Investigating Dynamics of the Spinal Cord Injury Adjustment Model: Mediation Model Analysis
Abstract
1. Introduction
1.1. SCI Rehabilitation
1.2. Factors That Impact Adjustment Following SCI
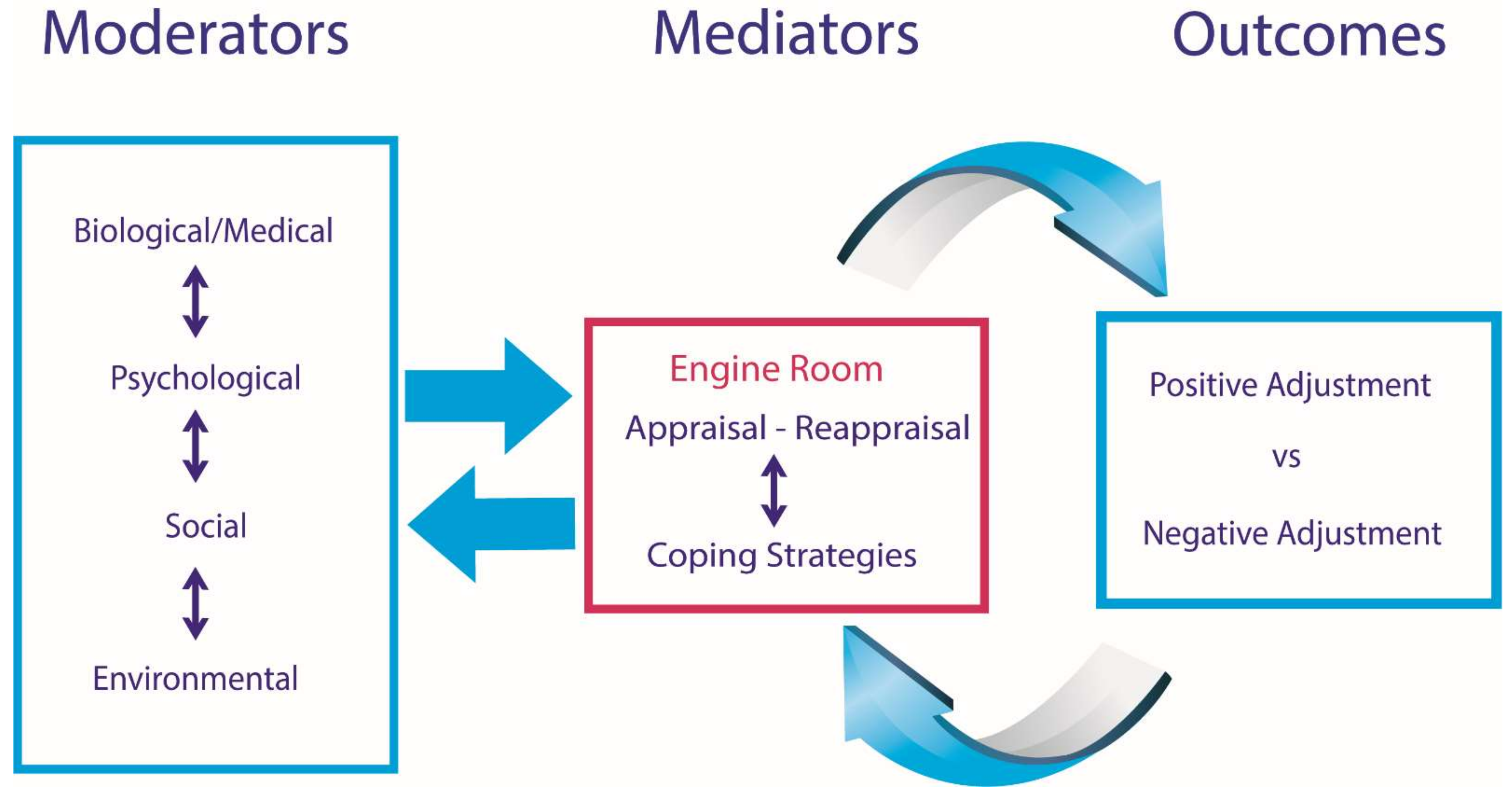
1.3. The SCI Adjustment Model (SCIAM)
1.4. Evidence Supporting SCIAM
1.5. Aims and Hypotheses
2. Methods
2.1. Participants, Study Setting, Procedure, and Ethics
2.2. Measures
2.3. Analyses
3. Results
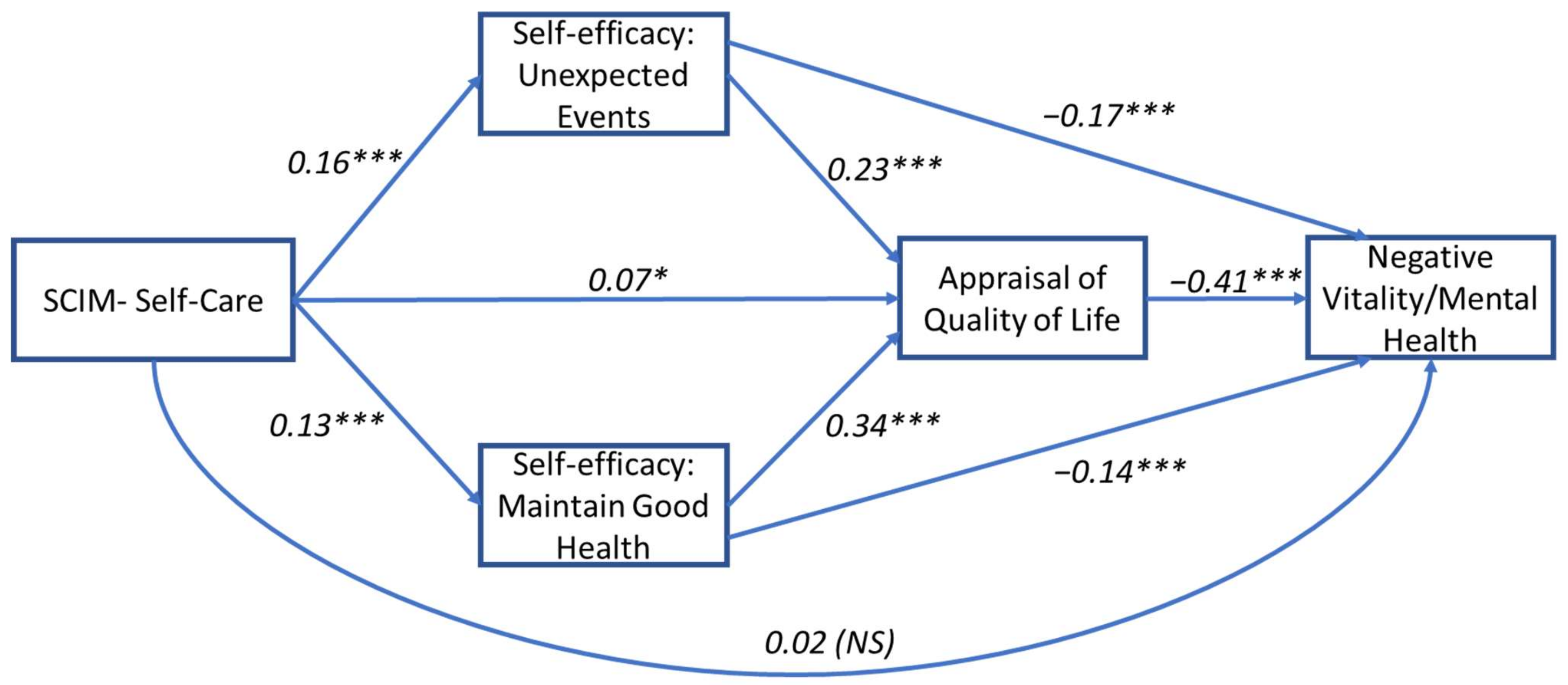
3.1. Model 1: Self-Care and Vitality/Mental Health
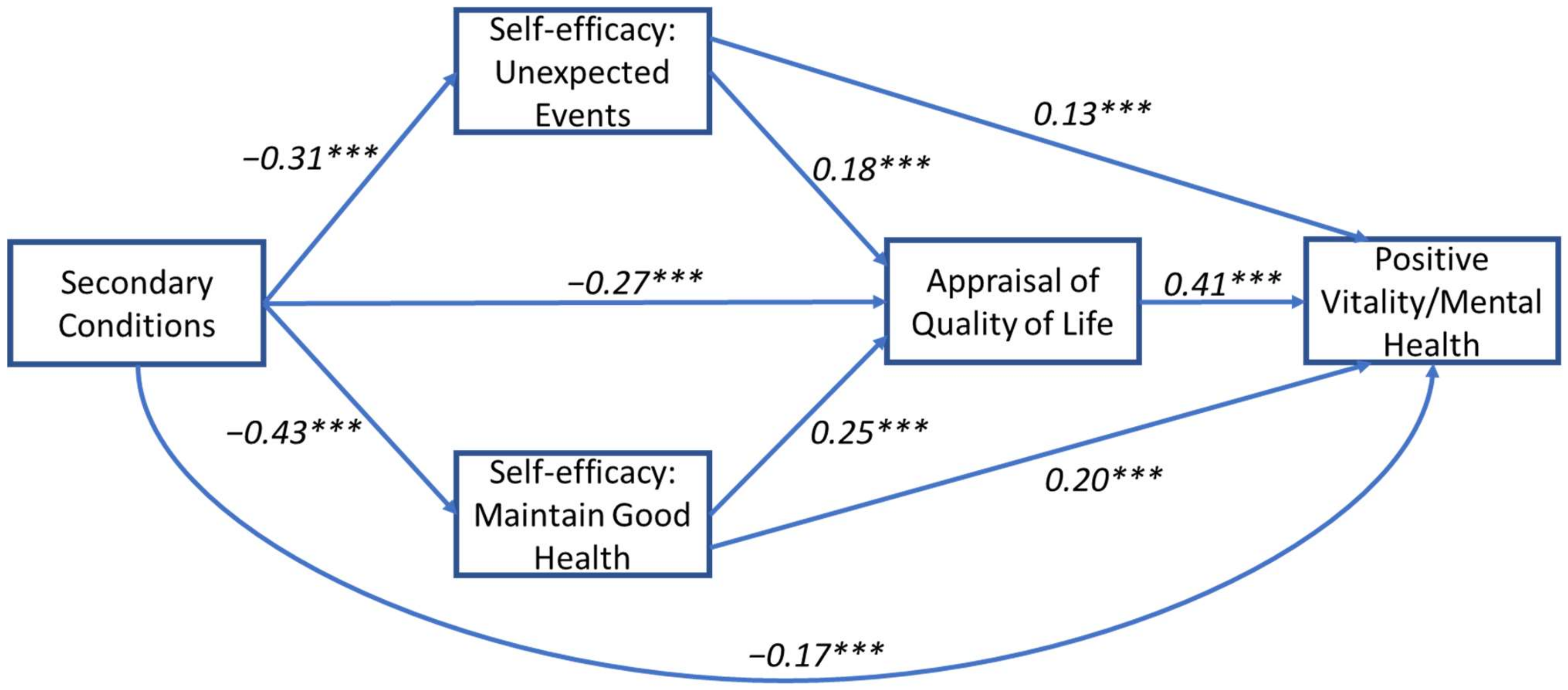
3.2. Model 2: Secondary Conditions and Vitality/Mental Health
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddall, P.J.; Cousins, M.J. Persistent pain as a disease entity: Implications for clinical management. Anesth. Analg. 2004, 99, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Siddall, P.J.; McClelland, J.M.; Rutkowski, S.B.; Cousins, M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 2003, 103, 249–257. [Google Scholar] [CrossRef]
- Elbasiouny, S.M.; Moroz, D.; Bakr, M.M.; Mushahwar, V.K. Management of spasticity after spinal cord injury: Current techniques and future directions. Neurorehabilit. Neural Repair 2010, 24, 23–33. [Google Scholar] [CrossRef]
- Cardenas, D.D.; Hooton, T.M. Urinary tract infection in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 1995, 76, 272–280. [Google Scholar] [CrossRef]
- Johns, J.S.; Krogh, K.; Ethans, K.; Chi, J.; Querée, M.; Eng, J.J. Pharmacological management of neurogenic bowel dysfunction after spinal cord injury and multiple sclerosis: A systematic review and clinical implications. J. Clin. Med. 2021, 10, 882. [Google Scholar] [CrossRef]
- Farkas, G.J.; Gordon, P.S.; Trewick, N.; Gorgey, A.S.; Dolbow, D.R.; Tiozzo, E.; Berg, A.S.; Gater, D.R. Comparison of various indices in identifying insulin resistance and diabetes in chronic spinal cord injury. J. Clin. Med. 2021, 10, 5591. [Google Scholar] [CrossRef]
- Sipski, M.L.; Alexander, C.J. Sexual Function and Dysfunction after Spinal Cord Injury. Phys. Med. Rehabil. Clin. N. Am. 1992, 3, 811–828. [Google Scholar] [CrossRef]
- Atkinson, R.A.; Cullum, N. Interventions for pressure ulcers: A summary of evidence for prevention and treatment. Spinal Cord 2018, 56, 186–198. [Google Scholar] [CrossRef]
- Krassioukov, A.; Claydon, V.E. The clinical problems in cardiovascular control following spinal cord injury: An overview. Prog. Brain Res. 2006, 152, 223–229. [Google Scholar] [CrossRef]
- Alilain, W.J.; Horn, K.P.; Hu, H.; Dick, T.E.; Silver, J. Functional regeneration of respiratory pathways after spinal cord injury. Nature 2011, 475, 196–200. [Google Scholar] [CrossRef]
