Traffic Noise and Ambient Air Pollution Are Risk Factorsfor Preeclampsia
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Exposure Assessment
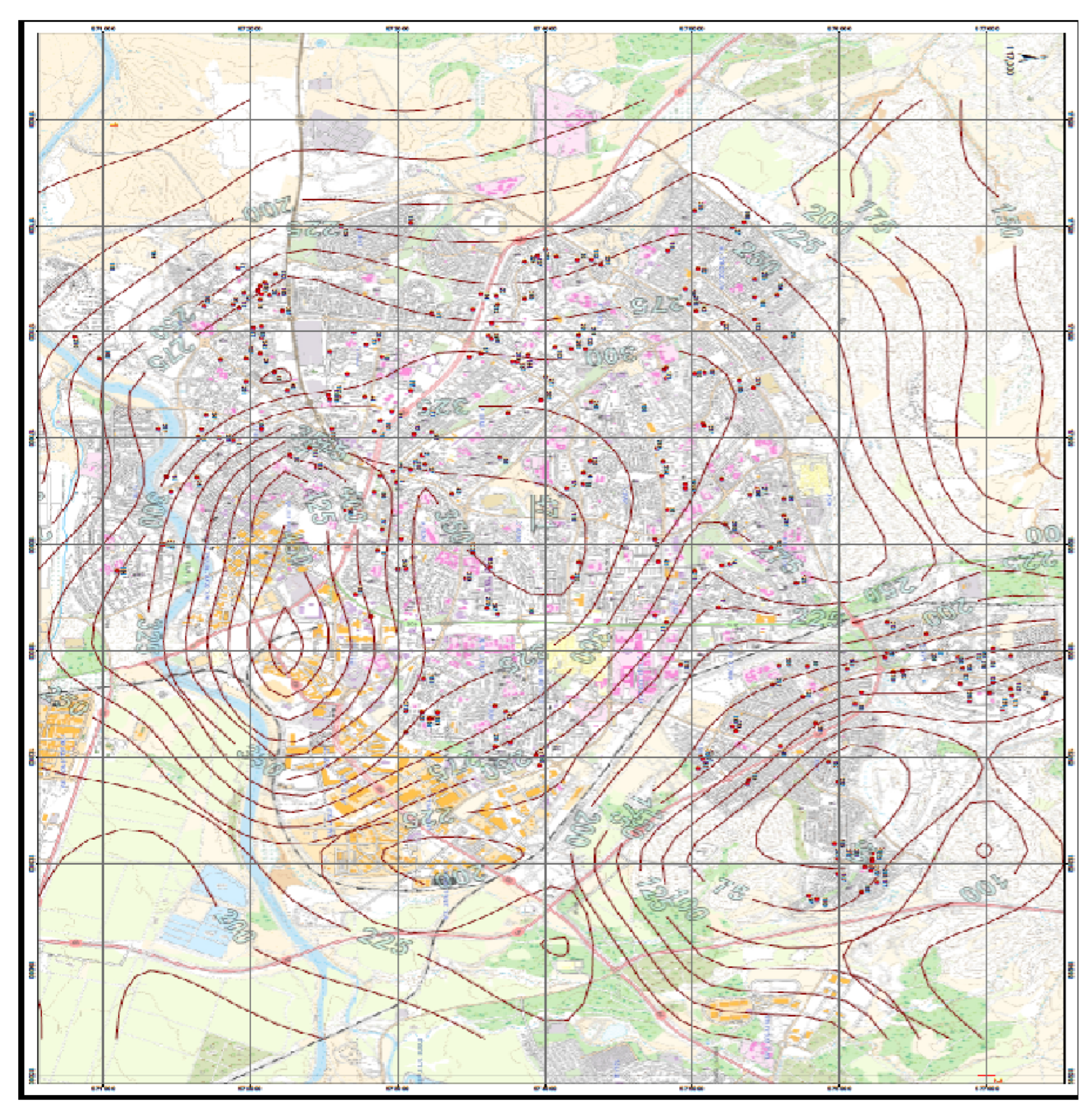
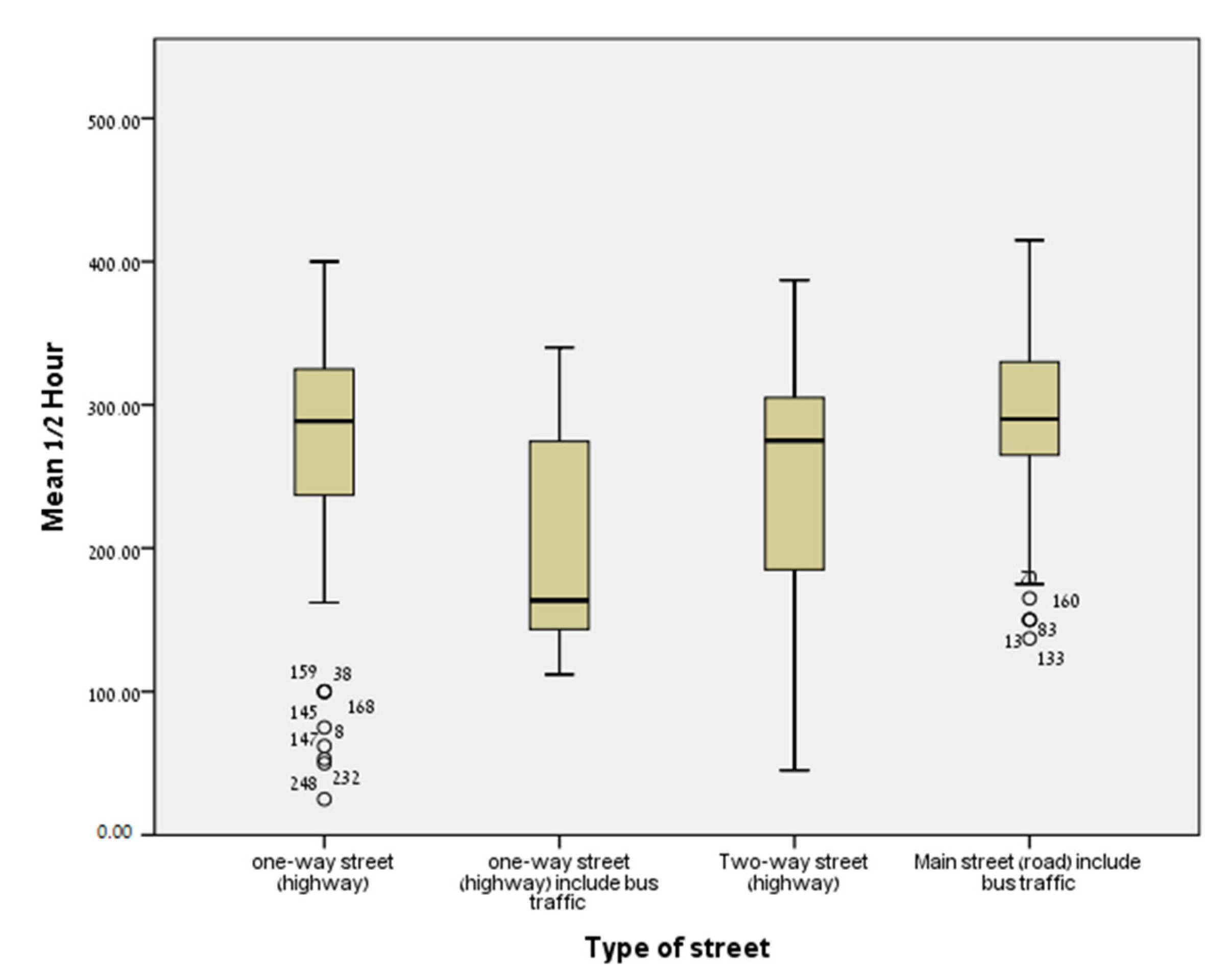
2.2.1. Noise Exposure
2.2.2. Traffic-Related Air Pollution (TRAP) Level
2.3. Outcome
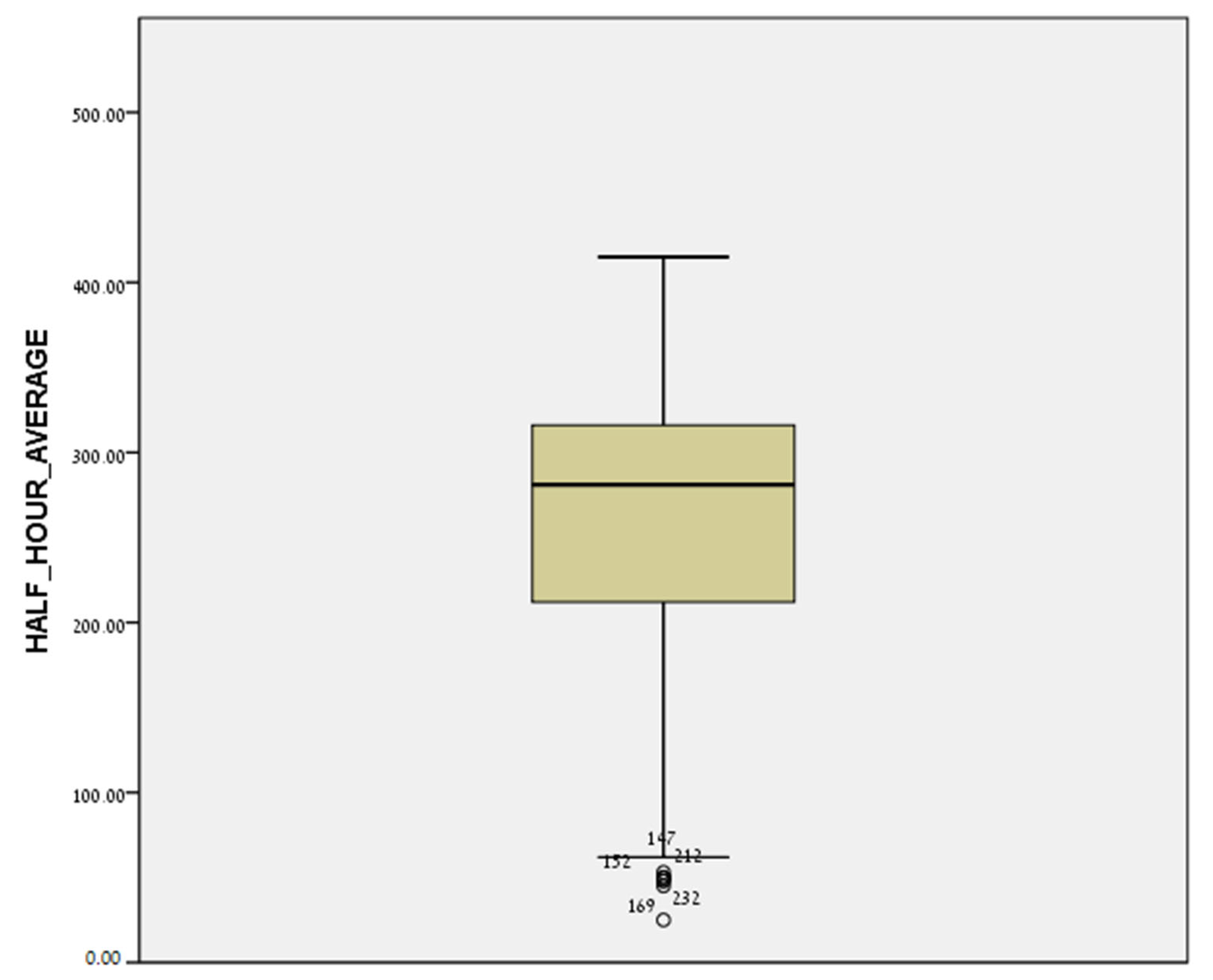
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wainstock, T.; Yoles, I.; Sergienko, R.; Kloog, I.; Sheiner, E. Prenatal particulate matter exposure and Intrauterine Fetal Death. Int. J. Hyg. Environ. Health 2021, 234, 113720. [Google Scholar] [CrossRef] [PubMed]
- Riediker, M.; Koren, H.S. The importance of environmental exposures to physical, mental and social well-being. Int. J. Hyg. Environ. Health 2004, 207, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, B.; Belojevic, G.; Paunovic, K.; Stojanov, V. Road traffic noise and sleep disturbances in an urban population: Cross-sectional study. Croat. Med. J. 2006, 47, 125–133. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2080382/ (accessed on 21 June 2022). [PubMed]
- Hume, K.I.; Brink, M.; Basner, M. Effects of environmental noise on sleep. Noise Health 2012, 14, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Babisch, W. Updated exposure-response relationship between road traffic noise and coronary heart diseases: A meta-analysis. Noise Health 2014, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barregard, L.; Blonde, E.; Ohrstrom, E. Risk of hypertension from exposure to road traffic noise in a population-based sample. Occup. Environ. Med. 2009, 66, 410–415. [Google Scholar] [CrossRef]
- Seidler, A.L.; Hegewald, J.; Schubert, M.; Weihofen, V.M.; Wagner, M.; Droge, P.; Swart, E.; Zeeb, H.; Seidler, A. The effect of aircraft, road, and railway traffic noise on stroke results of a case-control study based on secondary data. Noise Health 2018, 20, 152–161. [Google Scholar] [CrossRef]
