The Anemia Stress Index—Anemia, Transfusions, and Mortality in Patients with Continuous Flow Ventricular Assist Devices
Abstract
:1. Introduction
2. Methods
2.1. Study Population and Data Collection
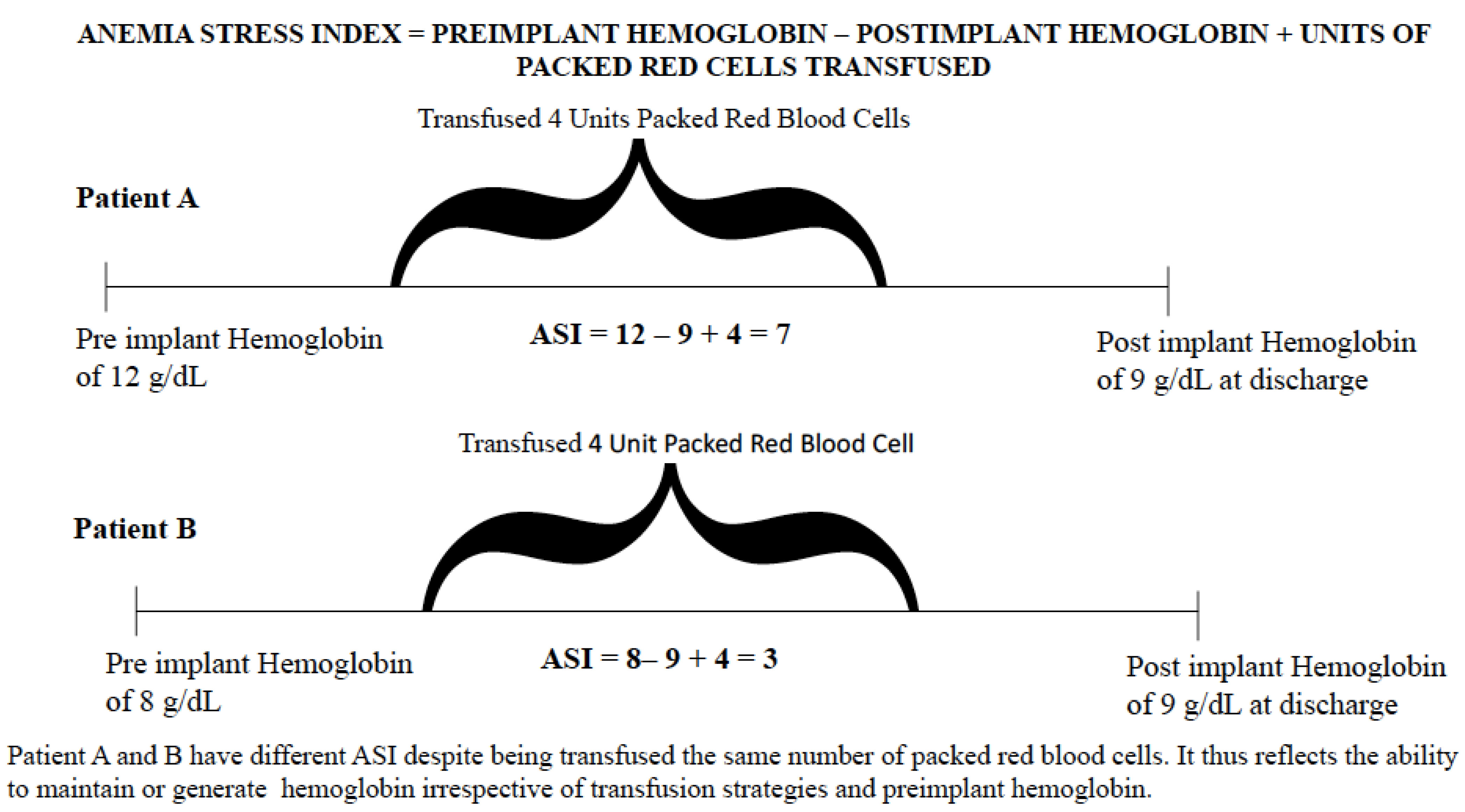
2.2. Independent Variable
2.3. Dependent Variable
2.4. Covariates
2.5. Statistical Analysis
3. Results
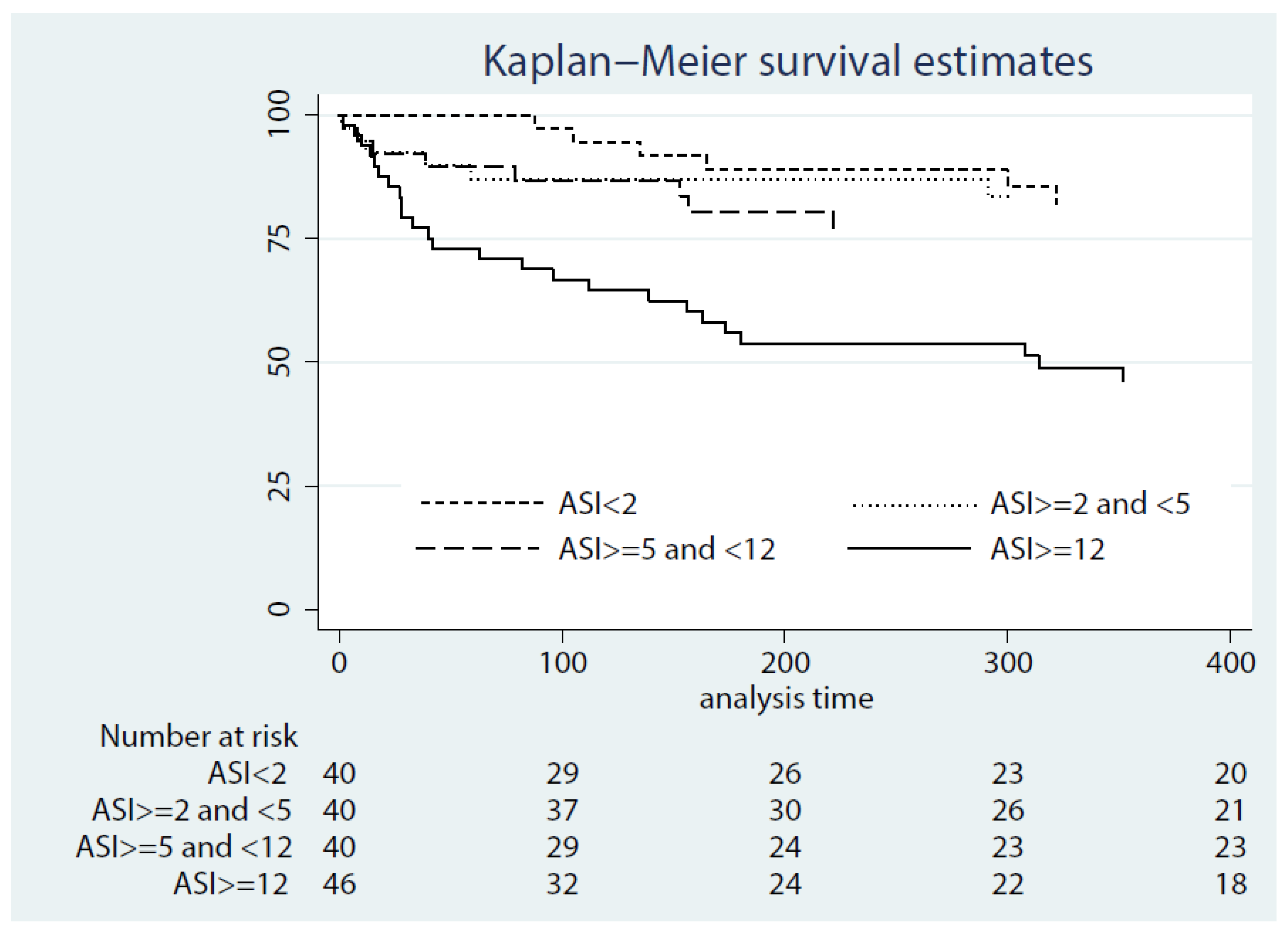
3.1. Association between the ASI at 24 h after LVAD Implant and All-Cause Mortality
3.2. Association between the ASI at 24 h after LVAD Implant and Early Right Ventricular Failure
3.3. Association between the ASI at Discharge and All-Cause Mortality
3.4. Association between the ASI at 3 Months after LVAD Implant and All-Cause Mortality
4. Discussion
4.1. Prior Studies
4.2. Study Implications
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tang, Y.D.; Katz, S.D. Anemia in chronic heart failure: Prevalence, etiology, clinical correlates, and treatment options. Circulation 2006, 113, 2454–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felker, G.M.; Adams, K.F.; Gattis, W.A., Jr.; O’Connor, C.M. Anemia as a risk factor and therapeutic target in heart failure. J. Am. Coll. Cardiol. 2004, 44, 959–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthiah, K.; Connor, D.; Ly, K.; Gardiner, E.E.; Andrews, R.K.; Qiao, J.; Rutgers, D.; Robson, D.; Low, J.; Jarvis, S.; et al. Longitudinal changes in hemostatic parameters and reduced pulsatility contribute to non-surgical bleeding in patients with centrifugal continuous-flow left ventricular assist devices. J. Heart Lung Transplant. 2016, 35, 743–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amione-Guerra, J.; Cruz-Solbes, A.S.; Bhimaraj, A.; Trachtenberg, B.H.; Pingali, S.R.; Estep, J.D.; Park, M.H.; Guha, A. Anemia after continuous-flow left ventricular assist device implantation: Characteristics and implications. Int. J. Artif. Organs 2017, 40, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Jennings, D.L.; Wagner, J.L.; To, L.; Nemerovski, C.W.; Kalus, J.S.; Morgan, J.A.; Lanfear, D.E. Epidemiology