- Craig, A.; Tran, Y.; Wijesuriya, N.; Middleton, J. Fatigue and tiredness in people with spinal cord injury. Psychosomatics 2012, 73, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Wijesuriya, N.; Tran, Y.; Middleton, J.; Craig, A. Impact of Fatigue on the Health-Related Quality of Life in Persons With Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2012, 93, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Guest, R.; Tran, Y.; Middleton, J. Cognitive Impairment and Mood States after Spinal Cord Injury. J. Neurotrauma 2017, 34, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Sandalic, D.; Craig, A.; Arora, M.; Pozzato, I.; Simpson, G.; Gopinath, B.; Kaur, J.; Shetty, S.; Weber, G.; Cameron, I.; et al. A prospective cohort study investigating contributors to mild cognitive impairment in adults with spinal cord injury: Study protocol. BMC Neurol. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Craig, A.; Perry, K.N.; Guest, R.; Tran, Y.; Dezarnaulds, A.; Hales, A.; Ephraums, C.; Middleton, J. Prospective Study of the Occurrence of Psychological Disorders and Comorbidities After Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2015, 96, 1426–1434. [Google Scholar] [CrossRef]
- Sandalic, D.; Tran, Y.; Craig, A.; Arora, M.; Pozzato, I.; Simpson, G.; Gopinath, B.; Kaur, J.; Shetty, S.; Weber, G.; et al. The Need for a Specialized Neurocognitive Screen and Consistent Cognitive Impairment Criteria in Spinal Cord Injury: Analysis of the Suitability of the Neuropsychiatry Unit Cognitive Assessment Tool. J. Clin. Med. 2022, 11, 3344. [Google Scholar] [CrossRef]
- Craig, A.; Nicholson Perry, K.; Guest, R.; Tran, Y.; Middleton, J. Adjustment following chronic spinal cord injury: Determining factors that contribute to social participation. Brit. J. Health Psychol. 2015, 20, 807–823. [Google Scholar] [CrossRef]
- Hilton, G.; Unsworth, C.; Murphy, G. The experience of attempting to return to work following spinal cord injury: A systematic review of the qualitative literature. Disabil. Rehabil. 2017, 40, 1745–1753. [Google Scholar] [CrossRef]
- Raguindin, P.F.; Bertolo, A.; Zeh, R.M.; Fränkl, G.; Itodo, O.A.; Capossela, S.; Bally, L.; Minder, B.; Brach, M.; Eriks-Hoogland, I.; et al. Body composition according to spinal cord injury level: A systematic review and meta-analysis. J. Clin. Med. 2021, 10, 3911. [Google Scholar] [CrossRef]
- Craig, A.; Tran, Y.; Guest, R.; Middleton, J. Excessive daytime sleepiness in adults with spinal cord injury and associations with pain catastrophizing and pain intensity. Spinal Cord 2020, 58, 831–839. [Google Scholar] [CrossRef]
