Association between the Use of Antibiotics and the Development of Acute Renal Injury in Patients Hospitalized for COVID-19 in a Hospital in the Peruvian Amazon
Abstract
:1. Introduction
2. Materials and Methods
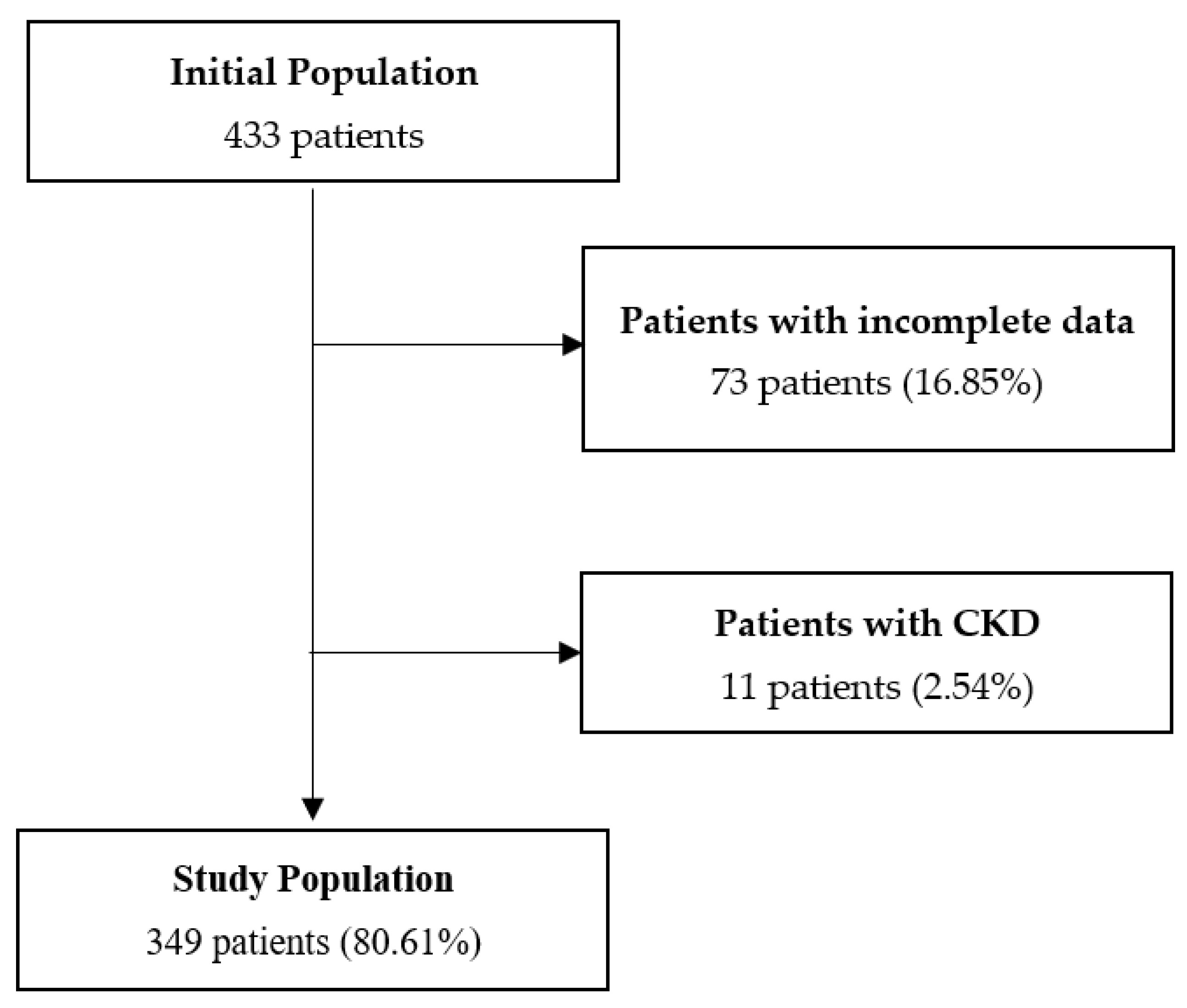
2.1. Study Design and Study Population
2.2. Data Collection
2.3. Statistical Analysis
2.4. Ethical Information
3. Results
4. Discussion
5. Conclusions
6. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Formeck, C.L.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Uncommon Causes of Acute Kidney Injury. Crit. Care Clin. 2022, 38, 317–347. [Google Scholar] [CrossRef] [PubMed]
- Zuber, K.; Davis, J. The ABCs of chronic kidney disease. JAAPA 2018, 31, 17–25. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Exp. Nephrol. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Sagnelli, C.; Sica, A.; Gallo, M.; Peluso, G.; Varlese, F.; D’Alessandro, V.; Ciccozzi, M.; Crocetto, F.; Garofalo, C.; Fiorelli, A.; et al. Renal involvement in COVID-19: Focus on kidney transplant sector. Infection 2021, 49, 1265–1275. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, H.; Li, X.; Li, H.; Xu, L.; Yu, Q.; Dong, Y.; Zhao, Y.; Wang, J.; Hou, W.; et al. Prevalence of Kidney Injury and Associations with Critical Illness and Death in Patients with COVID-19. Clin. J. Am. Soc. Nephrol. 2020, 15, 1549–1556. [Google Scholar] [CrossRef]
- Ronco, C.; Reis, T.; Husain-Syed, F. Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 2020, 8, 738–742. [Google Scholar] [CrossRef]
- Alenezi, F.K.; Almeshari, M.A.; Mahida, R.; Bangash, M.N.; Thickett, D.R.; Patel, J.M. Incidence and risk factors of acute kidney injury in COVID-19 patients with and without acute respiratory distress syndrome (ARDS) during the first wave of COVID-19: A systematic review and Meta-Analysis. Ren. Fail. 2021, 43, 1621–1633. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, C.; Luo, L.; Fang, F.; Chen, Y.; Li, J.; Peng, Z.; Pan, H. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J. Clin. Virol. 2020, 127, 104364. [Google Scholar] [CrossRef]
- Burgula, H.; Mohanty, L.; Mohanty, A.; Khujur, S.T.; Chaitanya, A.; Devasi, M. Study of Incidence, Clinical profile and Outcomes among patients hospitalized for COVID-19 with Acute Kidney Injury. J. Assoc. Physicians India 2022, 70, 11–12. [Google Scholar]
- Sullivan, M.K.; Lees, J.S.; Drake, T.M.; Docherty, A.B.; Oates, G.; E Hardwick, H.; Russell, C.D.; Merson, L.; Dunning, J.; Nguyen-Van-Tam, J.S.; et al. Acute kidney injury in patients hospitalized with COVID-19 from the ISARIC WHO CCP-UK Study: A prospective, multicentre cohort study. Nephrol. Dial. Transplant. 2021, 37, 271–284. [Google Scholar] [CrossRef]