- Dzhambov, A.M.; Dimitrova, D.D. Residential road traffic noise as a risk factor for hypertension in adults: Systematic review and meta-analysis of analytic studies published in the period 2011–2017. Environ. Pollut. 2018, 240, 306–318. [Google Scholar] [CrossRef]
- Bendokiene, I.; Grazuleviciene, R.; Dedele, A. Risk of hypertension related to road traffic noise among reproductive-age women. Noise Health 2011, 13, 371–377. [Google Scholar] [CrossRef]
- Fuks, K.; Moebus, S.; Hertel, S.; Vihaan, A.; Nonnemacher, M.; Dragano, S.; Mohlenkamp, H.; Jakobs, C.; Kessler, R.; Erbel, B.; et al. Long-term urban particulate air pollution, traffic noise and arterial blood pressure. Environ. Health Prespect. 2011, 119, 1706–1711. [Google Scholar] [CrossRef] [Green Version]
- Pitchika, A.; Hampel, R.; Wolf, K.; Kraus, U.; Cyrys, J.; Babisch, W.; Peters, A.; Schneider, A. Long-term associations of modeled and self-reported measures of exposure to air pollution and noise at residence on prevalent hypertension and blood pressure. Sci. Total Environ. 2017, 593–594, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.Y.; Wang, D.; Zou, X.P.; Yang, Z.H.; Li, T.C.; Chen, Y.Q. Does ambient air pollutants increase the risk of fetal loss? A case-control study. Arch. Gynecol. Obstet. 2014, 289, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Rudra, C.B.; Williams, M.A.; Sheppard, L.; Koenig, J.Q.; Chiff, M.A. Ambient carbon monoxide and fine particulate matter in relation to preeclampsia and preterm delivery in Westerm Washington State. Environ. Health Perspect. 2011, 118, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.G.; Braun, J.M.; Ryan, P.H.; Xu, Y.; Werner, E.F.; Lanphear, B.P.; Wellenius, G.A. The association of traffic-related air and noise pollution with maternal blood pressure and hypertensive disorders of pregnancy in the HOME study cohort. Environ. Int. 2018, 121, 574–581. [Google Scholar] [CrossRef]