- Berlowitz, D.; Brown, D.J.; Campbell, D.A.; Pierce, R.J. A Longitudinal Evaluation of Sleep and Breathing in the First Year After Cervical Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2005, 86, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Tran, Y.; Middleton, J. Theory of adjustment following severe neurological injury: Evidence supporting the Spinal Cord Injury Adjustment Model. In Horizons in Neuroscience Research; Costa, A., Villalba, E., Eds.; Nova Science Publishers: New York, NY, USA, 2017; Volume 29, pp. 117–139. [Google Scholar]
- Middleton, J.W.; Dayton, A.; Walsh, J.; Rutkowski, S.B.; Leong, G.; Duong, S. Life expectancy after spinal cord injury: A 50-year study. Spinal Cord 2012, 50, 803–811. [Google Scholar] [CrossRef]
- Savic, G.; DeVivo, M.J.; Frankel, H.L.; Jamous, M.A.; Soni, B.M.; Charlifue, S. Long-term survival after traumatic spinal cord injury: A 70-year British study. Spinal Cord 2017, 55, 651–658. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. A Glossary of Terms for Community Health Care and Services for Older Personnel; World Health Organisation Centre for Development, Ageing and Health Technical Report; WHO: Geneva, Switzerland, 2004; Volume 5. [Google Scholar]
- Middleton, J.; Nicholson Perry, K.; Craig, A. Development of a guide for the psychosocial care of people with spinal cord injury: Introduction and practice implications. Int. J. Phys. Med. Rehabil. 2014, 2, 226–231. [Google Scholar]
- Guest, R.; Craig, A.; Nicholson Perry, K.; Tran, Y.; Ephraums, C.; Hales, A.; Dezarnaulds, A.; Crino, R.; Middleton, J. Resilience following spinal cord injury: A prospective controlled study investigating the influence of the provision of group cognitive behavior therapy during inpatient rehabilitation. Rehabil. Psychol. 2015, 60, 311–321. [Google Scholar] [CrossRef]
- Middleton, J.; Tran, Y.; Craig, A. Relationship between quality of life and self-efficacy in persons with spinal cord injuries. Arch. Phys. Med. Rehabil. 2007, 88, 1643–1648. [Google Scholar] [CrossRef]
- Geyh, S.; Ballert, C.; Sinnott, A.; Charlifue, S.; Catz, A.; D’Andrea Greve, J.M.; Post, M.W. Quality of life after spinal cord injury: A comparison across six countries. Spinal Cord 2013, 51, 322–326. [Google Scholar] [CrossRef]
- Shojaei, M.H.; Alavinia, S.M.; Craven, B.C. Management of obesity after spinal cord injury: A systematic review. J. Spinal Cord Med. 2017, 40, 783–794. [Google Scholar] [CrossRef]
