Using Interpretable Machine Learning to Identify Baseline Predictive Factors of Remission and Drug Durability in Crohn’s Disease Patients on Ustekinumab
Abstract
1. Introduction
2. Materials and Methods
3. Results
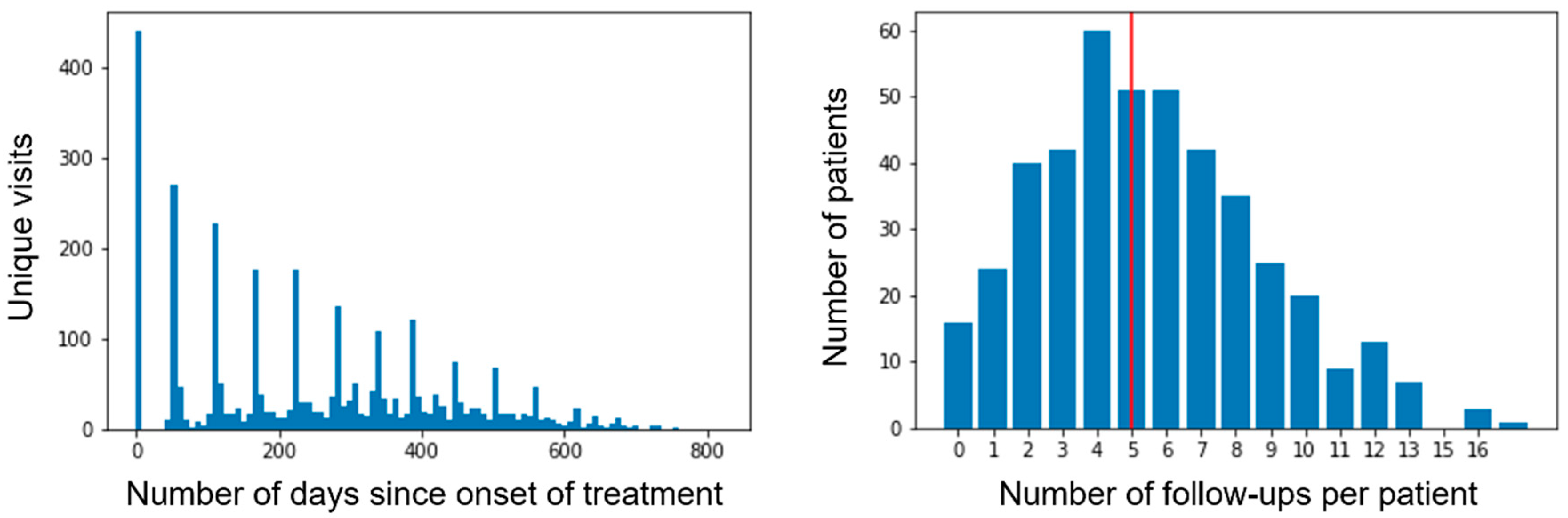
3.1. Performance of Models
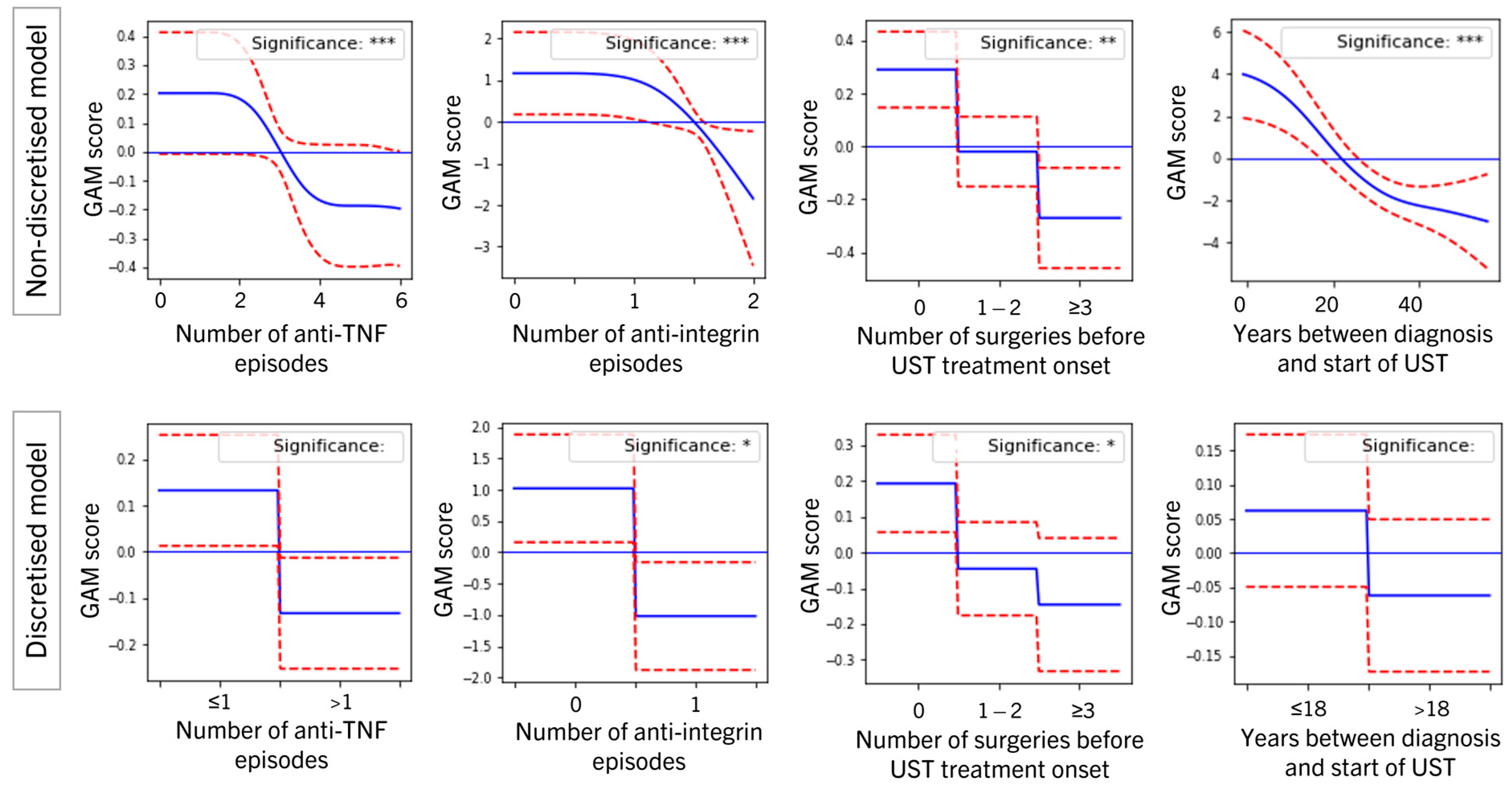
3.2. Significant Variables
3.3. Position of Ustekinumab in the Treatment Journey
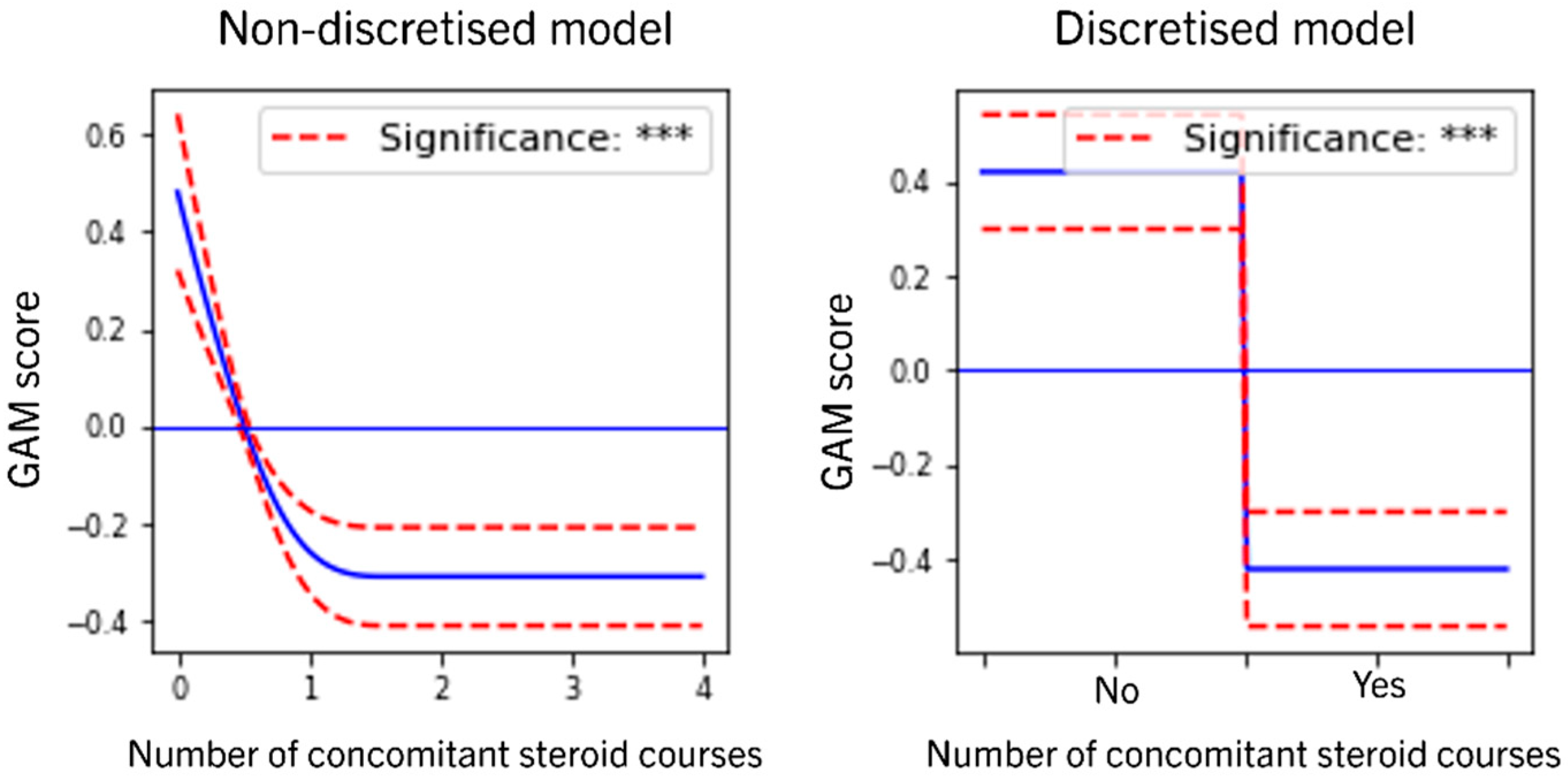
3.4. Role of Concomitant Steroids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Details of Statistical Methodology
Appendix A.1. Missing Values
Appendix A.2. Drop-Out in Longitudinal Analysis
Appendix A.3. Shape Constraints
Appendix A.4. Interaction Effects
| Feature Interactions | p-Value of Effect | ||
|---|---|---|---|
| Longitudinal Remission | Durability | ||
| Numeric Values Used for Several Features | All Features Discretized, Except for “Days since Start of UST Treatment” | ||
| (Baseline hemoglobin) × (Sex) | 0.626 | N/A | 0.821 |
| (HBI at the first UST dose, categorized) × (Baseline fecal calprotectin) | <0.05 | N/A | 0.307 |
| (HBI at the first UST dose, categorized) × (Baseline albumin) | <0.05 | N/A | 0.239 |
| (HBI at the first UST dose, categorized) × (Baseline hemoglobin) | <0.05 | N/A | 0.306 |
| (HBI at the first UST dose, categorized) × (Baseline CRP) | <0.05 | N/A | <0.05 |
| (Days since the start of UST treatment) × (Years between diagnosis and start of UST) | <0.05 | N/A | N/A |
| (Days since the start of UST treatment) × (BMI) | <0.05 | N/A | N/A |
References
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.L.; Miao, Y.; et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Bonovan, S.; Peyrin-Biroulet, L. Positioning ustekinumab in Crohn’s disease: From clinical evidence to clinical practice. J. Crohn’s Colitis 2017, 11, 1258–1266. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pacou, M.; Mesana, L.; Gauthier, A.; Naessens, D.; Sloan, S.; Danese, S.; Bonovas, S.; Abrams, K. Indirect treatment comparison of ustekinumab versus other biologics in moderate to severe Crohn’s disease: A 1-year treatment sequence analysis. Value Health 2016, 19, 576. [Google Scholar] [CrossRef]