- Abu-Rub, L.; Abdelrahman, H.; Johar, A.-R.; Alhussain, H.; Hadi, H.; Eltai, N. Antibiotics Prescribing in Intensive Care Settings during the COVID-19 Era: A Systematic Review. Antibiotics 2021, 10, 935. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Million, M.; Gautret, P.; Colson, P.; Cortaredona, S.; Giraud-Gatineau, A.; Honoré, S.; Gaubert, J.-Y.; Fournier, P.-E.; Tissot-Dupont, H.; et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med. Infect. Dis. 2020, 36, 101791. [Google Scholar] [CrossRef]
- Kumar, J.; Jain, S.; Meena, J.; Yadav, A. Efficacy and safety of hydroxychloroquine/chloroquine against SARS-CoV-2 infection: A systematic review and meta-analysis. J. Infect. Chemother. 2021, 27, 882–889. [Google Scholar] [CrossRef]
- Neto, A.G.M.; Lo, K.B.; Wattoo, A.; Salacup, G.; Pelayo, J.; DeJoy, R.; Do, R.B.; Gul, F.; Peterson, E.; Albano, J.; et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J. Med. Virol. 2020, 93, 1489–1495. [Google Scholar] [CrossRef]
- Cao, J.; Tu, W.-J.; Cheng, W.; Yu, L.; Liu, Y.-K.; Hu, X.; Liu, Q. Clinical Features and Short-term Outcomes of 102 Patients with Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 748–755. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85. [Google Scholar]
- Langham, R.G.; Bellomo, R.; D’ Intini, V.; Endre, Z.; Hickey, B.B.; McGuinness, S.; Phoon, K.S.; Salamon, K.; Woods, J.; Gallagher, M.P.; et al. KHA-CARI guideline: KHA-CARI adaptation of the KDIGO Clinical Practice Guideline for Acute Kidney Injury. Nephrology 2014, 19, 261–265. [Google Scholar] [CrossRef]
- Chand, S.; Kapoor, S.; Orsi, D.; Fazzari, M.J.; Tanner, T.G.; Umeh, G.C.; Islam, M.; Dicpinigaitis, P.V. COVID-19-Associated Critical Illness—Report of the First 300 Patients Admitted to Intensive Care Units at a New York City Medical Center. J. Intensive Care Med. 2020, 35, 963–970. [Google Scholar] [CrossRef]
- Bowe, B.; Cai, M.; Xie, Y.; Gibson, A.K.; Maddukuri, G.; Al-Aly, Z. Acute Kidney Injury in a National Cohort of Hospitalized US Veterans with COVID-19. Clin. J. Am. Soc. Nephrol. 2020, 16, 14–25. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Martín-Del-Campo, F.; Ruvalcaba-Contreras, N.; Velázquez-Vidaurri, A.L.; Cueto-Manzano, A.M.; Rojas-Campos, E.; Cortés-Sanabria, L.; Espinel-Bermúdez, M.C.; Hernández-González, S.O.; Nava-Zavala, A.H.; Fuentes-Orozco, C.; et al. Morbid obesity is associated with mortality and acute kidney injury in hospitalized patients with COVID-19. Clin. Nutr. ESPEN 2021, 45, 200–205. [Google Scholar] [CrossRef]
