I-FABP as a Potential Marker for Intestinal Barrier Loss in Porcine Polytrauma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals
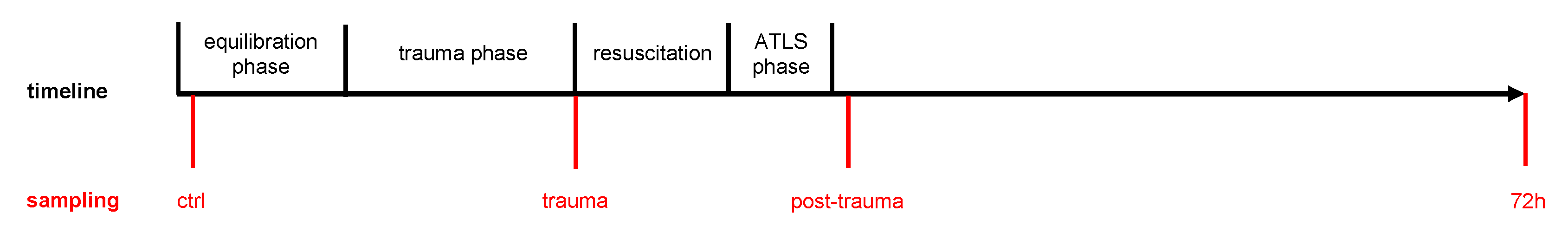
2.3. Porcine Polytrauma Model
2.4. Blood and Urine Sampling and Processing
2.5. ELISA Measurements
2.6. Immunohistochemical Staining of Intestine Tissue
2.7. Statistical Analyses
3. Results
3.1. Data Decription
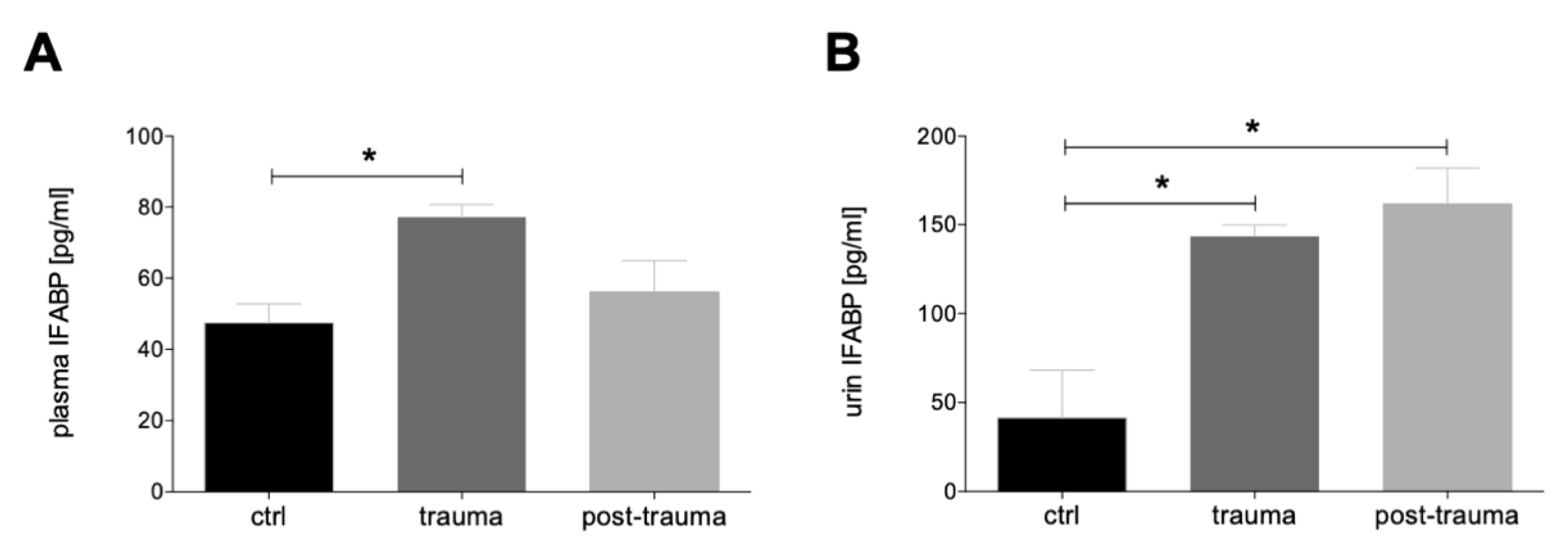
3.1.1. Plasma and Urine I-FABP Concentrations after Trauma
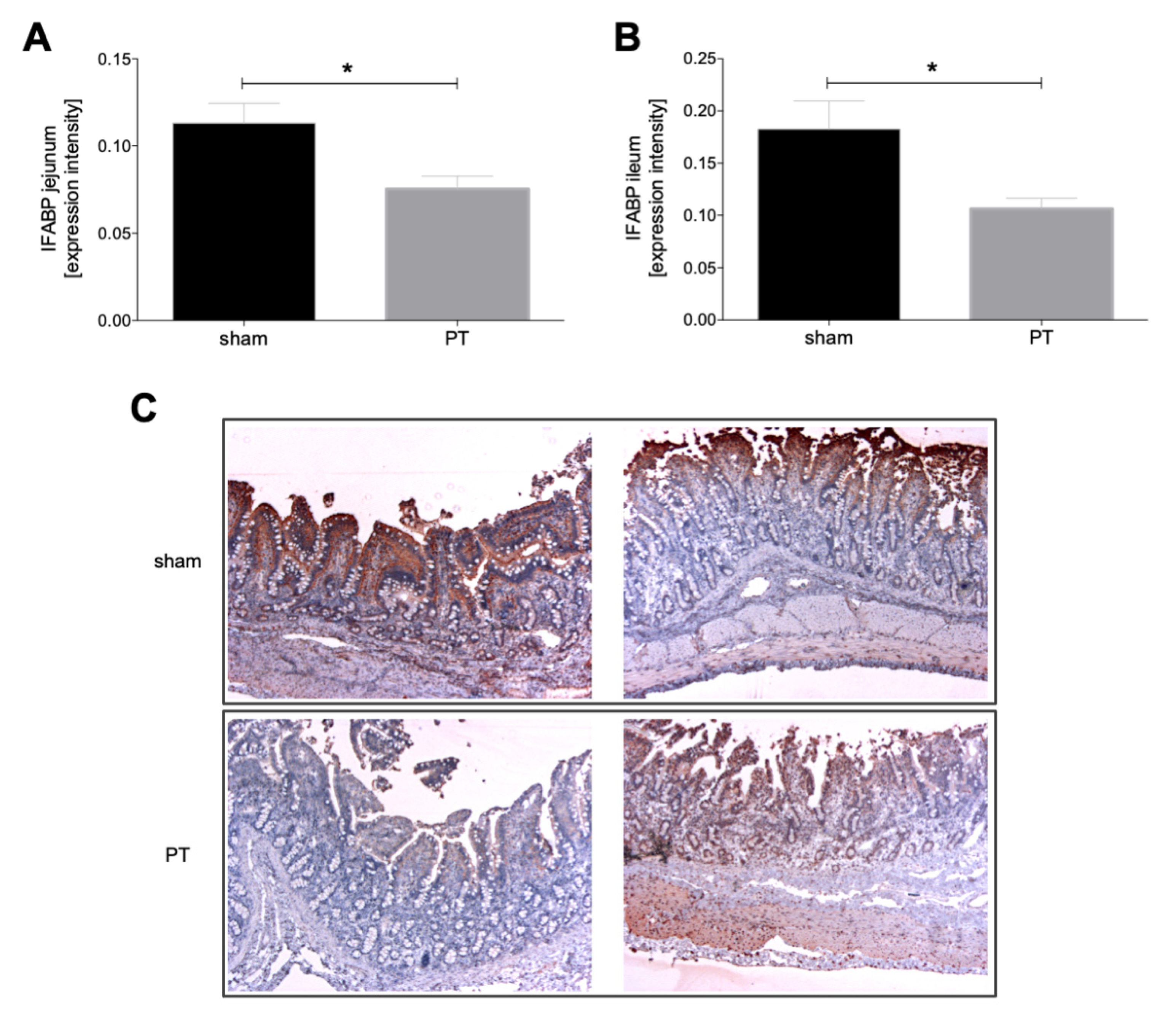
3.1.2. I-FABP Expression in Intestinal Tissue
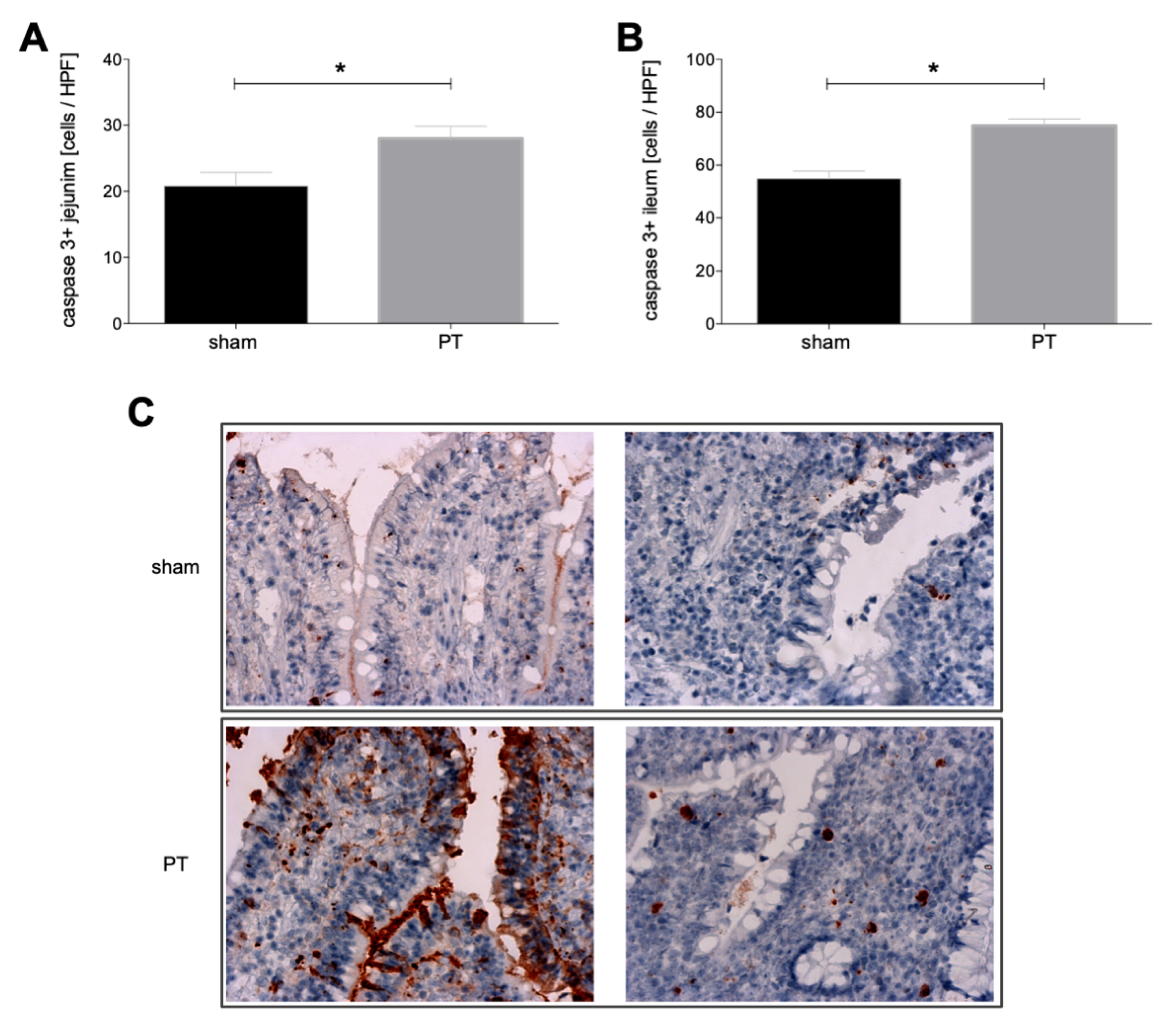
3.1.3. Caspase-3 Expression in Intestinal Tissue
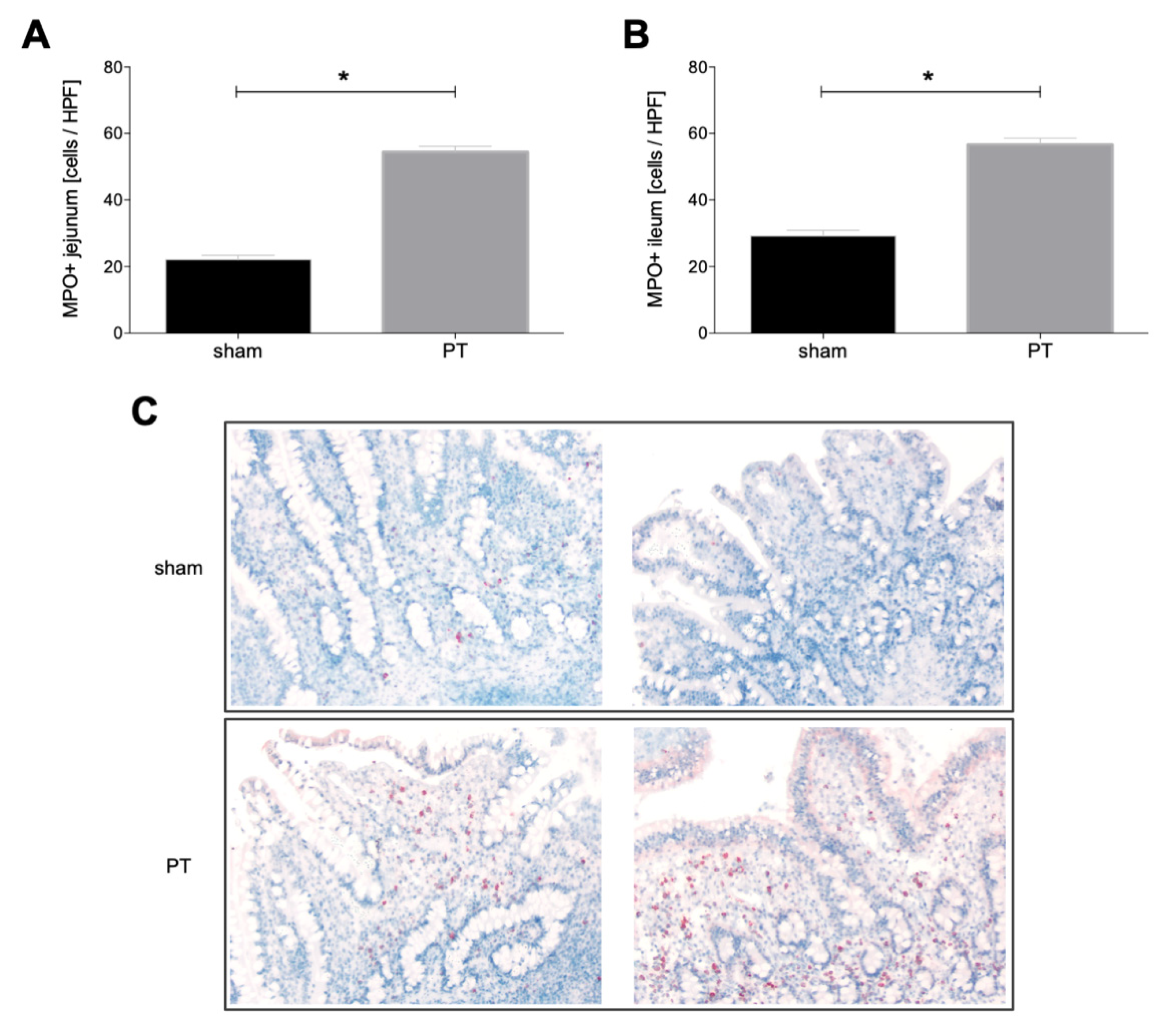
3.1.4. Myeloperoxidase Expression in Intestinal Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haagsma, J.A.; Graetz, N.; Bolliger, I.; Naghavi, M.; Higashi, H.; Mullany, E.C.; Abera, S.F.; Abraham, J.P.; Adofo, K.; Alsharif, U.; et al. The global burden of injury: Incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj. Prev. J. Int. Soc. Child. Adolesc. Inj. Prev. 2016, 22, 3–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nast-Kolb, D.; Aufmkolk, M.; Rucholtz, S.; Obertacke, U.; Waydhas, C. Multiple organ failure still a major cause of morbidity but not mortality in blunt multiple trauma. J. Trauma 2001, 51, 835–841; discussion 841–842. [Google Scholar] [CrossRef] [PubMed]
