What Is Currently Known about Intramedullary Spinal Cord Abscess among Children? A Concise Review
Abstract
1. Introduction
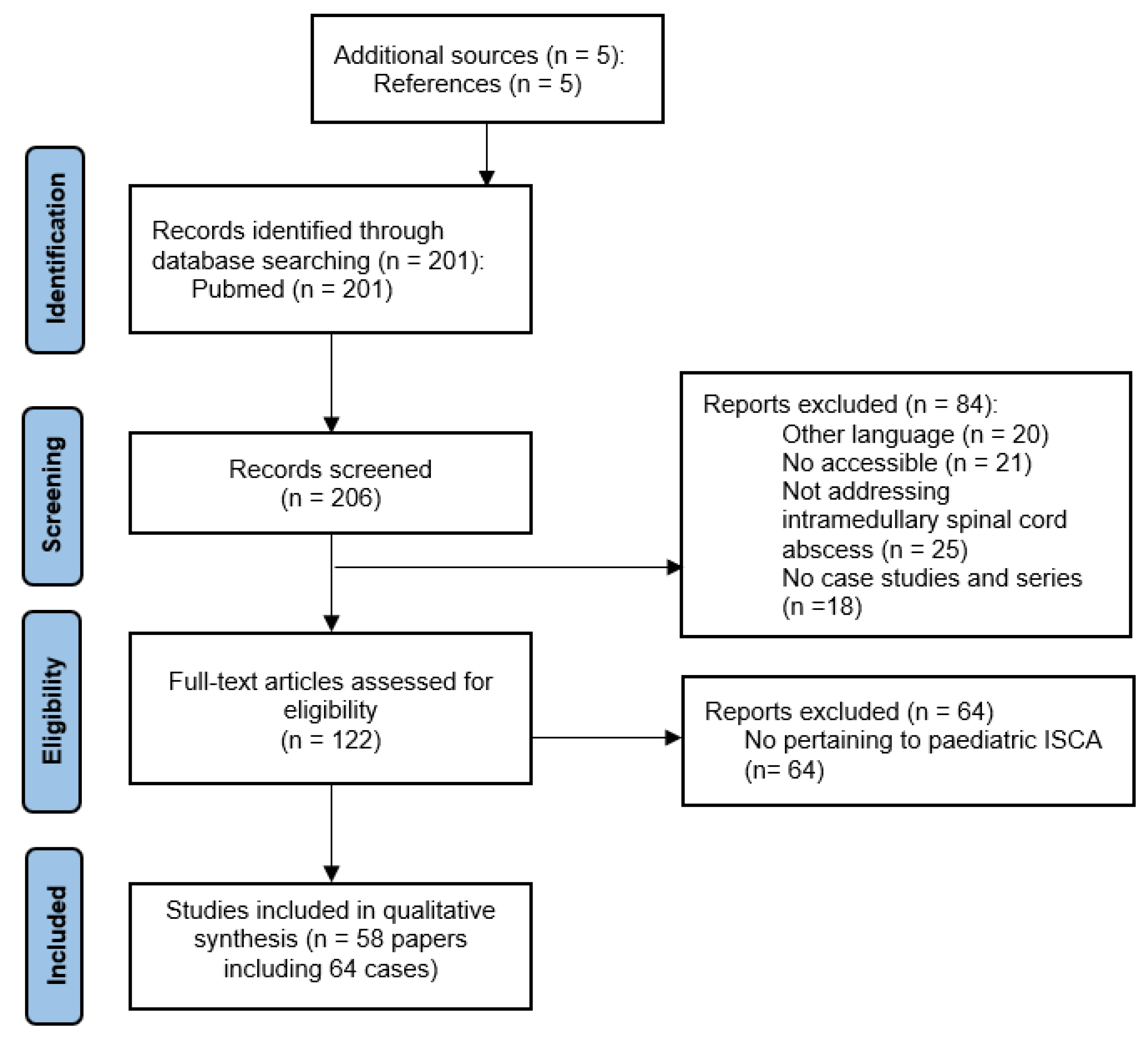
2. Literature Search
3. What Is Currently Known about Intramedullary Spinal Cord Abscesses in Children?
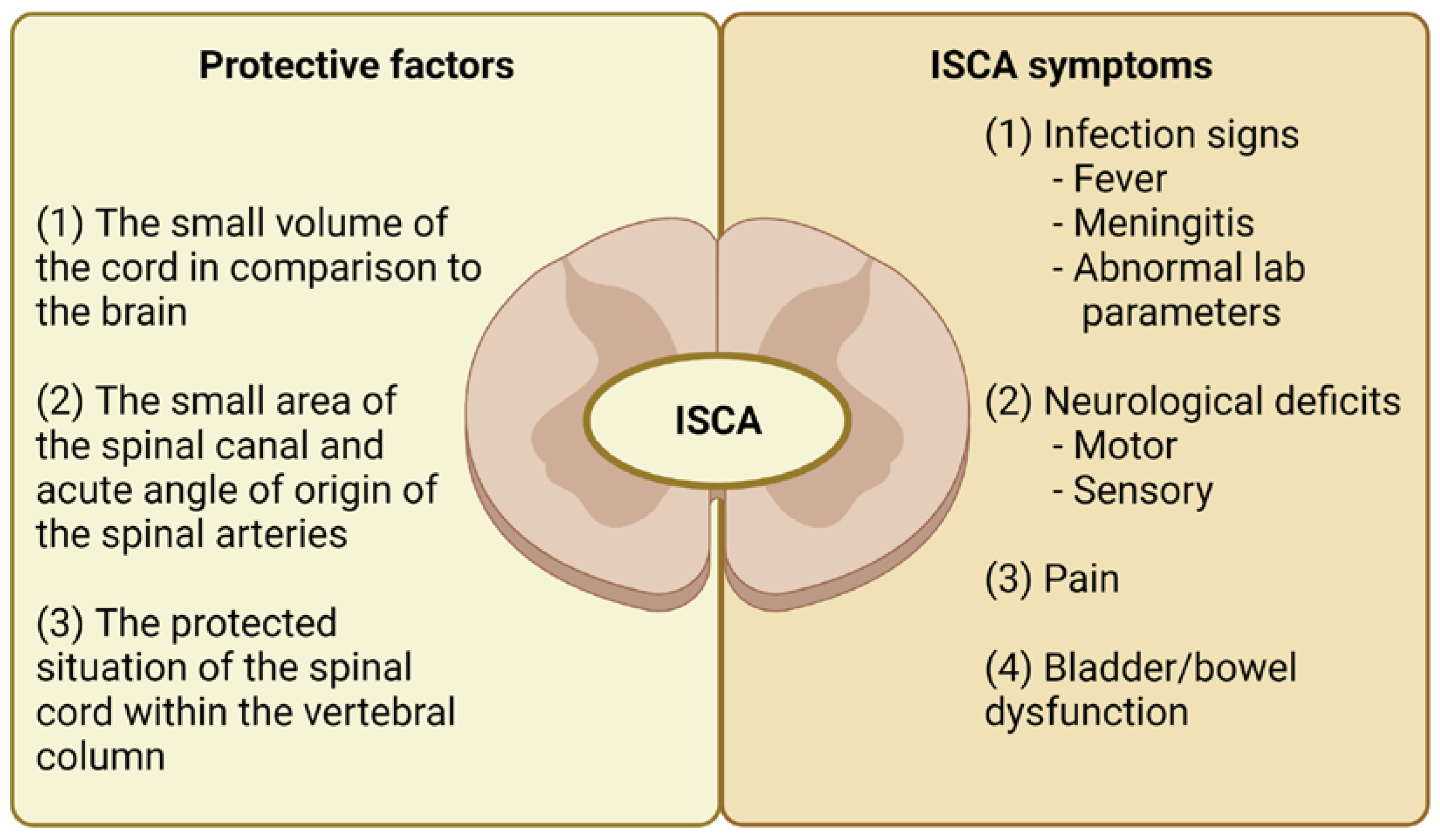
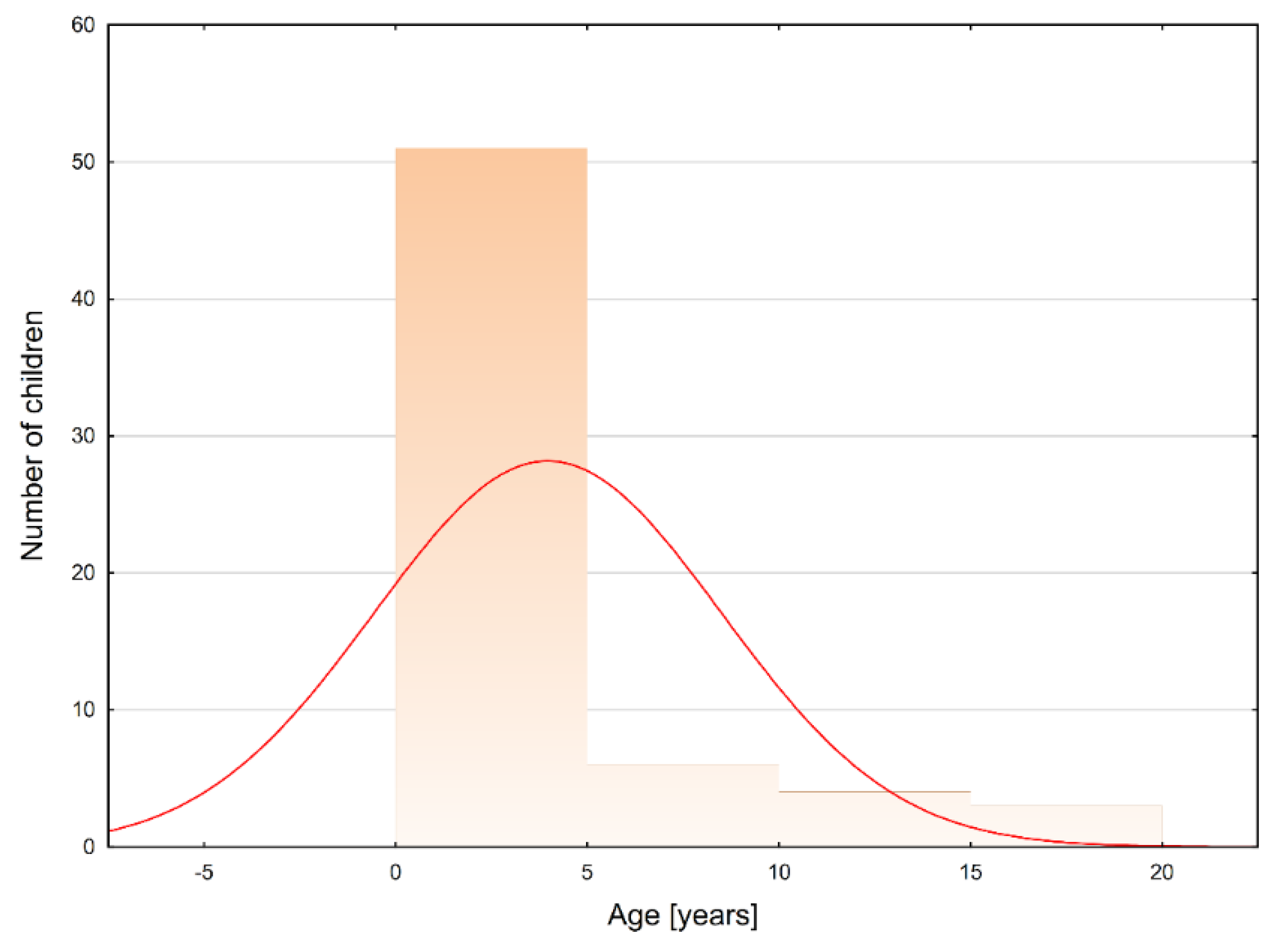
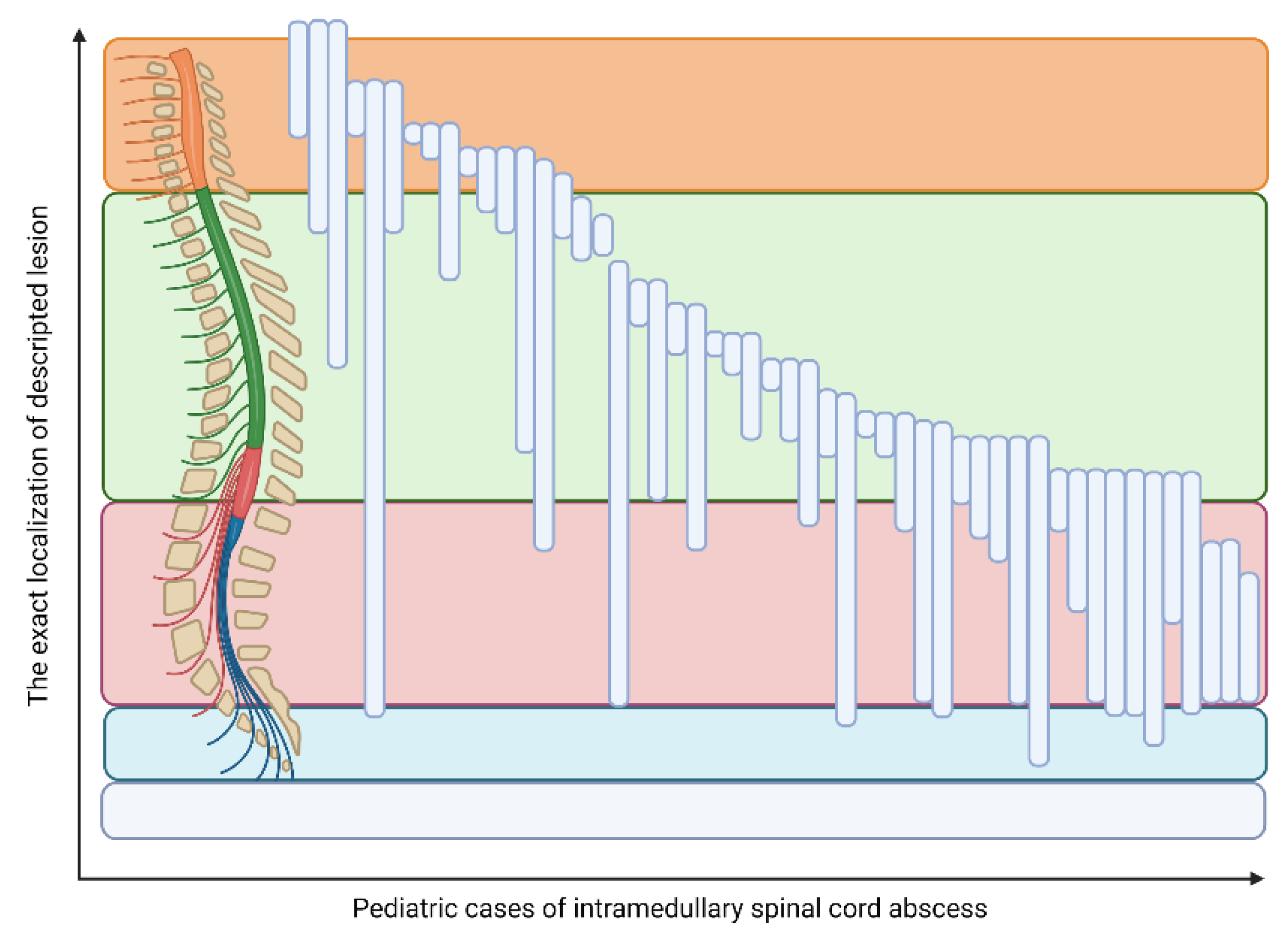
3.1. ISCA Course and Localization
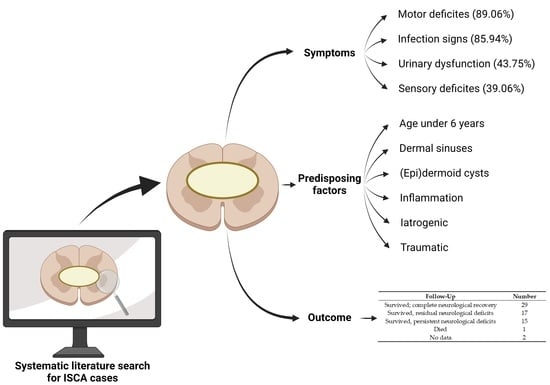
3.2. Symptoms Present in ISCA Patients
3.3. Predisposing Factors and Comorbidities
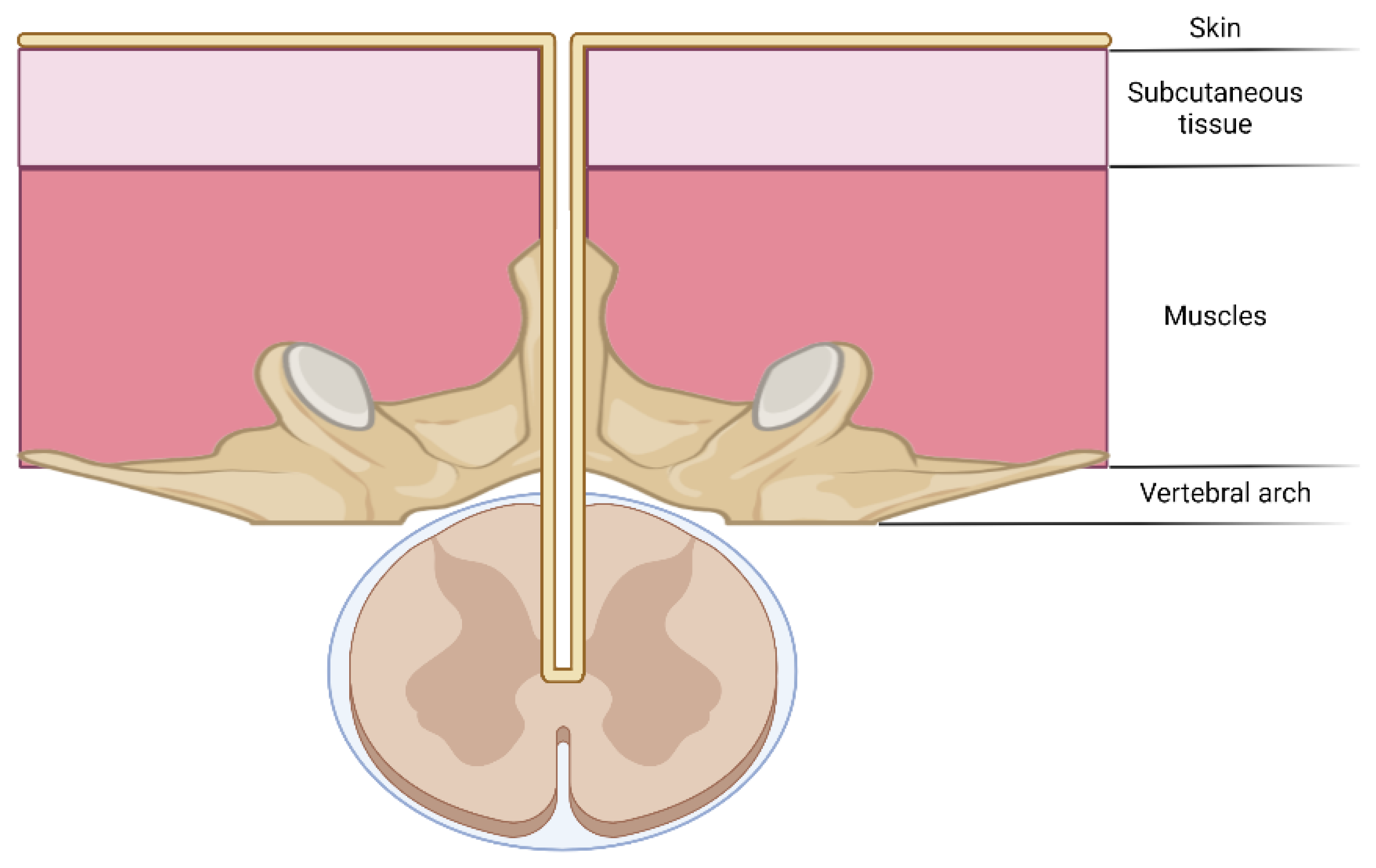
3.3.1. Dermal Sinus Tracts
3.3.2. (Epi)dermoid Cyst
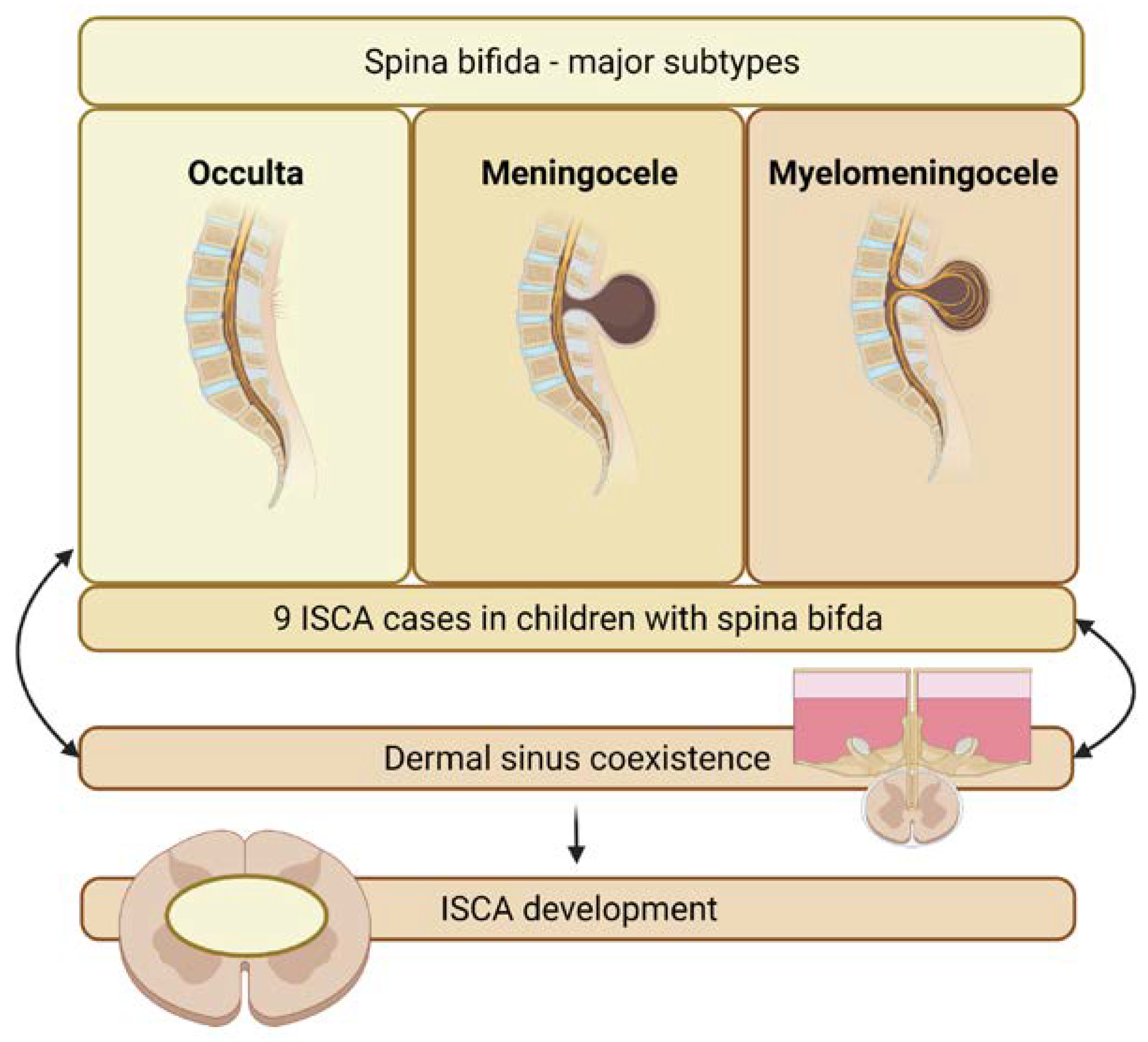
3.3.3. Spina Bifida
3.3.4. Prior Inflammation
3.3.5. Others
3.4. Available Treatments
3.5. Pathogens
3.6. Follow-Up
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hood, B.; Wolfe, S.Q.; Trivedi, R.A.; Rajadhyaksha, C.; Green, B. Intramedullary Abscess of the Cervical Spinal Cord in an Otherwise Healthy Man. World Neurosurg. 2011, 76, 361.e15–361.e19. [Google Scholar] [CrossRef] [PubMed]
- Hart, J. Case of Encysted Abscess in the Spinal Cord. Med.-Chir. Rev. 1831, 14, 284–286. [Google Scholar] [CrossRef]
- Foley, J. Intramedullary abscess of the spinal cord. Lancet 1949, 254, 193–195. [Google Scholar] [CrossRef]
- Chan, C.T.; Gold, W.L. Intramedullary Abscess of the Spinal Cord in the Antibiotic Era: Clinical Features, Microbial Etiologies, Trends in Pathogenesis, and Outcomes. Clin. Infect. Dis. 1998, 27, 619–626. [Google Scholar] [CrossRef] [PubMed]
- DiTullio, M.V.J. Intramedullary spinal abscess: A case report with a review of 53 previously described cases. Surg. Neurol. 1977, 7, 351–354. [Google Scholar] [PubMed]
- Bean, J.R.; Walsh, J.W.; Blacker, M.H. Cervical Dermal Sinus and Intramedullary Spinal Cord Abscess: Case report. Neurosurgery 1979, 5, 60–62. [Google Scholar] [CrossRef]
- Tewari, M.K.; Devi, B.I.; Thakur, R.C.; Pathak, A.; Khandelwal, N.; Kak, V.K. Intramedullary spinal cord abscess: A case report. Child’s Nerv. Syst. 1992, 8, 290–291. [Google Scholar] [CrossRef]