- Schiffl, H.; Lang, S.M. Obesity, acute kidney injury and outcome of critical illness. Int. Urol. Nephrol. 2016, 49, 461–466. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [Green Version]
- Alhazzani, W.; Evans, L.; Alshamsi, F.; Møller, M.H.; Ostermann, M.; Prescott, H.C.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; et al. Surviving Sepsis Campaign Guidelines on the Management of Adults with Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit. Care Med. 2021, 49, e219–e234. [Google Scholar] [CrossRef]
- Cui, X.; Yu, X.; Wu, X.; Huang, L.; Tian, Y.; Huang, X.; Zhang, Z.; Cheng, Z.; Guo, Q.; Zhang, Y.; et al. Acute Kidney Injury in Patients with the Coronavirus Disease 2019: A Multicenter Study. Kidney Blood Press. Res. 2020, 45, 612–622. [Google Scholar] [CrossRef]
- Bezerra, R.; Teles, F.; Mendonca, P.B.; Damte, T.; Likaka, A.; Ferrer-Miranda, E.; de Albuquerque, J.O.; Filho, J.L.D.L. Outcomes of critically ill patients with acute kidney injury in COVID-19 infection: An observational study. Ren. Fail. 2021, 43, 911–918. [Google Scholar] [CrossRef]
- Joannidis, M.; Truebsbach, S.; Bijuklic, K.; Schratzberger, P.; Dunzendorfer, S.; Wintersteiger, S.; Lhotta, K.; Mayer, G.; Wiedermann, C. Neutrophil Transmigration in Renal Proximal Tubular LLC-PK1 Cells. Cell. Physiol. Biochem. 2004, 14, 101–112. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Ronco, C.; Wald, R.; Mårtensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.; Lankadeva, Y.; Vaara, S.T.; et al. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.-L.; De Mendonca, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, D.C.; Franco, M.D.C.P.; dos Santos, D.R.P.; Santos, M.C.; Maltoni, I.S.; Mascotte, F.; de Souza, A.A.; Pietrobom, P.M.; Medeiros, E.A.; Ferreira, P.R.A.; et al. Acute kidney injury: Incidence, risk factors, and outcomes in severe COVID-19 patients. PLoS ONE 2021, 16, e0251048. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H.; Connors, J.M.; Warkentin, T.E.; Thachil, J.; Levi, M. The unique characteristics of COVID-19 coagulopathy. Crit. Care 2020, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Copaja-Corzo, C.; Hueda-Zavaleta, M.; Benites-Zapata, V.; Rodriguez-Morales, A. Antibiotic Use and Fatal Outcomes among Critically Ill Patients with COVID-19 in Tacna, Peru. Antibiotics 2021, 10, 959. [Google Scholar] [CrossRef]