- Klyce, D.W.; Bombardier, C.H.; Davis, T.J.; Hartoonian, N.; Hoffman, J.M.; Fann, J.R.; Kalpakjian, C.Z. Distinguishing grief from depression during acute recovery from spinal cord injury. Arch. Phys. Med. Rehabil. 2015, 96, 1419–1425. [Google Scholar] [CrossRef]
- Williams, R.; Murray, A. Prevalence of depression after spinal cord injury: A meta-analysis. Arch. Phys. Med. Rehabil. 2015, 96, 133–140. [Google Scholar] [CrossRef]
- Bombardier, C.H.; Fann, J.R.; Tate, D.G.; Richards, J.S.; Wilson, C.S.; Warren, A.M.; Temkin, N.R.; Heinemann, A.W.; PRISMS Investigators. An exploration of modifiable risk factors for depression after spinal cord injury: Which factors should we target? Arch. Phys. Med. Rehabil. 2012, 93, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Macciocchi, S.; Seel, R.T.; Warshowsky, A.; Thompson, N.; Barlow, K. Co-occurring traumatic brain injury and acute spinal cord injury rehabilitation outcomes. Arch. Phys. Med. Rehabil. 2012, 93, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, Z.; Kumar, A.; Lipinski, M.M.; Loane, D.J.; Stoica, B.A.; Faden, A.I. Endoplasmic reticulum stress and disrupted neurogenesis in the brain are associated with cognitive impairment and depressive-like behavior after spinal cord injury. J. Neurotrauma 2016, 33, 1919–1935. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Guest, R.; Tran, Y.; Nicholson Perry, K.; Middleton, J. Pain catastrophizing and negative mood states following spinal cord injury: Transitioning from inpatient rehabilitation into the community. J. Pain 2017, 18, 800–810. [Google Scholar] [CrossRef]
- Craig, A.; Rodrigues, D.; Tran, Y.; Guest, R.; Middleton, J. Daytime sleepiness and its relationships to fatigue and autonomic dysfunction in adults with spinal cord injury. J. Psychosom. Res. 2018, 112, 90–98. [Google Scholar] [CrossRef]
- Berlowitz, D.J.; Spong, J.; Gordon, I.; Howard, M.E.; Brown, D.J. Relationships between objective sleep indices and symptoms in a community sample of people with tetraplegia. Arch. Phys. Med. Rehabil. 2012, 93, 1246–1252. [Google Scholar] [CrossRef]
- Leduc, B.E.; Dagher, J.H.; Mayer, P.; Bellemare, F.; Lepage, Y. Estimated prevalence of obstructive sleep apnea–hypopnea syndrome after cervical cord injury. Arch. Phys. Med. Rehabi. 2007, 88, 333–337. [Google Scholar] [CrossRef]
- Jensen, M.P.; Gould, E.; Victor, T.W.; Gammaitoni, A.R.; White, R.E.; Galer, B.S. The relationship of changes in pain quality to pain interference and sleep quality. J. Pain. 2010, 11, 782–788. [Google Scholar] [CrossRef]
- Widerström-Noga, E.; Anderson, K.D.; Perez, S.; Hunter, J.P.; Martinez-Arizala, A.; Adcock, J.P.; Escalona, M. Living with chronic pain after spinal cord injury: A mixed-methods study. Arch. Phys. Med. Rehabil. 2017, 98, 856–865. [Google Scholar] [CrossRef]