- Relja, B.; Mörs, K.; Marzi, I. Danger signals in trauma. Eur. J. Trauma Emerg. Surg. Off. Publ. Eur. Trauma Soc. 2018, 44, 301–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sertaridou, E.; Papaioannou, V.; Kolios, G.; Pneumatikos, I. Gut failure in critical care: Old school versus new school. Ann. Gastroenterol. 2015, 28, 309–322. [Google Scholar]
- Mittal, R.; Coopersmith, C.M. Redefining the gut as the motor of critical illness. Trends Mol. Med. 2014, 20, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.J.; Rosenthal, M.D.; Miller, K.R.; Martindale, R.G. The gut in trauma. Curr. Opin. Crit. Care 2016, 22, 339–346. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Triantos, C.; Thomopoulos, K.; Fligou, F.; Maroulis, I.; Marangos, M.; Gogos, C.A. Gut-origin sepsis in the critically ill patient: Pathophysiology and treatment. Infection 2018, 46, 751–760. [Google Scholar] [CrossRef]
- Evennett, N.J.; Petrov, M.S.; Mittal, A.; Windsor, J.A. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J. Surg. 2009, 33, 1374–1383. [Google Scholar] [CrossRef]
- Voth, M.; Lustenberger, T.; Relja, B.; Marzi, I. Is I-FABP not only a marker for the detection abdominal injury but also of hemorrhagic shock in severely injured trauma patients? World J. Emerg. Surg. WJES 2019, 14, 49. [Google Scholar] [CrossRef] [Green Version]
- Glatz, J.F.; van der Vusse, G.J. Cellular fatty acid-binding proteins: Their function and physiological significance. Prog. Lipid Res. 1996, 35, 243–282. [Google Scholar] [CrossRef]