- Álvarez-Moreno, C.; Valderrama-Beltrán, S.; Rodriguez-Morales, A. Implications of Antibiotic Use during the COVID-19 Pandemic: The Example of Associated Antimicrobial Resistance in Latin America. Antibiotics 2021, 10, 328. [Google Scholar] [CrossRef]
- Movahed, S.M.M.; Akhavizadegan, H.; Dolatkhani, F.; Nejadghaderi, S.A.; Aghajani, F.; Gangi, M.F.; Ghazi, Z.; Ghasemi, H. Different incidences of acute kidney injury (AKI) and outcomes in COVID-19 patients with and without non-azithromycin antibiotics: A retrospective study. J. Med. Virol. 2021, 93, 4411–4419. [Google Scholar] [CrossRef]
- Petejova, N.; Martinek, A.; Zadrazil, J.; Kanova, M.; Klementa, V.; Sigutova, R.; Kacirova, I.; Hrabovsky, V.; Svagera, Z.; Stejskal, D. Acute Kidney Injury in Septic Patients Treated by Selected Nephrotoxic Antibiotic Agents—Pathophysiology and Biomarkers—A Review. Int. J. Mol. Sci. 2020, 21, 7115. [Google Scholar] [CrossRef]
- Elliott, B.P.; Tang, M.M.; Madden, J.A.; Markert, R.J.; Burdette, S.D.; Pleiman, C.M.; Speelmon, E.C. A retrospective cohort study assessing acute kidney injury and renal recovery among septic patients empirically treated with vancomycin piperacillin-tazobactam versus vancomycin cefepime. Intern Emerg. Med. 2022, 17, 91–99. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Qiu, S.; Dong, Z.; Xiang, X.; Zhang, D. MBD2 upregulates miR-301a-5p to induce kidney cell apoptosis during vancomycin-induced AKI. Cell Death Dis. 2017, 8, e3120. [Google Scholar] [CrossRef]
- Beović, B.; Doušak, M.; Ferreira-Coimbra, J.; Nadrah, K.; Rubulotta, F.; Belliato, M.; Berger-Estilita, J.; Ayoade, F.; Rello, J.; Erdem, H. Antibiotic use in patients with COVID-19: A ’snapshot’ Infectious Diseases International Research Initiative (ID-IRI) survey. J. Antimicrob. Chemother. 2020, 75, 3386–3390. [Google Scholar] [CrossRef]
- Liu, C.; Wen, Y.; Wan, W.; Lei, J.; Jiang, X. Clinical characteristics and antibiotics treatment in suspected bacterial infection patients with COVID-19. Int. Immunopharmacol. 2020, 90, 107157. [Google Scholar] [CrossRef]
- Chu, K.H.; Tsang, W.K.; Tang, C.S.; Lam, M.F.; Lai, F.M.; To, K.F.; Fung, K.S.; Tang, H.L.; Yan, W.W.; Chan, H.W.; et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005, 67, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.; Wen, Y.; Duan, Y.; Su, H.; Cao, W.; Xiao, M.; Ma, J.; Zhou, Y.; Chen, G.; Jiang, W.; et al. Clinicopathological Features and Outcomes of Acute Kidney Injury in Critically Ill COVID-19 with Prolonged Disease Course: A Retrospective Cohort. J. Am. Soc. Nephrol. 2020, 31, 2205–2221. [Google Scholar] [CrossRef]