- Barat, M.; Dehail, P.; De Seze, M. Fatigue after spinal cord injury. Ann. Réadaptation Méd. Phys. 2006, 49, 365–369. [Google Scholar] [CrossRef]
- Diemen, T.; Lankveld, W.; Leeuwen, C.; Post, M.; Nes, I. Multidimensional fatigue during rehabilitation in persons with recently acquired spinal cord injury. J. Rehabil. Med. 2016, 48, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Krassioukov, A. Autonomic function following cervical spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Larsson Lund, M.; Nordlund, A.; Nygård, L.; Lexell, J.; Bernspång, B. Perceptions of participation and predictors of perceived problems with participation in persons with spinal cord injury. J. Rehabil. Med. 2005, 37, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Hancock, K.; Dickson, H. Improving the long-term adjustment of spinal cord injured persons. Spinal Cord 1999, 37, 345–350. [Google Scholar] [CrossRef][Green Version]
- Bombardier, C.H.; Rimmele, C.T. Alcohol use and readiness to change after spinal cord injury. Arch. Phys. Med. Rehabil. 1998, 79, 1110–1115. [Google Scholar] [CrossRef]
- Engel, G.L. The need for a new medical model: A challenge for biomedicine. Science 1977, 196, 129–136. [Google Scholar] [CrossRef]
- Janz, N.K.; Becker, M.H. The health belief model: A decade later. Health Educ. Q. 1984, 11, 1–47. [Google Scholar] [CrossRef]
- Sprangers, M.A.; Schwartz, C.E. Integrating response shift into health-related quality of life research: A theoretical model. Soc. Sci. Med. 1999, 48, 1507–1515. [Google Scholar] [CrossRef]
- Lazarus, R.; Folkman, S. Stress Appraisal and Coping; Springer: New York, NY, USA, 1984. [Google Scholar]
- Duff, J.; Kennedy, P. Spinal cord injury. In Handbook of Clinical Health Psychology; Llewelyn, S., Kennedy, P., Eds.; John Wiley and Son Ltd: Chichester, UK, 2003; pp. 251–278. [Google Scholar]
- Craig, A.; Tran, Y.; Siddall, P.; Wijesuriya, N.; Lovas, J.; Bartrop, R.; Middleton, J. Developing a model of associations between chronic pain, depressive mood, chronic fatigue, and self-efficacy in people with spinal cord injury. J. Pain. 2013, 14, 911–920. [Google Scholar] [CrossRef]
- MacKinnon, D.P.; Luecken, L.J. How and for whom? Mediation and moderation in health psychology. Health Psychol. 2008, 27, S99. [Google Scholar] [CrossRef]