- Benzil, D.L.; Epstein, M.H.; Knuckey, N.W. Intramedullary Epidermoid Associated with an Intramedullary Spinal Abscess Secondary to a Dermal Sinus. Neurosurgery 1992, 30, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.; Bozzao, L.; Frasconi, F. Chronic intramedullary abscess of the spinal cord. J. Neurosurg. 1970, 33, 352–355. [Google Scholar] [CrossRef]
- Gindi, S.E.; Fairburn, B. Intramedullary Spinal Abscess as a Complication of a Congenital Dermal Sinus. J. Neurosurg. 1969, 30, 494–497. [Google Scholar] [CrossRef]
- Bartels, R.H.; Gonera, E.G.; Van Der Spek, J.A.N.; Thijssen, H.O.M.; Mullaart, R.A.; Gabreels, F.J.M. Intramedullary Spinal Cord Abscess—A Case Report. Spine 1995, 20, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Çokça, F.; Meço, O.; Arasil, E.; Ünlü, A. An intramedullary dermoid cyst abscess due to Brucella abortus biotype 3 at T11-L2 spinal levels. Infection 1994, 22, 359–360. [Google Scholar] [CrossRef]
- Rogg, J.M.; Benzil, D.L.; Haas, R.L.; Knuckey, N.W. Intramedullary abscess, an unusual manifestation of a dermal sinus. AJNR Am. J. Neuroradiol. 1993, 14, 1393–1395. [Google Scholar] [PubMed]
- Hanci, M.; Sarioglu, A.C.; Uzan, M.; Işlak, C.; Kaynar, M.Y.; Oz, B. Intramedullary Tuberculous Abscess: A case report. Spine 1996, 21, 766–769. [Google Scholar] [CrossRef]
- Dev, R.; Husain, M.; Gupta, A.; Gupta, R.K. MR of multiple intraspinal abscesses associated with congenital dermal sinus. AJNR Am. J. Neuroradiol. 1997, 18, 742–743. [Google Scholar]
- Morandi, X.; Mercier, P.; Fournier, H.-D.; Brassier, G. Dermal sinus and intramedullary spinal cord abscess. Child’s Nerv. Syst. 1999, 15, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Bavdekar, S.B.; Rao, N.; Kamat, J.R. Intramedullary spinal cord abscess. Indian J. Pediatr. 1997, 64, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, B.; Balasubramaniam, V. Intramedullary Abscess of the Spinal Cord. Pediatr. Neurosurg. 2001, 34, 43–44. [Google Scholar] [CrossRef]