- Zeshan, B.; Karobari, M.I.; Afzal, N.; Siddiq, A.; Basha, S.; Basheer, S.N.; Peeran, S.W.; Mustafa, M.; Daud, N.H.A.; Ahmed, N.; et al. The Usage of Antibiotics by COVID-19 Patients with Comorbidities: The Risk of Increased Antimicrobial Resistance. Antibiotics 2021, 11, 35. [Google Scholar] [CrossRef]
- Ramírez-Lozada, T.; Loranca-García, M.C.; Fuentes-Venado, C.E.; Rodríguez-Cerdeira, C.; Ocharan-Hernández, E.; Soriano-Ursúa, M.A.; Farfán-García, E.D.; Chávez-Gutiérrez, E.; Ramírez-Magaña, X.; Robledo-Cayetano, M.; et al. Does the Fetus Limit Antibiotic Treatment in Pregnant Patients with COVID-19? Antibiotics 2022, 11, 252. [Google Scholar] [CrossRef]
- Moyano, L.M.; Leon-Jimenez, F.; Cavalcanti, S.; Ocaña, V. Uso responsable de los antibióticos en COVID-19 en Perú: Ad portas de otra pandemia!! Atención Primaria 2021, 54, 102172. [Google Scholar] [CrossRef]
- Han, X.; Ye, Q. Kidney involvement in COVID-19 and its treatments. J. Med. Virol. 2020, 93, 1387–1395. [Google Scholar] [CrossRef]
- Pérez-Lazo, G.; Soto-Febres, F.; Morales-Moreno, A.; Cabrera-Enríquez, J.A.; Díaz-Agudo, J.; Rojas-Tovar, R.; Arenas-Ramírez, B.; Illescas-Mucha, R. Uso racional de antimicrobianos en tiempos de COVID-19 en Perú: Rol de los programas de optimización del uso de antimicrobianos e intervenciones desde el punto de vista de control de infecciones. Horiz. Médico 2021, 21, e1254. [Google Scholar] [CrossRef]
| Variable | n = 349 | % |
|---|---|---|
| Sex | ||
| Female | 120 | 34.38 |
| Male | 229 | 65.62 |
| Age | 64 | (55–71) * |
| Creatinine † | 0.76 | (0.58–0.98) * |
| Weight † | 75 | (67–83.5) * |
| Size † | 1.69 | (1.65–1.73) * |
| BMI † | 26.28 | (24.14–29.06) * |
| Time of illness (days) † | 8 | (7–13) * |
| Days of hospitalization † | 5 | (2–10) * |
| Comorbidities † | ||
| Hypertension | 147 | 42.24 |
| Diabetes Mellitus | 93 | 26.72 |
| Neoplasms | 1 | 0.29 |
| Cardiovascular disease | 31 | 8.91 |
| Pulmonary Disease | 16 | 4.60 |
| Diabetes + Hypertension | 63 | 18.05 |
| Complications of COVID-19 | ||
| Sepsis | 36 | 10.32 |
| Shock | 33 | 9.46 |
| Mechanical ventilation ¥ | 148 | 42.41 |
| Venous thromboembolism | 5 | 1.43 |
| Medications † | ||
| Aminoglycosides | 14 | 4.01 |
| Vancomycin | 51 | 14.61 |
| Ivermectin | 40 | 11.46 |
| Azithromycin | 59 | 16.91 |
| Tociluzumab | 9 | 2.58 |
| Corticosteroids | 326 | 93.41 |
| Ivermectin + Azithromycin | 27 | 7.74 |
| Ivermectin + Azithromycin + Corticosteroids | 23 | 6.59 |
| Azithromycin + Corticosteroids | 55 | 15.76 |
| Renal Replacement Therapy † | 51 | 14.61 |