- Pelsers, M.M.A.L.; Hermens, W.T.; Glatz, J.F.C. Fatty acid-binding proteins as plasma markers of tissue injury. Clin. Chim. Acta Int. J. Clin. Chem. 2005, 352, 15–35. [Google Scholar] [CrossRef]
- Pelsers, M.M.A.L.; Namiot, Z.; Kisielewski, W.; Namiot, A.; Januszkiewicz, M.; Hermens, W.T.; Glatz, J.F.C. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin. Biochem. 2003, 36, 529–535. [Google Scholar] [CrossRef]
- Voth, M.; Duchene, M.; Auner, B.; Lustenberger, T.; Relja, B.; Marzi, I. I-FABP is a Novel Marker for the Detection of Intestinal Injury in Severely Injured Trauma Patients. World J. Surg. 2017, 41, 3120–3127. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 2011. Available online: http://www.ncbi.nlm.nih.gov/books/NBK54050/ (accessed on 1 April 2022).
- Kilkenny, C.; Browne, W.J.; Cuthi, I.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 2012, 41, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Horst, K.; Simon, T.P.; Pfeifer, R.; Teuben, M.; AlMahmoud, K.; Zhi, Q.; Santos, S.A.; Wembers, C.C.; Leonhardt, S.; Heussen, N.; et al. Characterization of blunt chest trauma in a long-term porcine model of severe multiple trauma. Sci. Rep. 2016, 6, 39659. [Google Scholar] [CrossRef] [Green Version]
- ATLS Subcommittee; American College of Surgeons’ Committee on Trauma; International ATLS Working Group. Advanced trauma life support (ATLS®): The ninth edition. J. Trauma Acute Care Surg. 2013, 74, 1363–1366. [Google Scholar]
