahsheh, A.S. Short-Term Cardiovascular Complications of Multi-System Inflammatory Syndrome in Children (MIS-C) in Adolescents and Children. Curr. Pediatr. Rep. 2021, 9, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Bagri, N.K.; Deepak, R.K.; Meena, S.; Gupta, S.K.; Prakash, S.; Setlur, K.; Satapathy, J.; Chopra, K.; Upadhyay, A.D.; Ramakrishnan, S.; et al. Outcomes of Multisystem Inflammatory Syndrome in Children Temporally Related to COVID-19: A Longitudinal Study. Rheumatol. Int. 2022, 42, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, F.; Baldini, L.; Dellepiane, M.; Covizzi, C.; Mogni, R.; Pruccoli, G.; Orsi, C.; Rabbone, I.; Parodi, E.; Mignone, F.; et al. MIS-C Treatment: Is IVIG Always Necessary? Front. Pediatr. 2021, 9, 753123. [Google Scholar] [CrossRef]
- McArdle, A.J.; Vito, O.; Patel, H.; Seaby, E.G.; Shah, P.; Wilson, C.; Broderick, C.; Nijman, R.; Tremoulet, A.H.; Munblit, D.; et al. Treatment of Multisystem Inflammatory Syndrome in Children. N. Engl. J. Med. 2021, 385, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Villacis-Nunez, D.S.; Jones, K.; Jabbar, A.; Fan, L.; Moore, W.; Peter, A.S.; Henderson, M.; Xiang, Y.; Kelleman, M.S.; Sherry, W.; et al. Short-Term Outcomes of Corticosteroid Monotherapy in Multisystem Inflammatory Syndrome in Children. JAMA Pediatr. 2022, 176, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, L.; Piñeres-Olave, B.E.; Niño-Serna, L.F.; Vega, L.M.; Gomez, I.J.A.; Chacón, S.; Jaramillo-Bustamante, J.C.; Mulett-Hoyos, H.; González-Pardo, O.; Zemanate, E.; et al. Mortality and Clinical Characteristics of Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Covid-19 in Critically Ill Patients: An Observational Multicenter Study (MISCO Study). BMC Pediatr. 2021, 21, 516. [Google Scholar] [CrossRef]
- Tiwari, A.; Balan, S.; Rauf, A.; Kappanayil, M.; Kesavan, S.; Raj, M.; Sivadas, S.; Vasudevan, A.K.; Chickermane, P.; Vijayan, A.; et al. COVID-19 Related Multisystem Inflammatory Syndrome in Children (MIS-C): A Hospital-Based Prospective Cohort Study from Kerala, India. BMJ Paediatr. Open 2021, 5, e001195. [Google Scholar] [CrossRef]
- Buonsenso, D.; Mariani, F.; Pierri, L.; Morello, R.; Yock-Corrales, A.; Del Aguila, O.; Lazzareschi, I.; Zampino, G.; Nunziata, F.; Valentini, P.; et al. Association between Coagulation Profile and Clinical Outcome in Children with SARS-CoV-2 Infection or MIS-C: A Multicenter Cross-Sectional Study. Children 2022, 9, 279. [Google Scholar] [CrossRef]
- Lapp, S.A.; Abrams, J.; Lu, A.T.; Hussaini, L.; Kao, C.M.; Hunstad, D.A.; Rosenberg, R.B.; Zafferani, M.J.; Ede, K.C.; Ballan, W.; et al. Serologic and Cytokine Signatures in Children with Multisystem Inflammatory Syndrome and Coronavirus Disease 2019. Open Forum Infect. Dis. 2022, 9, ofac070. [Google Scholar] [CrossRef]
| All MIS-C | Low-Risk Group | High-Risk Group | p * | |
|---|---|---|---|---|
| Number of patients, n (%) | 99 (100) | 59 (59.6) | 40 (40.4) | |
| Age, month, median (min.–max.) | 83 (3–205) | 73 (16–191) | 95.5 (3–205) | 0.07 |
| Age groups, n (%) | 0.03 | |||
| <72-month-old | 37 (37.4) | 27 (45.8) | 10 (25.0) | |