- Helvaci, M.; Kasirga, E.; Cetin, N.; Yaprak, I. Intramedullary spinal cord abscess suspected of Brucella infection. Pediatr. Int. 2002, 44, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Tsurubuchi, T.; Matsumura, A.; Nakai, K.; Fujita, K.; Enomoto, T.; Iwasaki, N.; Nose, T. Reversible holocord edema associated with intramedullary spinal abscess secondary to an infected dermoid cyst. Pediatr. Neurosurg. 2002, 37, 282–286. [Google Scholar] [CrossRef]
- Simon, J.K.; Lazareff, J.A.; Diament, M.J.; Kennedy, W.A. Intramedullary abscess of the spinal cord in children: A case report and review of the literature. Pediatr. Infect. Dis. J. 2003, 22, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, T.; Kizilkilic, O.; Hanci, M.; Kaynar, M.Y.; Ünalan, H.; Oz, B. Atypical intradural spinal tuberculosis: Report of three cases. Spinal Cord 2003, 41, 403–409. [Google Scholar] [CrossRef]
- Morimoto, K.; Takemoto, O.; Nakamura, H.; Takeuchi, M. Spinal Dermal Sinus Associated with Intramedullary Abscess and Dermoid. Pediatr. Neurosurg. 2003, 39, 225–226. [Google Scholar] [CrossRef]
- Dutton, J.E.M.; Alexander, G.L. Intramedullary spinal abscess. J. Neurol. Neurosurg. Psychiatry 1954, 17, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Guzel, N.; Eras, M.; Guzel, D.K. A child with spinal intramedullary abscess. Child’s Nerv. Syst. 2003, 19, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Betty, M.; Lorber, J. Intramedullary abscess of the spinal cord. J. Neurol. Neurosurg. Psychiatry 1963, 26, 236–240. [Google Scholar] [CrossRef]
- Yuceer, N.; Senoglu, M.; Arda, M.N. Intramedullary spinal cord abscess in a 4-year old child. Acta Neurochir. 2004, 146, 1273–1274. [Google Scholar] [CrossRef]
- Bunyaratavej, K.; Desudchit, T.; Pongpunlert, W. Holocord intramedullary abscess due to dermal sinus in a 2-month-old child successfully treated with limited myelotomy and aspiration. J. Neurosurg. Pediatr. 2006, 104, 269–274. [Google Scholar] [CrossRef]