and outcomes associated with anemia during long-term support with continuous-flow left ventricular assist devices. J. Card. Fail. 2014, 20, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.; Hanff, T.C.; Mazurek, J.A.; Seigerman, M.; Zhang, R.; Grandin, E.W.; Vorovich, E.; Mather, P.; Olt, C.; Howard, J.; et al. The effect of transfusion of blood products on ventricular assist device support outcomes. ESC Heart Fail. 2020, 7, 3573–3581. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Carless, P.A.; Hebert, P.C. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012, 10, CD002042. [Google Scholar]
- Hajjar, L.A.; Vincent, J.L.; Galas, F.R.; Nakamura, R.E.; Silva, C.M.; Santos, M.H.; Fukushima, J.; Kalil Filho, R.; Sierra, D.B.; Lopes, N.H.; et al. Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. JAMA 2010, 304, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Lampert, B.C.; Teuteberg, J.J. Right ventricular failure after left ventricular assist devices. J. Heart Lung Transplant. 2015, 34, 1123–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabashnikov, A.; Mohite, P.N.; Zych, B.; García, D.; Popov, A.F.; Weymann, A.; Patil, N.P.; Hards, R.; Capoccia, M.; Wahlers, T.; et al. Outcomes and predictors of early mortality after continuous-flow left ventricular assist device implantation as a bridge to transplantation. ASAIO J. 2014, 60, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Groenveld, H.F.; Januzzi, J.L.; Damman, K.; van Wijngaarden, J.; Hillege, H.L.; van Veldhuisen, D.J.; van der Meer, P. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2008, 52, 818–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCullough, P.A.; Lepor, N.E. Piecing together the evidence on anemia: The link between chronic kidney disease and cardiovascular disease. Rev. Cardiovasc. Med. 2005, 6 (Suppl. 3), S4–S12. [Google Scholar] [PubMed]