| ≥72-month-old | 62 (62.6) | 32 (54.2) | 30 (75.0) | |
| Gender male, n (%) | 63 (63.6) | 38 (64.4) | 25 (62.5) | 0.84 |
| Pre-existing medical condition, n (%) | 10 (10.1) | 1 (1.7) | 9 (22.5) | N/A ** |
| Autoinflammatory diseases | 2 (2.0) | - | 2 (5.0) | |
| Asthma | 2 (2.0) | 1 (1.7) | 1 (2.5) | |
| Metabolic diseases | 2 (2.0) | - | 2 (5.0) | |
| Malignancy | 2 (2.0) | - | 2 (5.0) | |
| Congenital heart disease | 1 (1.0) | - | 1 (2.5) | |
| Chronic kidney disease | 1 (1.0) | - | 1 (2.5) | |
| Kawasaki Disease symptoms, n (%) | ||||
| Fever | 99 (100) | 59 (100) | 40 (100) | - |
| Conjunctivitis | 69 (69.7) | 41 (69.5) | 28 (70.0) | 0.97 |
| Rash | 64 (64.6) | 41 (69.5) | 23 (57.5) | 0.22 |
| Oral mucous membrane changes | 48 (48.5) | 27 (45.8) | 21 (52.5) | 0.51 |
| Peripheral extremity changes | 30 (30.3) | 14 (23.7) | 16 (40.0) | 0.08 |
| Cervical lymphadenopathy (>1.5 cm) | 13 (13.1) | 8 (13.6) | 5 (12.5) | 0.87 |
| Number of positive criteria (instead of fever) for Kawasaki Disease, n (%) | 0.53 | |||
| ≤1 | 32 (32.3) | 20 (33.9) | 12 (30.0) | |
| 2–3 | 52 (52.5) | 32 (54.2) | 20 (50.0) | |
| ≥4 | 15 (15.2) | 7 (11.9) | 8 (20.0) | |
| Other symptoms and findings, n (%) | ||||
| Abdominal pain *** | 59 (67.8) | 36 (70.6) | 23 (63.9) | 0.51 |
| Nausea/vomiting | 64 (64.6) | 38 (64.4) | 26 (65.0) | 0.95 |
| Diarrhea | 47 (47.5) | 29 (49.2) | 18 (45.0) | 0.68 |
| Headache *** | 22 (30.6) | 15 (37.5) | 7 (21.9) | 0.15 |
| Respiratory distress | 15 (15.2) | 0 (0) | 15 (37.5) | N/A ** |
| Arthralgia/arthritis | 14 (14.1) | 9 (15.3) | 5 (12.5) | 0.70 |
| Organomegaly (hepatomegaly or splenomegaly) | 13 (13.1) | 5 (8.5) | 8 (20.0) | 0.09 |
| Neurological symptoms/findings (except headache) | 7 (6.1) | 0 (0) | 7 (17.5) | N/A ** |
| COVID-19 history, n (%) | 0.97 | |||
| Previous SARS-CoV-2 infection § | 25 (25.3) | 15 (25.4) | 10 (25.0) | |
| Close contact with a case but no infection | 43 (43.4) | 26 (44.1) | 17 (42.5) | |
| No close contact or a history of infection | 31 (31.3) | 18 (30.5) | 13 (32.5) | |
| The severity of previous SARS-CoV-2 infection †, n (%) | 0.15 | |||
| Non-severe (no need for hospitalization) | 23 (92.0) | 15 (100) | 8 (80.0) | |
| Need of hospitalization | 2 (8.0) | 0 (0) | 2 (20.0) | |
| Duration between COVID-19 (or contact) and MIS-C ‡ week, median (min.–max.) | 4 (2–16) | 4 (2–16) | 4 (2–9) | 0.34 |
| Duration between the onset of the MIS-C symptoms and admission, day median (min.–max.) | 5 (1–10) | 5 (1–10) | 5.5 (1–10) | 0.39 |
| SARS-CoV-2 PCR and serology results at the admission, n (%) | 0.23 | |||
| PCR and serology are both positive | 4 (4.0) | 1 (1.7) | 3 (7.5) | |
| PCR positive—serology negative | 1 (1.0) | 0 | 1 (2.5) | |
| PCR negative—serology positive | 93 (93.9) | 57 (96.6) | 36 (90.0) | |
| PCR and serology are both negative | 1 (1.0) | 1 (1.7) | 0 | |
| Chest X-ray abnormal findings, n (%) | 24 (24.2) | 5 (8.5) | 19 (47.5) | <0.001 |
| Low blood pressure (hypotension/shock), n (%) | 13 (13.1) | 0 (0) | 13 (32.5) | N/A ** |
| Respiratory support (high flow oxygen or invasive mechanical ventilation) need, n (%) | 15 (15.2) | 0 (0) | 15 (37.5) | N/A ** |