- Kalia, V.; Vibhuti; Aggarwal, T. Holocord intramedullary abscess. Indian J. Pediatr. 2007, 74, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Kamgarpour, A.; Izadfar, M.-A.; Razmkon, A. Neglected intramedullary cord abscess in a 3-year old child: A case report. Child’s Nerv. Syst. 2008, 24, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.-C.; Wang, H.-S.; Wu, C.-T.; Lui, T.-N.; Wong, A.M.-C. Spinal Intramedullary Abscess with an Epidermoid Secondary to a Dermal Sinus. Pediatr. Neurol. 2007, 37, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, R.; Zimmermann, M.; Hermann, E.; Kieslich, M.; Weidauer, S.; Seifert, V. Large intramedullary abscess of the spinal cord associated with an epidermoid cyst without dermal sinus. Case report. J. Neurosurg. Spine 2007, 7, 357–361. [Google Scholar] [CrossRef]
- Du Plessis, J.; Andronikou, S.; Theron, S.; Wieselthaler, N.; Hayes, M. Unusual forms of spinal tuberculosis. Child’s Nerv. Syst. 2008, 24, 453–457. [Google Scholar] [CrossRef]
- Baradaran, N.; Ahmadi, H.; Nejat, F.; Khashab, M.; Mahdavi, A.; Rahbarimanesh, A.A. Recurrent meningitis caused by cervico-medullary abscess, a rare presentation. Child’s Nerv. Syst. 2008, 24, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Tufan, K.; Cekinmez, M.; Sener, L.; Erdogan, B. Infected Lumbar Dermoid Cyst Presenting with Tetraparesis Secondary to Holocord Central Lesion. J. Child Neurol. 2008, 23, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Al Barbarawi, M.; Khriesat, W.; Qudsieh, S.; Qudsieh, H.; Loai, A.A. Management of intramedullary spinal cord abscess: Experience with four cases, pathophysiology and outcomes. Eur. Spine J. 2009, 18, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Houx, L.; Brochard, S.; Peudenier, S.; Hieu, P.D.; Rémy-Néris, O. Recovery after Tetraplegia Caused by Dermal Sinus Infection: Intramedullary Abscess and Tetraparesis. Pediatr. Neurol. 2011, 44, 229–232. [Google Scholar] [CrossRef]
- Mohindra, S.; Sodhi, H.S.; Aggarwal, A. Management problems of intramedullary holocord abscess: An illustration in a pediatric case. Child’s Nerv. Syst. 2011, 28, 637–640. [Google Scholar] [CrossRef]