- Chaparro, M.; Baston-Rey, I.; Fernández-Salgado, E.; González García, J.; Ramos, L.; Diz-Lois Palomares, M.T.; Argüelles-Arias, F.; Iglesias Flores, E.; Cabello, M.; Rubio Iturria, S.; et al. Long-Term Real-World Effectiveness and Safety of Ustekinumab in Crohn’s Disease Patients: The SUSTAIN Study. Inflamm. Bowel Dis. 2022; online ahead of print. [Google Scholar]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models; CRC Press: Boca Raton, FL, USA, 1990; Volume 43. [Google Scholar]
- Argyropoulos, C.; Unruh, M.L. Analysis of time to event outcomes in randomized controlled trials by generalized additive models. PLoS ONE 2015, 10, e0123784. [Google Scholar] [CrossRef]
- Berhane, K.; Tibshirani, R.J. Generalized Additive Models for Longitudinal Data. Can. J. Stat. 1998, 26, 517–535. [Google Scholar] [CrossRef]
- Waljee, A.K.; Wallace, B.I.; Cohen-Mekelburg, S.; Liu, Y.; Liu, B.; Sauder, K.; Stidham, R.W.; Zhu, J.; Higgins, P.D. Development and validation of machine learning models in prediction of remission in patients with moderate to severe Crohn disease. JAMA Netw. Open 2019, 2, e193721. [Google Scholar] [CrossRef] [PubMed]
- Biemans, V.B.; van der Meulen-de Jong, A.E.; Van Der Woude, C.J.; Löwenberg, M.; Dijkstra, G.; Oldenburg, B.; De Boer, N.K.; Van Der Marel, S.; Bodelier, A.G.; Jansen, J.M.; et al. Ustekinumab for Crohn’s Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study. J. Crohn’s Colitis 2020, 14, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Straatmijer, T.; Biemans, V.B.; Hoentjen, F.; De Boer, N.K.; Bodelier, A.G.; Dijkstra, G.; Van Dop, W.A.; Haans, J.J.; Jansen, J.M.; Maljaars, P.J.; et