| ICU admission, n (%) | 15 (15.2) | 0 (0) | 15 (37.5) | N/A ** |
| Length of stay, day median (min.–max.) | 7 (3–24) | 6 (3–9) | 10,5 (6–24) | N/A ** |
| Mortality, ratio (%) | ||||
| General mortality | 3/99 (3.0) | 0 (0) | 3/40 (7.5) | 0.03 |
| Mortality with a pre-existing medical condition | 2/10 (20) | 2/9 (22.2) | ||
| Mortality in previously healthy children | 1/89 (1.1) | 1/31 (3.2) |
| Variables * | Patients with MIS-C ** (n = 93) | Reference Ranges |
|---|---|---|
| WBC (cell/mm3) | 6281 ± 2592 | |
| Absolute lymphocyte count (cell/mm3) | 930 (200–4920) | |
| Absolute neutrophil count (cell/mm3) | 4365 ± 2255 | |
| Platelet count (cell × 103/mm3) | 152 (27–490) | |
| ESR (mm/h) | 42.6 ± 24.4 | 0–20 |
| C-reactive protein (mg/dL) | 155.5 ± 90.0 | 0–0.5 |
| Procalcitonin (ng/mL) | 6.0 (0.1–100) | 0–0.5 |
| Ferritin (ng/mL) | 534 (108–7800) | 14–124 |
| BNP (pg/mL) | 4611 (52–48050) | 0–125 |
| LDH (U/L) | 343 (61–4285) | 120–300 |
| ALT (U/L) | 39 (7–838) | 0–33 |
| AST (U/L) | 45 (9–965) | 0–32 |
| Total bilirubin (mg/dL) | 0.41 (0.1–8.37) | 0–0.9 |
| Direct bilirubin (mg/dL) | 0.20 (0.06–7.06) | 0–0.3 |
| Total protein (g/dL) | 5.84 ± 0.84 | 6–8 |
| Albumin (g/dL) | 2.80 ± 0.49 | 3.2–4.5 |
| Sodium (mEq/L) | 131.7 ± 3.7 | 136–145 |
| BUN (mg/dL) | 13.4 (5.6–87.9) | 5–18 |
| Creatinine (mg/dL) | 0.52 (0.24–3.19) | 0.4–0.9 |
| aPTT (s) | 31.1 (22.3–58.0) | 25–36 |
| PT (s) | 14.1 (11.4–62.3) | 10–14 |
| INR | 1.26 (0.96–5.85) | 0.8–1.2 |
| D-dimer (ng/mL) | 4620 (600–63000) | 0–500 |
| Fibrinogen (mg/dL) | 486.9 ± 173.4 | 180–350 |
| Findings n (%) * | All MIS-C (n = 99) | Low-Risk MIS-C (n = 59) | High-Risk MIS-C (n = 40) | p ** |
|---|---|---|---|---|
| Valvulitis | 72 (72.7) | 41 (69.49) | 31 (77.5) | 0.38 |
| Valve involved Mitral Tricuspid Aortic Pulmonary | 71 (71.7) 25 (25.3) 10 (10.1) 3 (3.0) | 41 (69.5) 8 (13.6) 2 (3.4) 0 (0) | 30 (75.0) 17 (42.5) 8 (20.0) 3 (7.1) | 0.55 0.001 0.007 0.06 |
| Number of valves involved None 1 2 >2 | 27 (27.3) 41 (41.4) 24 (24.2) 7 (7.1) | 18 (30.5) 30 (50.8) 11 (18.6) 0 (0) | 9 (22.5) 11 (27.5) 13 (32.5) 7 (17.5) | 0.004 |
| Valvular maximum insufficiency degree None 1 2 3 | 27 (27.3) 65 (65.7) 6 (6.1) 1 (1.0) | 18 (30.5) 41 (69.5) 0 (0) 0 (0) | 9 (22.5) 24 (60.0) 6 (15.0) 1 (2.5) | 0.011 |
| Left ventricular dysfunction | 10 (10.1) | 0 (0) | 10 (25.0) | N/A |
| Troponin levels Normal or <10-fold of the upper limit Increased by ≥10-fold of upper limit | 90 (90.9) 9 (9.1) | 57 (96.6) 2 (3.4) | 33 (82.5) 7 (17.5) | 0.017 |
| Myocarditis | 14 (14.1) | 3 (5.1) | 11 (27.5) | 0.002 |
| Pericardial effusion | 12 (12.1) | 4 (6.8) | 8 (20.0) | 0.048 |
| Coronary artery involvement | 10 (10.1) | 0 (0) | 10 (25.0) | N/A |
| Transient sinus bradycardia | 62 (62.6) | 34 (57.6) | 28 (70.0) | 0.21 |
| Duration between fever ending and bradycardia starting, median day (min.–max.) | 2 (0–6) | 1.5 (0–3) | 2 (0–6) | 0.15 |
| Treatment Regime, n (%) | Low-Risk (n = 59) | High-Risk (n = 40) | p |
|---|---|---|---|
| IVIG plus steroid | 46 (78.0) | 39 (97.5) | 0.045 * |