- Environmental Protection Department, Scott Wilson Ltd. Additional Work for a Comparative Study of Noise Levels in Hong Kong; Environmental Protection Department, Scott Wilson Ltd.: Hong Kong, China, 2007. [Google Scholar]
- Allen, R.W.; Davies, H.; Cohen, M.A.; Mallach, G.; Kaufman, J.; Adar, S.D. The spatial relationship between traffic-generated air pollution and noise in 2 US cities. Environ. Res. 2009, 109, 334–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Environmental Protection Agency. Revision to guideline on air quality models: Adoption of a preferred general purpose (flat and complex terrain) dispersion model and other revisions; fine rule. Fed. Regist. 2005, 70, 68218–68261. [Google Scholar]
- Jeyabalan, A. Epidemiology of preeclampsia: Impact of obesity. Nutr. Rev. 2013, 71 (Suppl. 1), S18–S25. [Google Scholar] [CrossRef] [Green Version]
- Auger, N.; Duplaix, M.; Bilodeau-Bertrand, M.; Lo, E.; Smargiassi, A. Environmental noise pollution and risk of preeclampsia. Environ. Pollut. 2018, 239, 599–606. [Google Scholar] [CrossRef]
- Jonson, J.E.; Broken-Kleefeld, J.; Simpson, D.; Nyiri, A.; Posch, M.; Heyes, C. Impact of excess NOx emissions from diesel cars on air quality, public health and eutrophication in Europe. Environ. Res. Lett. 2017, 12, 094017. [Google Scholar] [CrossRef] [Green Version]
- Okby, R.; Harlev, A.; Sacks, K.N.; Sergienko, R.; Sheiner, E. Preeclampsia acts differently in in vitro fertilization versus spontaneous twins. Arch. Gynecol. Obstet. 2018, 297, 653–658. [Google Scholar] [CrossRef]
- Kessous, R.; Shoham-Vardi, I.; Pariente, G.; Sergienko, R.; Sheiner, E. Long-term maternal atherosclerotic morbidity in women with pre-eclampsia. Heart 2015, 101, 442–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shental, O.; Friger, M.; Sheiner, E. Ethnic differences in the monthly variation of preeclampsia among Bedouin and Jewish parturient in the Negev. Hypertens. Pregnancy 2010, 29, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Katz, O.; Paz-Tal, O.; Lazer, T.; Aricha-Tamir, B.; Mazor, M.; Wiznitzer, A.; Sheiner, E. Severe