- Malik, N.; Behari, S.; Ansari, M.S.; Jaiswal, A.K.; Gupta, P.; Jain, M. An Intramedullary Tuberculous Abscess of the Conus in a 5-Year-Old Child Presenting with Urinary Dysfunction. World Neurosurg. 2011, 76, 592.e15–592.e18. [Google Scholar] [CrossRef]
- Aggarwal, M.; Aggarwal, K.C.; Karamchand; Aggarwal, A. Intramedullary spinal cord abscess masquerading as spinal tumor. Indian Pediatr. 2011, 48, 973–974. [Google Scholar] [PubMed]
- Khalid, M.; Khalid, S.; Mittal, S.; Ahmad, U. Intramedullary tubercular abscess with syrinx formation. J. Pediatr. Neurosci. 2012, 7, 61–63. [Google Scholar] [CrossRef]
- Chopra, A.; Patra, B.; Aneja, S.; Mukherjee, S.; Maheswari, A.; Seth, A. Spinal Congenital Dermal Sinus Presenting as a Diagnostic Conundrum. Pediatr. Neurosurg. 2012, 48, 187–190. [Google Scholar] [CrossRef]
- Bukhari, E.E.; Alotibi, F.E. Fatal Streptococcus melleri Meningitis Complicating Missed Infected Intramedullary Dermoid Cyst Secondary to Dermal Sinus in a Saudi Child. J. Trop. Pediatr. 2013, 59, 246–249. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.S.L.; Loduca, R.D.D.S. Intramedullary spinal cord abscess as complication of lumbar puncture: A case-based update. Child’s Nerv. Syst. 2013, 29, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Nicola, Z.; Antonio, C.; De Tommasi, A. Cervical dermal sinus complicated with intramedullary abscess in a child: Case report and review of literature. Eur. Spine J. 2014, 23 (Suppl. S2), 192–196. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, V.G.; Karthikeyan, K.V.; Kitchanan, S.; Sriraman, B. Holocord abscess in association with congenital dermal sinus. J. Pediatr. Neurosci. 2013, 8, 198–200. [Google Scholar] [CrossRef]
- Whitson, W.J.; Ball, P.A.; Lollis, S.S.; Balkman, J.D.; Bauer, D.F. Postoperative Mycoplasma hominis infections after neurosurgical intervention. J. Neurosurg. Pediatr. 2014, 14, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Kanaheswari, Y.; Lai, C.; Lope, R.J.R.; Azizi, A.B.; Zulfiqar, M.A. Intramedullary spinal cord abscess: The result of a missed congenital dermal sinus. J. Paediatr. Child Health 2015, 51, 223–225. [Google Scholar] [CrossRef]