- Van Diemen, T.; Crul, T.; van Nes, I.; Group, S.S.; Geertzen, J.H.; Post, M.W. Associations between self-efficacy and secondary health conditions in people living with spinal cord injury: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2017, 98, 2566–2577. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.W.; Tran, Y.; Lo, C.; Craig, A. Reexamining the validity and dimensionality of the Moorong self-efficacy scale: Improving its clinical utility. Arch. Phys. Med. Rehabil. 2016, 97, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Van Diemen, T.; Craig, A.; van Nes, I.J.W.; van Laake, C.; Bloemen, J.; Stolwijk-Swuste, J.M.; Scholten, E.; Faber, W.; Boerrigter, J.; Beurskens, M.; et al. Enhancing our conceptual understanding of state and trait self-efficacy by correlational analysis of four self-efficacy scales in people with spinal cord injury. BMC Psychol. 2020, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Hancock, K.; Chang, E.; Dickson, H. The effectiveness of group psychological intervention in enhancing perceptions of control following spinal cord injury. Aust. N. Z. J. Psychiatry 1998, 32, 112–118. [Google Scholar] [CrossRef]
- Peter, C.; Müller, R.; Post, M.W.; van Leeuwen, C.M.; Werner, C.S.; Geyh, S. Psychological resources, appraisals, and coping and their relationship to participation in spinal cord injury: A path analysis. Arch. Phys. Med. Rehabil. 2014, 95, 1662–1671. [Google Scholar] [CrossRef]
- Guest, R.; Craig, A.; Tran, Y.; Middleton, J. Factors predicting resilience in people with spinal cord injury during transition from inpatient rehabilitation to the community. Spinal Cord 2015, 53, 682–686. [Google Scholar] [CrossRef]
- Middleton, J.W.; Arora, M.; Kifley, A.; Clark, J.; Borg, S.J.; Tran, Y.; Atresh, S.; Kaur, J.; Shetty, S.; Nunn, A.; et al. Australian arm of the International Spinal Cord Injury (Aus-InSCI) Community Survey: 2. Understanding the lived experience in people with spinal cord injury. Spinal Cord 2022, 1–11. [Google Scholar] [CrossRef]
- Borg, S.J.; Geraghty, T.; Arora, M.; Foster, M.; Marshall, R.; Nunn, A.; Middleton, J.W. Employment outcomes following spinal cord injury: A population-based cross-sectional study in Australia. Spinal Cord 2021, 59, 1120–1131. [Google Scholar] [CrossRef]
- E Ware, J.; Kosinski, M.; Bayliss, M.S.; A McHorney, C.; Rogers, W.H.; Raczek, A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the Medical Outcomes Study. Med. Care 1995, 33, AS264–AS279. [Google Scholar]
- Schwarzer, R.; Jerusalem, M. Generalized Self-Efficacy scale. In Measures in Health Psychology: A User’s Portfolio. Causal and Control Beliefs; Weinman, J., Wright, S., Johnston, M., Eds.; NFER-Nelson: Windsor, UK, 1995; pp. 35–37. [Google Scholar]
- Geyh, S.; Fellinghauer, B.A.; Kirchberger, I.; Post, M. Cross-cultural validity of four quality of life scales in persons with spinal cord injury. Health Qual. Life Outcomes 2010, 8, 94. [Google Scholar] [CrossRef]