| IVIG only | 8 (13.6) | 1 (2.5) | |
| Steroid only | 3 (5.1) | 0 (0) | |
| IVIG or steroid not used | 2 (3.4) | 0 (0) |
| Variable | OR | 95% CI for OR | p * |
|---|---|---|---|
| BNP (10−3) | 1.125 | 1.047–1.209 | 0.001 |
| Total protein ** | 2.717 | 1.130–6.536 | 0.026 |
| Troponin level (Increased by ≥10-fold upper normal limit) | 9.491 | 1.287–70.018 | 0.027 |
| Method | Cut-Off Value | Accuracy | Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|---|---|
| Univariate ROC Analysis | |||||||
| Procalcitonin | 4.5 | 0.607 | 0.706 | 0.509 | 0.453 | 0.75 | 0.614 |
| Platelet | 73,000 | 0.429 | 0.824 | 0.034 | 0.329 | 0.25 | 0.566 |
| Ferritin | 546 | 0.721 | 0.765 | 0.678 | 0.578 | 0.833 | 0.742 |
| BNP | 8332 | 0.77 | 0.676 | 0.864 | 0.742 | 0.823 | 0.772 |
| ALC | 780 | 0.606 | 0.5 | 0.712 | 0.5 | 0.712 | 0.627 |
| D-dimer | 2890 | 0.674 | 0.912 | 0.407 | 0.478 | 0.923 | 0.681 |
| Total protein | 5.48 | 0.69 | 0.618 | 0.763 | 0.6 | 0.776 | 0.75 |
| Combined Tests Analysis (Machine Learning) | |||||||
| Decision Tree (CART) | 0.77 | 0.676 | 0.864 | 0.742 | 0.823 | ||
| SVM w/radial basis function | 0.859 | 0.735 | 0.983 | 0.962 | 0.866 | ||
| Logistic regression | 0.785 | 0.823 | 0.746 | 0.651 | 0.88 | ||
| Naïve Bayes | 0.836 | 0.706 | 0.966 | 0.923 | 0.851 | ||
| LDA | 0.746 | 0.559 | 0.932 | 0.826 | 0.786 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cetin, B.S.; Kısaarslan, A.P.; Tekin, S.; Goksuluk, M.B.; Baykan, A.; Akyıldız, B.N.; Seçilmiş, Y.; Poyrazoglu, H.; on behalf of the Erciyes MIS-C Study Group. Evaluation of Baseline Characteristics and Prognostic Factors in Multisystemic Inflammatory Syndrome in Children: Is It Possible to Foresee the Prognosis in the First Step? J. Clin. Med. 2022, 11, 4615. https://doi.org/10.3390/jcm11154615
Cetin BS, Kısaarslan AP, Tekin S, Goksuluk MB, Baykan A, Akyıldız BN, Seçilmiş Y, Poyrazoglu H, on behalf of the Erciyes MIS-C Study Group. Evaluation of Baseline Characteristics and Prognostic Factors in Multisystemic Inflammatory Syndrome in Children: Is It Possible to Foresee the Prognosis in the First Step? Journal of Clinical Medicine. 2022; 11(15):4615. https://doi.org/10.3390/jcm11154615
Chicago/Turabian StyleCetin, Benhur Sirvan, Ayşenur Paç Kısaarslan, Sedanur Tekin, Merve Basol Goksuluk, Ali Baykan, Başak Nur Akyıldız, Yılmaz Seçilmiş, Hakan Poyrazoglu, and on behalf of the Erciyes MIS-C Study Group. 2022. "Evaluation of Baseline Characteristics and Prognostic Factors in Multisystemic Inflammatory Syndrome in Children: Is It Possible to Foresee the Prognosis in the First Step?" Journal of Clinical Medicine 11, no. 15: 4615. https://doi.org/10.3390/jcm11154615
APA StyleCetin, B. S., Kısaarslan, A. P., Tekin, S., Goksuluk, M. B., Baykan, A., Akyıldız, B. N., Seçilmiş, Y., Poyrazoglu, H., & on behalf of the Erciyes MIS-C Study Group. (2022). Evaluation of Baseline Characteristics and Prognostic Factors in Multisystemic Inflammatory Syndrome in Children: Is It Possible to Foresee the Prognosis in the First Step? Journal of Clinical Medicine, 11(15), 4615. https://doi.org/10.3390/jcm11154615