- Anand, I.S.; Kuskowski, M.A.; Rector, T.S.; Florea, V.G.; Glazer, R.D.; Hester, A.; Chiang, Y.T.; Aknay, N.; Maggioni, A.P.; Opasich, C.; et al. Anemia and change in hemoglobin over time related to mortality and morbidity in patients with chronic heart failure: Results from Val-HeFT. Circulation 2005, 112, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, C.N.; Larson, D.F. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion 2005, 20, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Rauchhaus, M.; Doehner, W.; Francis, D.P.; Davos, C.; Kemp, M.; Liebenthal, C.; Niebauer, J.; Hooper, J.; Volk, H.D.; Coats, A.J.; et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 2000, 102, 3060–3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanas, J.N.; Matsouka, C.; Karageorgopoulos, D.; Leonti, A.; Tsolakis, E.; Drakos, S.G.; Tsagalou, E.P.; Maroulidis, G.D.; Alexopoulos, G.P.; Kanakakis, J.E.; et al. Etiology of anemia in patients with advanced heart failure. J. Am. Coll. Cardiol. 2006, 48, 2485–2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrtovec, B.; Radovancevic, R.; Delgado, R.M.; Radovancevic, B.; Bracey, A.W.; Gregoric, I.D.; Frazier, O.H. Significance of anaemia in patients with advanced heart failure receiving long-term mechanical circulatory support. Eur. J. Heart Fail. 2009, 11, 1000–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birati, E.Y.; Hanff, T.C.; Mazurek, J.A.; Banerji, S.; Grandin, E.; Pedrotty, D.; Vorovich, E.; Howard, J.L.; Acker, M.; Kirkpatrick, J.N.; et al. The Effect of Pre and Post Implant Anemia on Outcomes of Patients with Left Ventricular Assist Device. J. Heart Lung Transplant. 2015, 34, S58–S59. [Google Scholar] [CrossRef]
- Schaffer, J.M.; Arnaoutakis, G.J.; Allen, J.G.; Weiss, E.S.; Patel, N.D.; Russell, S.D.; Shah, A.S.; Conte, J.V. Bleeding complications and blood product utilization with left ventricular assist device implantation. Ann. Thorac. Surg. 2011, 91, 740–747, discussion 7–9. [Google Scholar] [CrossRef] [PubMed]