- Bhanage, A.; Katkar, A.; Ghate, P.; Ratta, B. Intra-medullary tubercular abscess with spinal dysraphism: An unusual case. J. Pediatr. Neurosci. 2015, 10, 73–75. [Google Scholar] [CrossRef]
- Papaevangelou, G.; Tsitsopoulos, P.P.; Flaris, N.; Iliadis, C.; Tsonidis, C. Dermal Sinus Tract of the Thoracic Spine Presenting with Intramedullary Abscess and Cranial Nerve Deficits. Pediatr. Neurosurg. 2015, 50, 339–343. [Google Scholar] [CrossRef]
- Kamat, A.S.; Thango, N.S.; Ben Husein, M. Proteus mirabilis abscess involving the entire neural axis. J. Clin. Neurosci. 2016, 30, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, B.; Ülkü, G.; Ucar, M.; Demirdağ, T.B.; İnan, A.; Börcek, A.Ö. Intramedullary dermoid cyst infection mimicking holocord tumor: Should radical resection be mandatory?—A case report. Child’s Nerv. Syst. 2016, 32, 2249–2253. [Google Scholar] [CrossRef]
- Sahu, R.N.; Bhaisora, K.S.; Godbole, C.; Das, K.K.; Mehrotra, A.; Jayesh, S.; Behari, S.; Srivastava, A.K.; Jaiswal, A.K. Delayed intramedullary abscess in operated case of spinal lipoma. J. Pediatr. Neurosci. 2016, 11, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Tassigny, D.; Fomekong, E.; Koerts, G.; Raftopoulos, C. Intramedullary holocord abscess secondary to infected dermoid cyst. Acta Neurochir. 2018, 160, 209–212. [Google Scholar] [CrossRef]
- Vankipuram, S.; Sahoo, S.K.; Srivastava, C.; Ojha, B.K. Spinal cord abscess secondary to infected dorsal dermal sinus in an infant: Uncommon presentation of a known entity. BMJ Case Rep. 2017, 2017. [Google Scholar] [CrossRef]
- Shaikh, S.; Joshi, R. Pyomyelia presenting as acute flaccid paralysis. Oxf. Med. Case Rep. 2016, 2016, omw052. [Google Scholar] [CrossRef]
- Verdier, E.P.; Konsol, O.; Portillo, S. Intramedullary cervical abscess mimicking a spinal cord tumor in a 10-year-old girl: A case-based review. Child’s Nerv. Syst. 2018, 34, 2143–2147. [Google Scholar] [CrossRef]
- Alsubaie, S.; Dolgum, S.; Binkhamis, K.; Alweijri, I.; Bugshan, A.; Alzamil, F.; Bakhshan, A. Finegoldia magna causing intramedullary thoracic spinal cord abscess in an infant. Anaerobe 2019, 56, 57–60. [Google Scholar] [CrossRef]