- Nikolaou, N.I.; Welsford, M.; Beygui, F.; Bossaert, L.; Ghaemmaghami, C.; Nonogi, H.; O’Connor, R.E.; Pichel, D.R.; Scott, T.; Walters, D.L.; et al. Part 5: Acute coronary syndromes: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 2015, 132, S146–S176. [Google Scholar]
- Khadaroo, R.G.; Fortis, S.; Salim, S.Y.; Streutker, C.; Churchill, T.A.; Zhang, H. I-FABP as biomarker for the early diagnosis of acute mesenteric ischemia and resultant lung injury. PLoS ONE 2014, 9, e115242. [Google Scholar] [CrossRef] [Green Version]
- Niewold, T.A.; Meinen, M.; van der Meulen, J. Plasma intestinal fatty acid binding protein (I-FABP) concentrations increase following intestinal ischemia in pigs. Res. Vet. Sci. 2004, 77, 89–91. [Google Scholar] [CrossRef]
- Uzun, O.; Turkmen, S.; Eryigit, U.; Mentese, A.; Turkyilmaz, S.; Turedi, S.; Karahan, S.C.; Gunduz, A. Can Intestinal Fatty Acid Binding Protein (I-FABP) Be a Marker in the Diagnosis of Abdominal Pathology? Turk. J. Emerg. Med. 2014, 14, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Tsukahara, A.; Ueki, K.; Sakai, Y.; Tani, T.; Nishimura, A.; Yamazaki, T.; Tamiya, Y.; Tada, T.; Hirota, M.; et al. Diagnosis of ischemic small bowel disease by measurement of serum intestinal fatty acid-binding protein in patients with acute abdomen: A multicenter, observer-blinded validation study. J. Gastroenterol. 