- Birati, E.Y.; Hanff, T.C.; Mazurek, J.A.; Banerji, S.; Grandin, E.; Vorovich, E.; Pedrotty, D.; Kaiser, A.; Phillips, E.; Acker, M.; et al. Blood Transfusions Affect the Panel of Reactive Antibodies and Survival After Ventricular Assist Device Implantation. J. Heart Lung Transplant. 2015, 34, S167. [Google Scholar] [CrossRef]
| Characteristic | Quartile 1 ASI < 2 (n = 40) | Quartile 2 ASI > 2 to <5 (n = 40) | Quartile 3 ASI > 5 to <12 (n = 40) | Quartile 4 ASI > 12 (n = 46) | p Value |
|---|---|---|---|---|---|
| Age, years | 51.7 ± 14.9 | 52.5 ± 17.9 | 61.1 ± 15.4 | 59.6 ± 12.6 | <0.01 |
| Male sex | 28 (70%) | 30 (75%) | 32 (80%) | 43 (93.5%) | 0.04 |
| Race | 0.26 | ||||
| Caucasian | 17 (42.5%) | 16 (40%) | 15 (37.5%) | 29 (63%) | |
| African-American | 6 (15.1%) | 10 (25%) | 6 (15%) | 9 (19.6%) | |
| Other | 3 (7.5) | 1 (2.5%) | 1 (2.5%) | 0 (0) | |
| Unknown | 14 (35%) | 13 (32.5%) | 18 (45%) | 8 (17.4%) | |
| BMI (mean ± SD) | 30.5 ± 7.4 | 27.3 ± 6.1 | 27.7 ± 7.3 | 28.6 ± 5.4 | 0.13 |
| Diabetes | 19 (47.5%) | 16 (40%) | 18 (45%) | 22 (47.8%) | 0.88 |
| Hypertension | 25 (62.5%) | 15 (37.5%) | 23 (57.5%) | 35 (76.1%) | <0.01 |
| Dyslipidemia | 26 (65%) | 25 (62.5%) | 27 (67.5%) | 30 (65.2%) | 0.97 |
| Atrial fibrillation | 13 (32.5%) | 14 (35%) | 18 (45%) | 23 (50%) | 0.31 |
| Stroke/TIA | 8 (20%) | 3 (7.5%) | 4 (10%) | 8 (17.4%) | 0.31 |
| Chronic renal disease | 10 (25%) | 15 (37.5%) | 13 (32.5%) | 20 (43.5%) | 0.30 |
| Prior CT surgery | 10 (25%) | 10 (25.0%) | 18 (45%) | 18 (39.1%) | 0.13 |
| HF etiology | 0.60 | ||||
| Ischemic cardiomyopathy | 17 (42.5%) | 16 (40%) | 19 (47.5%) | 26 (56.5%) | |
| Non ischemic cardiomyopathy: | |||||
| -Idiopathic | 18 | 19 | 16 | 17 | |
| -Congenital heart disease | 0 | 1 | 0 | 0 | |
| -Viral cardiomyopathy | 2 | 0 | 0 | 0 | |
| -Peripartum cardiomyopathy | 1 | 2 | 1 | 0 | |
| -Alcohol-induced cardiomyopathy | 0 | 1 | 0 | 0 | |
| -Myocarditis | 0 | 0 | 1 | 1 | |
| -Chemotherapy-induced cardiomyopathy | 1 | 1 | 2 | 1 | |
| -Valvular cardiomyopathy | 1 | 0 | 1 | 1 | |