- Fekete, C.; Eriks-Hoogland, I.; Baumberger, M.; Catz, A.; Itzkovich, M.; Lüthi, H.; Post, M.W.; von Elm, E.; Wyss, A.; Brinkhof, M.W. Development and validation of a self-report version of the Spinal Cord Independence Measure (SCIM III). Spinal Cord 2013, 51, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Itzkovich, M.; Gelernter, I.; Biering-Sørensen, F.; Weeks, C.; Laramee, M.T.; Craven, B.; Tonack, M.; Hitzig, S.L.; Glaser, E.; Zeilig, G.; et al. The Spinal Cord Independence Measure (SCIM) version III: Reliability and validity in a multi-center international study. Disabil. Rehabil. 2007, 29, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Kalpakjian, C.Z.; Scelza, W.M.; Mpp, M.B.F.; Toussaint, L.L. Preliminary Reliability and Validity of a Spinal Cord Injury Secondary Conditions Scale. J. Spinal Cord Med. 2007, 30, 131–139. [Google Scholar] [CrossRef]
- Baron, R.M.; Kenny, D.A. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Person. Soc. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef]
- Bullock, J.G.; Green, D.P. The Failings of Conventional Mediation Analysis and a Design-Based Alternative. Adv. Methods Pr. Psychol. Sci. 2021, 4, 1–8. [Google Scholar] [CrossRef]
- Hayes, A.F. Introduction to mediation, moderation, and conditional process analysis. In A Regression-Based Approach, 3rd ed.; Guildford Press: New York, NY, USA, 2018. [Google Scholar]
- Kock, N. Common Method Bias in PLS-SEM: A Full Collinearity Assessment Approach. Int. J. e-Collab. 2015, 11, 1–10. [Google Scholar] [CrossRef]
- Ditlevsen, S.; Christensen, U.; Lynch, J.; Damsgaard, M.T.; Keiding, N. The mediation proportion: A structural equation approach for estimating the proportion of exposure effect on outcome explained by an intermediate variable. Epidemiology 2005, 16, 114–120. [Google Scholar] [CrossRef]
- Craig, A.; Hancock, K.; Dickson, H.; Chang, E. Immunizing against depression and anxiety following spinal cord injury. Arch. Phys. Med. Rehabil. 1998, 79, 375–377. [Google Scholar] [CrossRef]
- Craig, A.; Moses, P.; Tran, Y.; McIsaac, P.; Kirkup, L. The effectiveness of a hands-free environmental control system for the profoundly disabled. Arch. Phys. Med. Rehabil. 2002, 83, 1455–1458. [Google Scholar] [CrossRef]



| Characteristics | Values | |
|---|---|---|
| Age (years), mean (SD) | 57.5 (14.4) | |
| Years since injury (years), mean (SD) | 17.2 (13.9) | |
| Level of injury * | Paraplegia, n (%) | 912 (57.8) |
| Tetraplegia, n (%) | 580 (36.7) | |
| Completeness of injury ** | Complete, n (%) | 498 (31.5) |