- Bevan, R.; Leach, P. Infected congenital cervical dermal sinuses leading to spinal cord abscess: Two case reports and a review of the literature. Child’s Nerv. Syst. 2021, 37, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, T.; Hirose, S.; Mizoguchi, T.; Yokota, Y.; Hara, M.; Ishihara, M.; Morita, A.; Nakajima, H. Ruptured long intramedullary spinal cord abscess successfully treated with antibiotic treatment. J. Clin. Neurosci. 2020, 82, 249–251. [Google Scholar] [CrossRef]
- Sehgal, R.; Budnik, E.; Mallik, A.; Bonwit, A.; Leischner, M. A Rare Case of an Intramedullary Spinal Cord Abscess Due to Escherichia coli in a Pediatric Patient. Child Neurol. Open 2021, 8, 2329048X2110118. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Murakami, N.; Kurogi, A.; Lee, S.; Mukae, N.; Shimogawa, T.; Shono, T.; Suzuki, S.O.; Yoshimoto, K.; Morioka, T. Intramedullary abscess at thoracolumbar region transmitted from infected dermal sinus and dermoid through retained medullary cord. Surg. Neurol. Int. 2022, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Chi Square Calculator—Up To 5x5, with Steps. Available online: https://www.socscistatistics.com/tests/chisquare2/default2.aspx (accessed on 8 May 2022).
- Van Schoor, A.N.; Bosman, M.C.; Bosenberg, A.T. Descriptive study of the differences in the level of the conus medullaris in four different age groups. Clin. Anat. 2015, 28, 638–644. [Google Scholar] [CrossRef]
- Foster, M.T.; Moxon, C.A.; Weir, E.; Sinha, A. Dermal sinus tracts. BMJ 2019, 366, l5202. [Google Scholar] [CrossRef] [PubMed]
- Chandran, R.S.; Bhanuprabhakar, R.; Sumukhan, S. Intramedullary Spinal Cord Abscess: Illustration of Two Cases and Review of Literature. Indian J. Neurosurg. 2017, 6, 31–35. [Google Scholar] [CrossRef][Green Version]
- Maurice-Williams, R.S.; Pamphilon, D.; Coakham, H.B. Intramedullary abscess--A rare complication of spinal dysraphism. J. Neurol. Neurosurg. Psychiatry 1980, 43, 1045–1048. [Google Scholar] [CrossRef]