al. Ustekinuma b for Crohn’s Disease: Two-Year Results of the Initiative on Crohn and Colitis (ICC) Registry, a Nationwide Prospective Observational Cohort Study. J. Crohn’s Colitis 2021, 15, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Iborra, M.; Beltrán, B.; Fernández-Clotet, A.; Iglesias-Flores, E.; Navarro, P.; Rivero, M.; Gutiérrez, A.; Sierra-Ausin, M.; Mesonero, F.; Ferreiro-Iglesias, R.; et al. Real-world long-term effectiveness of ustekinumab in Crohn’s disease: Results from the ENEIDA registry. Aliment. Pharm. Ther. 2020, 52, 1017–1030. [Google Scholar]
- Park, J.; Chun, J.; Yoon, H.; Cheon, J.H. Feasibility of a Clinical Decision Support Tool for Ustekinumab to Predict Clinical Remission and Relapse in Patients with Crohn’s Disease: A Multicenter Observational Study. Inflamm. Bowel Dis. 2022, izac105. [Google Scholar] [CrossRef] [PubMed]
- Little, R.J.; Rubin, D.B. Statistical Analysis with Missing Data; John Wiley and Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Garcia-Laencina, P.J.; Sancho-Gomez, J.-L.; Figueiras-Vidal, A.R.; Verleysen, M. K Nearest Neighbours with Mutual Information for Simultaneous Classification and Missing Data Imputation. Neurocomputing 2009, 72, 1483–1493. [Google Scholar] [CrossRef]

| Variable | Original (Non-Discretized) | Post-Processed (Discretized) | Description of Discretized Version | Descriptives |
|---|---|---|---|---|
| Days since the start of UST treatment (longitudinal, not baseline) | Numeric | Numeric | - | |
| HBI at baseline, categorized | Ordinal (3) | - | [5–7] (mild), [8–16] (moderate), >16 (severe) | Distribution: Mild 50%, Moderate 45%, Severe 5% % missing values: 0% |
| Years between diagnosis and start of UST | Numeric | Dichotomous | ≤18, >18 | Median: 10.0 % missing values: 0% |
| Number of surgeries before UST treatment onset | Numeric | Ordinal (3) | 0, 1–2, ≥3 | Distribution: 0 (47%), 1–2 (43%), ≥3 (10%) |