| Incomplete, n (%) | 1042 (66.0) | |
| Sex | Male, n (%) | 1157 (73.3) |
| Female, n (%) | 422 (26.7) | |
| Mediation Pathway | Standardised Indirect Effect | S.E. | 95% CI | Mediation Proportion * |
|---|---|---|---|---|
| A | ||||
| SC > UE > PVMH | 0.022 | 0.006 | 0.011, 0.035 | 0.23 |
| SC > MGH > PVMH | 0.033 | 0.008 | 0.018, 0.051 | 0.36 |
| SC > QoL > PVMH | 0.029 | 0.013 | 0.004, 0.055 | 0.31 |
| SC > UE > QoL > PVMH | 0.017 | 0.004 | 0.010, 0.025 | 0.18 |
| SC > MGH > QoL > PVMH | 0.022 | 0.005 | 0.012, 0.033 | 0.23 |
| B | ||||
| SC > UE > NVMH | −0.027 | 0.007 | −0.04, −0.01 | 0.33 |
| SC > MGH > NVMH | −0.018 | 0.006 | −0.03, −0.008 | 0.22 |
| SC > QoL > NVMH | −0.027 | 0.011 | −0.05, −0.005 | 0.33 |
| SC > UE > QoL > NVMH | −0.014 | 0.004 | −0.02, −0.008 | 0.18 |
| SC > MGH > QoL > NVMH | −0.019 | 0.005 | −0.03, −0.010 | 0.22 |
| Mediation Pathway | Standardised Indirect Effect | S.E. | 95% CI | Mediation Proportion * |
|---|---|---|---|---|
| A | ||||
| SC > UE > PVMH | −0.041 | 0.009 | −0.06, −0.02 | 0.09 |
| SC > MGH > PVMH | −0.088 | 0.013 | −0.11, −0.06 | 0.19 |
| SC > QoL > PVMH | −0.108 | 0.014 | −0.14, 0.08 | 0.24 |
| SC > UE > QoL > PVMH | −0.023 | 0.004 | −0.03, −0.02 | 0.05 |
| SC > MGH > QoL > PVMH | −0.044 | 0.006 | −0.06, −0.03 | 0.10 |
| B | ||||
| SC > UE > NVMH | 0.054 | 0.010 | 0.036, 0.074 | 0.11 |
| SC > MGH > NVMH | 0.037 | 0.012 | 0.013, 0.062 | 0.08 |
| SC > QoL > NVMH | 0.090 | 0.013 | 0.066, 0.116 | 0.19 |
| SC > UE > QoL > NVMH | 0.019 | 0.004 | 0.012, 0.026 | 0.04 |
| SC > MGH > QoL > NVMH | 0.036 | 0.006 | 0.025, 0.048 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craig, A.; Tran, Y.; Arora, M.; Pozzato, I.; Middleton, J.W. Investigating Dynamics of the Spinal Cord Injury Adjustment Model: Mediation Model Analysis. J. Clin. Med. 2022, 11, 4557. https://doi.org/10.3390/jcm11154557
Craig A, Tran Y, Arora M, Pozzato I, Middleton JW. Investigating Dynamics of the Spinal Cord Injury Adjustment Model: Mediation Model Analysis. Journal of Clinical Medicine. 2022; 11(15):4557. https://doi.org/10.3390/jcm11154557
Chicago/Turabian StyleCraig, Ashley, Yvonne Tran, Mohit Arora, Ilaria Pozzato, and James W. Middleton. 2022. "Investigating Dynamics of the Spinal Cord Injury Adjustment Model: Mediation Model Analysis" Journal of Clinical Medicine 11, no. 15: 4557. https://doi.org/10.3390/jcm11154557
APA StyleCraig, A., Tran, Y., Arora, M., Pozzato, I., & Middleton, J. W. (2022). Investigating Dynamics of the Spinal Cord Injury Adjustment Model: Mediation Model Analysis. Journal of Clinical Medicine, 11(15), 4557. https://doi.org/10.3390/jcm11154557