- Hardwidge, C.; PalSingh, J.; Williams, B. Pyomyelia: An intramedullary spinal abscess complicating lumbar lipoma with spina bifida. Br. J. Neurosurg. 1993, 7, 419–422. [Google Scholar] [CrossRef] [PubMed]
| Microbiological Examination | Number |
|---|---|
| Culture-negative | 15 |
| Staphylococcus aureus | 8 |
| Mycobacterium tuberculosis | 6 |
| Escherichia coli | 4 |
| Proteus mirabilis | 4 |
| Others | 11 |
| No data | 4 |
| More than one pathogen | 12 |
| Follow-Up | Number |
|---|---|
| Survived; complete neurological recovery | 29 |
| Survived, residual neurological deficits | 17 |
| Survived, persistent neurological deficits | 15 |
| Died | 1 |
| No data | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szmyd, B.; Jabbar, R.; Lusa, W.; Karuga, F.F.; Pawełczyk, A.; Błaszczyk, M.; Jankowski, J.; Sołek, J.; Wysiadecki, G.; Tubbs, R.S.; et al. What Is Currently Known about Intramedullary Spinal Cord Abscess among Children? A Concise Review. J. Clin. Med. 2022, 11, 4549. https://doi.org/10.3390/jcm11154549
Szmyd B, Jabbar R, Lusa W, Karuga FF, Pawełczyk A, Błaszczyk M, Jankowski J, Sołek J, Wysiadecki G, Tubbs RS, et al. What Is Currently Known about Intramedullary Spinal Cord Abscess among Children? A Concise Review. Journal of Clinical Medicine. 2022; 11(15):4549. https://doi.org/10.3390/jcm11154549
Chicago/Turabian StyleSzmyd, Bartosz, Redwan Jabbar, Weronika Lusa, Filip Franciszek Karuga, Agnieszka Pawełczyk, Maciej Błaszczyk, Jakub Jankowski, Julia Sołek, Grzegorz Wysiadecki, R. Shane Tubbs, and et al. 2022. "What Is Currently Known about Intramedullary Spinal Cord Abscess among Children? A Concise Review" Journal of Clinical Medicine 11, no. 15: 4549. https://doi.org/10.3390/jcm11154549
APA StyleSzmyd, B., Jabbar, R., Lusa, W., Karuga, F. F., Pawełczyk, A., Błaszczyk, M., Jankowski, J., Sołek, J., Wysiadecki, G., Tubbs, R. S., Iwanaga, J., & Radek, M. (2022). What Is Currently Known about Intramedullary Spinal Cord Abscess among Children? A Concise Review. Journal of Clinical Medicine, 11(15), 4549. https://doi.org/10.3390/jcm11154549