pre-eclampsia is associated with abnormal trace elements concentrations in maternal and fetal blood. J. Matern. Fetal. Neonatal. Med. 2012, 25, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lio, Y.; Huang, X.; Rong, Y.; He, M.; Wang, Y.; Yuan, J.; Wu, T.; Chen, W. The effect of shift work on sleeping quality, hypertension and diabetes in retired workers. PLoS ONE 2013, 8, e71107. [Google Scholar] [CrossRef] [Green Version]
- Jakovljevic, B.; Belojevic, G.; Paunovic, K. Acoustical factors influencing noise annoyance of urban population. In Proceedings of the 9th International Congress on noise (ICBEN), Foxwoods, CT, USA, 21–28 July 2008. [Google Scholar]
- Vodonos, A.; Friger, M.; Katra, I.; Avnon, L.; Krasnov, H.; Koutrakis, P.; Schwartz, J.; Novack, V. The impact of desert dust exposures on hospitalizations due to exacerbation of chronic obstructive pulmonary disease. Air Qual. Atmos. Health 2014, 7, 433–439. [Google Scholar] [CrossRef]
- Davies, H.W.; Vlaanderen, J.J.; Henderson, S.B.; Brauer, M. Correlation between co-exposures to noise and air pollution from traffic sources. Occup. Environ. Med. 2009, 66, 347–350. [Google Scholar] [CrossRef]
- Fernández-Somoano, A.; Llop, S.; Aguilera, I.; Tamayo-Uria, I.; Martínez, M.D.; Foraster, M.; Ballester, F.; Tardón, A. Annoyance cause by noise and air pollution during pregnancy: Associated factors and correlation with outdoor NO2 and benzene estimations. Int. J. Environ. Res. Public Health 2015, 12, 7044–7058. [Google Scholar] [CrossRef] [Green Version]
- Savitz, D.A.; Elston, B.; Bobb, J.F.; Clougherty, J.E.; Dominicj, F.; Ito, K.; Johnson, S.; McAlexander, T.; Ross, Z.; Shmool, J.; et al. Ambient fine particulate matter, nitrogen dioxide, and hypertensive disorders of pregnancy in New York City. Epidemiology 2015, 26, 748–757. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, M.; Halldorsson, T.I.; Olsen, S.F.; Hjortebjerg, D.; Ketzel, M.; Grandstrom, C.; Raaschou-Nielsen, O.; Sorensen, M. Impact of road traffic pollution on pre-eclampsia and pregnancy-induced hypertensive disorders. Epidemiology 2017, 28, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Ndrepepa, A.; Twardella, D. Relationship between noise annoyance from road traffic noise and cardiovascular diseases: A meta-analysis. Noise Health 2011, 13, 251–259. [Google Scholar] [CrossRef]
- Ristovska, G.; Laszlo, H.E.; Hansell, A.L. Reproductive outcomes associated with noise exposure—A systematic review of the literature. Int. J. Environ. Res. Public Health 2014, 11, 7931–7952. [Google Scholar] [CrossRef] [PubMed]
- Nepomnaschy, P.A.; Sheiner, E.; Mastorakos, G.; Arck, P.C. Stress, immune function, and women’s reproduction. Ann. N. Y. Acad. Sci. 2007, 1113, 350–364. [Google Scholar] [CrossRef]
- Cwikel, J.; Gidron, Y.; Sheiner, E. Psychological interactions with infertility among women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 117, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Babisch, W. Stress hormones in the research on cardiovascular effects of noise. Noise Health 2003, 5, 1–11. [Google Scholar] [PubMed]
- Rasmussen, S.; Glickman, G.; Norinsky, R.; Quimby, F.W.; Tolwani, R.J. Construction noise decreases reproductive efficiency in mice. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 363–370. [Google Scholar]
- Wu, J.; Ren, C.; Delfino, R.J.; Chung, J.; Wilhelm, M.; Ritz, B. Association between local traffic- generated air pollution and preeclampsia and preterm delivery in the south coast air basin of California. Environ. Health Perspect. 2009, 117, 1773–1779. [Google Scholar] [CrossRef]
- Pedersen, M.; Stayner, L.; Slime, R.; Sorensen, M.; Figueras, F.; Nieuwenhuijsen, M.J.; Raaschou-Nielsen, O.; Dadvand, P. Ambiet air pollution and pregnancy-induced hypertensive disorders: A systematic review and meta-analysis. Hypertension 2014, 64, 494–500. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Preeclampsia | p | |
|---|---|---|---|