2011, 46, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.L.; Cen, Y.Y.; Li, S.M.; Li, W.M.; Lu, Q.P.; Xu, P.Y. Accuracy of the serum intestinal fatty-acid-binding protein for diagnosis of acute intestinal ischemia: A meta-analysis. Sci. Rep. 2016, 6, 34371. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Schmieg, R.E.; Swanson, P.E.; Freeman, B.D.; Tinsley, K.W.; Cobb, J.P.; Karl, I.E.; Buchman, T. Rapid onset of intestinal epithelial and lymphocyte apoptotic cell death in patients with trauma and shock. Crit. Care Med. 2000, 28, 3207–3217. [Google Scholar] [CrossRef] [PubMed]
- Sturm, R.; Haag, F.; Janicova, A.; Xu, B.; Vollrath, J.T.; Bundkirchen, K.; Dunay, I.R.; Neunaber, C.; Marzi, I.; Relja, B. Acute alcohol consumption increases systemic endotoxin bioactivity for days in healthy volunteers-with reduced intestinal barrier loss in female. Eur. J. Trauma Emerg. Surg. Off. Publ. Eur. Trauma Soc. 2022, 48, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Xu, D.Z.; Davidson, M.T.; Haskó, G.; Deitch, E.A. Hemorrhagic shock induces endothelial cell apoptosis, which is mediated by factors contained in mesenteric lymph. Crit. Care Med. 2004, 32, 2464–2470. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Haan, J.J.; Lubbers, T.; Derikx, J.P.; Relja, B.; Henrich, D.; Greve, J.W.; Marzi, I.; Buurman, W.A. Rapid development of intestinal cell damage following severe trauma: A prospective observational cohort study. Crit. Care 2009, 13, R86. [Google Scholar] [CrossRef] [Green Version]