| LVAD reason | 0.93 | ||||
| Cardiogenic shock | 21 (52.5%) | 17 (43.6%) | 19 (47.5%) | 22 (50%) | |
| Inotrope-dependent heart failure | 14 (35%) | 14 (35.9%) | 15 (37.5%) | 14 (31.8%) | |
| Worsening, non-inotrope-dependent heart failure | 4 (10%) | 7 (17.9%) | 5 (12.5%) | 7 (15.9%) | |
| Intractable ventricular arrhythmia | 0 (0) | 1 (2.6%) | 1 (2.5%) | 1 (2.3%) | |
| Pump exchange | 1 (2.5%) | 0 (0) | 0 (0) | 0 (0) | |
| LVAD indication | 0.01 | ||||
| Bridge to transplant | 23 (57.5%) | 13 (32.5%) | 11 (27.5%) | 17 (36.9%) | |
| Bridge to decision | 6 (15%) | 2 (5%) | 4 (10%) | 4 (8.7) | |
| Destination therapy | 11 (27.5%) | 21 (52.5%) | 24 (60%) | 25 (54.3%) | |
| Bridge to recovery | 0 (0) | 4 (10%) | 1 (2.5%) | 0 (0) | |
| LVAD type | 0.28 | ||||
| HeartMate II | 30 (75%) | 36 (90%) | 34 (85%) | 40 (86.9%) | |
| HeartWare | 10 (25%) | 4 (10%) | 6 (15%) | 6 (13%) | |
| Pulmonary hypertension | 13 (32.5%) | 16 (40%) | 16 (40%) | 11 (24.4%) | 0.37 |
| COPD | 9 (23.1%) | 3 (7.5%) | 8 (20%) | 9 (19.6%) | 0.27 |
| History of smoking | 16 (40%) | 17 (42.5%) | 16 (40%) | 19 (41.3%) | 0.99 |
| Hemoglobin baseline (median, IQR) | 11.2 (8.9–12.3) | 11.4 (10.0–12.9) | 11.1 (10.2–12.2) | 10.6 (9.4–12.0) | 0.18 |
| Platelet baseline (mean ± SD) | 194.6 ± 79.6 | 178.3 ± 58.2 | 191.3 ± 64.2 | 192.4 ±75.1 | 0.72 |
| Albumin (mean ± SD) | 3.4 ± 0.6 | 3.4 ± 0.5 | 3.4 ± 0.5 | 3.3 ± 0.7 | 0.71 |
| Unadjusted HR (95% CI) for Mortality | Adjusted HR (95% CI) for Mortality | |
|---|---|---|
| Anemia stress index at 24 h post-implant | 1.09 (1.04–1.14) | 1.08 (1.03–1.14) |
| Anemia stress index at discharge | 1.04 (1.01–1.07) | 1.08 (1.00–1.16) |
| Anemia stress index at 3 months after LVAD implant | 1.05 (1.02–1.07) | 1.09 (1.02–1.16) |
| Characteristic | OR (95% CI) | p Value |
|---|---|---|
| Anemia stress index * at 24 h | 1.09 (1.04–1.14) | <0.01 |
| Age * | 0.99 (0.95–1.03) | 0.53 |
| Male sex | 1.25 (0.37–4.24) | 0.72 |
| BMI * | 0.99 (0.91–1.06) | 0.73 |