| Number of anti-TNF episodes | Numeric | Dichotomous | ≤1, >1 | Median: 2.0 96.1% of patients ≥1 anti-TNF |
| Number of anti-integrin episodes | Numeric | Dichotomous | 0, 1 | Median: 0.0 23.7% of patients ≥1 anti-integrin |
| Age at the time of signing consent | Numeric | Dichotomous | ≤40, >40 | Median: 46.0 % missing values: 0% |
| BMI at baseline | Numeric | Ordinal (3) | <27, 27–38, >38 | Median: 23.81 % missing values: 16% |
| Sex | Dichotomous | Dichotomous | Female, Male | Distribution: Female 49%, Male 51% % missing values: 0% |
| Number of comorbidities | Numeric | Ordinal (4) | 0–1, 2–3, ≥4 | Median: 1.0 |
| Perianal disease | Categorical | Dichotomous | Never/Previous, Current | Distribution: Never/Previous 86%, Current 14% % missing values: 0% |
| CD location | Categorical | Dichotomous | Ileocolic/Ileum (L1/L3), Colon (L2) | Distribution: Ileocolic/Ileum 88%, Colon 12% % missing values: 0% |
| Patient ever had EIMs | Dichotomous | Dichotomous | Yes/No | Distribution: No 59%, Yes 41% |
| Family history of CD | Dichotomous | Dichotomous | Yes/No | Distribution: No 13%, Yes 87% % missing values: 16% |
| Upper gastrointenstinal tract (L4) involved | Dichotomous | Dichotomous | Yes/No | Distribution: No 92%, Yes 8% % missing values: 0% |
| Baseline albumin (g/L) | Numeric | Dichotomous | ≤3.8, >3.8 | Median: 4.0 % missing values: 33% |
| Baseline faecal calprotectin (mcg/g) | Numeric | Ordinal (3) | ≤80, 81–650, >650 | Median: 667.33 % missing values: 52% |
| Baseline haemoglobin (g/L) | Numeric | Dichotomous | ≤10.9, >10.9 | Median: 13.2 % missing values: 10% |
| Baseline CRP (mg/L) | Numeric | Ordinal (3) | ≤0.5, 0.6–6.1, >6.1 | Median: 7.2 % missing values: 10% |
| Steroid use at first UST dose | Dichotomous | Dichotomous | Yes/No | Distribution: No 72%, Yes 28% |
| Under Immunosuppressants at first UST dose | Dichotomous | Dichotomous | Yes/No | Distribution: No 70%, Yes 30% |