- Timmermans, K.; Özcan, S.; Kox, M.; Vaneker, M.; De Jong, C.; Gerretsen, J.; Edwards, M.; Scheffer, G.J.; Pickkers, P. Circulating iFABP Levels as a marker of intestinal damage in trauma patients. Shock 2015, 43, 117–120. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vollrath, J.T.; Klingebiel, F.; Bläsius, F.; Greven, J.; Bolierakis, E.; Nowak, A.J.; Simic, M.; Hildebrand, F.; Marzi, I.; Relja, B. I-FABP as a Potential Marker for Intestinal Barrier Loss in Porcine Polytrauma. J. Clin. Med. 2022, 11, 4599. https://doi.org/10.3390/jcm11154599
Vollrath JT, Klingebiel F, Bläsius F, Greven J, Bolierakis E, Nowak AJ, Simic M, Hildebrand F, Marzi I, Relja B. I-FABP as a Potential Marker for Intestinal Barrier Loss in Porcine Polytrauma. Journal of Clinical Medicine. 2022; 11(15):4599. https://doi.org/10.3390/jcm11154599
Chicago/Turabian StyleVollrath, Jan Tilmann, Felix Klingebiel, Felix Bläsius, Johannes Greven, Eftychios Bolierakis, Aleksander J. Nowak, Marija Simic, Frank Hildebrand, Ingo Marzi, and Borna Relja. 2022. "I-FABP as a Potential Marker for Intestinal Barrier Loss in Porcine Polytrauma" Journal of Clinical Medicine 11, no. 15: 4599. https://doi.org/10.3390/jcm11154599
APA StyleVollrath, J. T., Klingebiel, F., Bläsius, F., Greven, J., Bolierakis, E., Nowak, A. J., Simic, M., Hildebrand, F., Marzi, I., & Relja, B. (2022). I-FABP as a Potential Marker for Intestinal Barrier Loss in Porcine Polytrauma. Journal of Clinical Medicine, 11(15), 4599. https://doi.org/10.3390/jcm11154599