| Yes (n = 24) | No (n = 229) | ||
| n (%) or Mean ± SD | |||
| Maternal age, y (mean ± SD) | 31.3 ± 5.7 | 31.3 ± 5.0 | 0.992 1 |
| BMI before pregnancy | 25.8 ± 5.7 | 23.8 ± 4.7 | 0.057 1 |
| Number of birth | 1.8 ± 0.8 | 2.8 ± 1.8 | 0.007 2 |
| Education (years) | 14.5 ± 2.5 | 14.7 ± 2.7 | 0.525 1 |
| Pregnancy week | 33.5 ± 3.7 | 31.0 ± 3.1 | 0.249 1 |
| Fetal sex | 0.159 3 | ||
| Male | 15 (10.8) | 124 (89.2) | |
| Female | 9 (6.2) | 137 (93.8) | |
| Chronic Illness *, yes | 6 (33.3) | 17 (7.4) | <0.001 3 |
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Joint air pollution and noise exposure (Exposed to both noise and air pollution vs. non-exposed) | 4.11 | 1.31; 12.94 | 0.016 |
| Age, years | 1.06 | 0.95; 1.16 | 0.363 |
| BMI before pregnancy | 1.08 | 0.97; 1.19 | 0.169 |
| Number of births | 0.48 | 0.28; 0.83 | 0.008 |
| Fetal sex (male vs. female) | 1.98 | 0.69; 5.70 | 0.208 |
| Chronic illness (yes vs. no) | 8.27 | 1.93; 23.01 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilenko, N.; Ashin, M.; Friger, M.; Fischer, L.; Sergienko, R.; Sheiner, E. Traffic Noise and Ambient Air Pollution Are Risk Factorsfor Preeclampsia. J. Clin. Med. 2022, 11, 4552. https://doi.org/10.3390/jcm11154552
Bilenko N, Ashin M, Friger M, Fischer L, Sergienko R, Sheiner E. Traffic Noise and Ambient Air Pollution Are Risk Factorsfor Preeclampsia. Journal of Clinical Medicine. 2022; 11(15):4552. https://doi.org/10.3390/jcm11154552
Chicago/Turabian StyleBilenko, Natalya, Michal Ashin, Michael Friger, Laura Fischer, Ruslan Sergienko, and Eyal Sheiner. 2022. "Traffic Noise and Ambient Air Pollution Are Risk Factorsfor Preeclampsia" Journal of Clinical Medicine 11, no. 15: 4552. https://doi.org/10.3390/jcm11154552
APA StyleBilenko, N., Ashin, M., Friger, M., Fischer, L., Sergienko, R., & Sheiner, E. (2022). Traffic Noise and Ambient Air Pollution Are Risk Factorsfor Preeclampsia. Journal of Clinical Medicine, 11(15), 4552. https://doi.org/10.3390/jcm11154552