| Number of concomitant steroid courses | Numeric | Dichotomous | Yes/No | Median: 0.0 |
| Longitudinal Remission | Durability | ||
|---|---|---|---|
| Feature | Non-Discretized | Discretized * | |
| Days since start of UST treatment | <0.05 | <0.05 | <0.05 |
| HBI at the first UST dose, categorized | <0.05 | <0.05 | 0.083 |
| Years between diagnosis and start of UST | <0.05 | 0.547 | 0.077 |
| Number of surgeries before UST treatment onset | <0.05 | <0.05 | 0.24 |
| Number of anti-TNF episodes | <0.05 | 0.09 | <0.05 |
| Number of anti-integrin episodes | <0.05 | <0.05 | <0.05 |
| Age at the time of signing consent | <0.05 | <0.05 | <0.05 |
| BMI at baseline | <0.05 | <0.05 | <0.05 |
| Sex | 0.888 | N/A | 0.842 |
| Number of comorbidities | <0.05 | <0.05 | 0.296 |
| Perianal disease | <0.05 | <0.05 | 0.541 |
| Location of CD | <0.05 | <0.05 | 0.137 |
| Patient ever had EIMs | 0.806 | N/A | 0.648 |
| Family history of CD | 0.958 | N/A | 0.998 |
| Upper gastrointestinal tract (L4) involved | 0.999 | N/A | 0.828 |
| Baseline albumin | <0.05 | 0.431 | 0.357 |
| Baseline fecal calprotectin | <0.05 | 0.26 | <0.05 |
| Baseline hemoglobin | <0.05 | 0.77 | <0.05 |
| Baseline CRP | <0.05 | <0.05 | <0.05 |
| Under Steroids at first UST dose | <0.05 | <0.05 | 0.237 |
| Under Immunosuppressants at first UST dose | <0.05 | <0.05 | 0.813 |
| Number of concomitant Steroid courses | <0.05 | <0.05 | <0.05 |
| Direction of Effect in Non-Discretized Longitudinal Remission Model | Exact Effect Size in Discretized Longitudinal Remission Model (Lowest vs. Highest) | Effect for Group with Lowest Value | Effect for Group with Highest Value | |
|---|---|---|---|---|
| HBI at the first UST dose, categorised | Negative | Negative: −2.32 | 1.09 (0.91, 1.27) | 0.14 (−0.04, 0.32) |
| Years between diagnosis and start of UST | Negative | Not significant, trending negative | 0.06 (−0.05, 0.17) | −0.06 (−0.17, 0.05) |