| Diabetes | 1.07 (0.42–2.74) | 0.89 |
| COPD | 2.30 (0.74–7.21) | 0.74 |
| Pulmonary hypertension | 1.06 (0.40–2.80) | 0.40 |
| Smoking history | 0.62 (0.24–1.64) | 0.34 |
| Atrial fibrillation | 1.35 (0.51–3.57) | 0.55 |
| Chronic renal disease | 1.50 (0.55–4.10) | 0.55 |
| Prior CT surgery | 1.01 (0.39–2.64) | 0.98 |
| LVAD indication ** | ||
| Bridge to transplant | Reference | |
| Bridge to decision | 1.06 (0.22–5.16) | 0.94 |
| Destination therapy | 0.76 (0.26–2.23) | 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shore, S.; Hanff, T.C.; Mazurek, J.A.; Fox, A.; Tanna, M.S.; Grandin, E.W.; Zhang, R.; Wald, J.; Peters, C.; Acker, M.A.; et al. The Anemia Stress Index—Anemia, Transfusions, and Mortality in Patients with Continuous Flow Ventricular Assist Devices. J. Clin. Med. 2022, 11, 4517. https://doi.org/10.3390/jcm11154517
Shore S, Hanff TC, Mazurek JA, Fox A, Tanna MS, Grandin EW, Zhang R, Wald J, Peters C, Acker MA, et al. The Anemia Stress Index—Anemia, Transfusions, and Mortality in Patients with Continuous Flow Ventricular Assist Devices. Journal of Clinical Medicine. 2022; 11(15):4517. https://doi.org/10.3390/jcm11154517
Chicago/Turabian StyleShore, Supriya, Thomas C. Hanff, Jeremy A. Mazurek, Arieh Fox, Monique S. Tanna, Edward W. Grandin, Robert Zhang, Joyce Wald, Carli Peters, Michael A. Acker, and et al. 2022. "The Anemia Stress Index—Anemia, Transfusions, and Mortality in Patients with Continuous Flow Ventricular Assist Devices" Journal of Clinical Medicine 11, no. 15: 4517. https://doi.org/10.3390/jcm11154517
APA StyleShore, S., Hanff, T. C., Mazurek, J. A., Fox, A., Tanna, M. S., Grandin, E. W., Zhang, R., Wald, J., Peters, C., Acker, M. A., Atluri, P., Rame, J. E., Goldberg, L. R., Jessup, M., Margulies, K. B., & Birati, E. Y. (2022). The Anemia Stress Index—Anemia, Transfusions, and Mortality in Patients with Continuous Flow Ventricular Assist Devices. Journal of Clinical Medicine, 11(15), 4517. https://doi.org/10.3390/jcm11154517