| Number of surgeries before UST treatment onset | Negative | −0.33 | 0.19 (0.06, 0.33) | −0.14 (−0.33, 0.05) |
| Number of anti-TNF episodes | Negative | Borderline insignificant, trending negative | 0.11 (−0.01, 0.24) | −0.11 (−0.24, 0.01) |
| Number of anti-integrin episodes | Negative | Negative: −1.96 | 0.7 (0.19, 1.22) | −1.26 (−2.27, −0.25) |
| Age at the time of signing consent | Negative | Negative: −0.31 | 0.16 (0.06, 0.26) | −0.16 (−0.26, −0.06) |
| BMI at baseline | Negative | Negative: −2 | 0.8 (0.52, 1.09) | −1.2 (−1.73, −0.68) |
| Sex | Insignificant | N/A | N/A | N/A |
| Number of comorbidities | Negative | Negative: −0.41 | 0.22 (0.06, 0.38) | −0.19 (−0.44, 0.07) |
| Perianal disease | Negative | Negative: −0.7 | 0.35 (0.21, 0.48) | −0.35 (−0.48, −0.21) |
| Location of CD (no ileal involvement) | Positive | Positive: 0.74 | −0.37 (−0.52, −0.23) | 0.37 (0.23, 0.52) |
| Patient ever had EIMs | Insignificant | N/A | N/A | N/A |
| Family history of CD | Insignificant | N/A | N/A | N/A |
| Upper gastrointenstinal tract (L4) involved | Insignificant | N/A | N/A | N/A |
| Baseline albumin | Negative | Negative: −0.13 | 0.07 (−0.03, 0.16) | −0.07 (−0.16, 0.03) |
| Baseline faecal calprotectin | Negative | Negative: −0.45 | 0.28 (0.02, 0.53) | −0.17 (−0.33, −0.0) |
| Baseline hemoglobin | Positive | Positive: 0.08 | −0.04 (−0.19, 0.11) | 0.04 (−0.11, 0.19) |
| Baseline CRP | Positive | Positive: 0.6 | −0.32 (−0.53, −0.11) | 0.28 (0.14, 0.43) |
| Under Steroids at first UST dose | Positive | Positive: 0.69 | −0.35 (−0.48, −0.22) | 0.35 (0.22, 0.48) |
| Under Immunosuppressants at first UST dose | Negative | Negative: −0.28 | 0.14 (0.04, 0.23) | −0.14 (−0.23, −0.04) |
| Number of concomitant Steroid courses | Negative | Negative: −0.83 | 0.42 (0.29, 0.54) | −0.42 (−0.54, −0.29) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaparro, M.; Baston-Rey, I.; Fernández Salgado, E.; González García, J.; Ramos, L.; Diz-Lois Palomares, M.T.; Argüelles-Arias, F.; Iglesias Flores, E.; Cabello, M.; Rubio Iturria, S.; et al. Using Interpretable Machine Learning to Identify Baseline Predictive Factors of Remission and Drug Durability in Crohn’s Disease Patients on Ustekinumab. J. Clin. Med. 2022, 11, 4518. https://doi.org/10.3390/jcm11154518
Chaparro M, Baston-Rey I, Fernández Salgado E, González García J, Ramos L, Diz-Lois Palomares MT, Argüelles-Arias F, Iglesias Flores E, Cabello M, Rubio Iturria S, et al. Using Interpretable Machine Learning to Identify Baseline Predictive Factors of Remission and Drug Durability in Crohn’s Disease Patients on Ustekinumab. Journal of Clinical Medicine. 2022; 11(15):4518. https://doi.org/10.3390/jcm11154518
Chicago/Turabian StyleChaparro, María, Iria Baston-Rey, Estela Fernández Salgado, Javier González García, Laura Ramos, María Teresa Diz-Lois Palomares, Federico Argüelles-Arias, Eva Iglesias Flores, Mercedes Cabello, Saioa Rubio Iturria, and et al. 2022. "Using Interpretable Machine Learning to Identify Baseline Predictive Factors of Remission and Drug Durability in Crohn’s Disease Patients on Ustekinumab" Journal of Clinical Medicine 11, no. 15: 4518. https://doi.org/10.3390/jcm11154518
APA StyleChaparro, M., Baston-Rey, I., Fernández Salgado, E., González García, J., Ramos, L., Diz-Lois Palomares, M. T., Argüelles-Arias, F., Iglesias Flores, E., Cabello, M., Rubio Iturria, S., Núñez Ortiz, A., Charro, M., Ginard, D., Dueñas Sadornil, C., Merino Ochoa, O., Busquets, D., Iyo, E., Gutiérrez Casbas, A., Ramírez de la Piscina, P., ... Gisbert, J. P. (2022). Using Interpretable Machine Learning to Identify Baseline Predictive Factors of Remission and Drug Durability in Crohn’s Disease Patients on Ustekinumab. Journal of Clinical Medicine, 11(15), 4518. https://doi.org/10.3390/jcm11154518