| Recovery † | 39 | 11.27 |
| Death † | 273 | 78.22 |
| Variables | Acute Renal Failure | p | |
|---|---|---|---|
| No n (%) | Yes n (%) | ||
| Sex | |||
| Female | 109 (90.83) | 11 (9.17) | 0.455 * |
| Male | 202 (88.21) | 27 (11.79) | |
| Age | 64 (55–72) ¥ | 59 (55–66) ¥ | 0.034 † |
| Weight | 75 (67–82) ¥ | 76 (68–89) ¥ | 0.289 † |
| Size | 1.69 (1.65–1.73) ¥ | 1.65 (1.59–1.70) ¥ | 0.001 † |
| BMI | 26.07 (23.74–28.73) ¥ | 27.69 (25.71–31.23) ¥ | 0.002 † |
| Time of illness (days) | 9 (7–13) ¥ | 7 (5–7) ¥ | <0.001 † |
| Days of hospitalization | 4 (2–8) ¥ | 28 (19–43) ¥ | <0.001 † |
| Comorbidities | |||
| Hypertension | 132 (89.80) | 15 (10.20) | 0.714 * |
| Diabetes Mellitus | 85 (91.40) | 8 (8.60) | 0.403 * |
| Neoplasms | -- | --- | --- |
| Cardiovascular disease | 27 (87.10) | 4 (12.90) | 0.761 ** |
| Pulmonary Disease | 15 (93.75) | 1 (6.25) | 1.000 ** |
| Diabetes + Hypertension | 59 (93.65) | 4(6.35) | 0.201 * |
| Complications of COVID-19 | |||
| Sepsis | 8 (22.22) | 28 (77.78) | <0.001 ** |
| Shock | 8 (24.24) | 25 (75.76) | <0.001 ** |
| Mechanical ventilation | 114 (76.51) | 35 (23.49) | <0.001 * |
| Venous thromboembolism | 2 (40.00) | 3 (60.00) | 0.010 ** |
| Drugs used in the treatment of COVID-19 | |||
| Aminoglycosides | 13 (92.86) | 1 (7.14) | 1.000 ** |
| Vancomycin | 21 (41.18) | 30 (58.82) | <0.001 * |
| Ivermectin | 33 (82.50) | 7 (17.50) | 0.174 ** |
| Azithromycin | 46 (77.97) | 13 (22.03) | 0.003 * |
| Tociluzumab | 8 (88.89) | 1 (11.11) | 1.000 ** |
| Corticosteroids | 293 (89.88) | 33 (10.12) | 0.090 ** |
| Ivermectin + Azithromycin | 20 (74.06) | 7 (25.93) | 0.018 ** |
| Ivermectin + Azithromycin + Corticosteroids | 18 (78.26) | 5 (21.74) | 0.090 ** |
| Azithromycin + Corticosteroids | 44 (80.00) | 11 (20.00) | 0.018 * |
| Variables | Simple Regression | Multiple Regression | ||||
|---|---|---|---|---|---|---|
| cPR | 95% CI | p | aPR | 95% CI | p | |
| Sex | ||||||
| Female | Ref. | - | - | - | - | - |
| Male | 1.29 | 0.66–2.50 | 0.459 | - | - | - |
| Age | 0.98 | 0.97–0.99 | 0.033 | 1.00 | 0.98–1.03 | 0.938 |
| BMI | 1.11 | 1.05–1.17 | <0.001 | 1.00 | 0.96–1.06 | 0.785 |
| Time of illness (days) | 0.81 | 0.72–0.91 | <0.001 | 0.92 | 0.84–1.00 | 0.078 |
| Days of hospitalization | 1.04 | 1.03–1.04 | <0.001 | 1.00 | 0.99–1.02 | 0.596 |
| Comorbidities | ||||||
| Hypertension | 0.89 | 0.48–1.65 | 0.715 | - | - | - |
| Diabetes Mellitus | 0.73 | 0.35–1.54 | 0.409 | - | - | - |
| Cardiovascular disease | 1.20 | 0.46–3.17 | 0.709 | - | - | - |
| Pulmonary Disease | 0.56 | 0.08–3.84 | 0.556 | - | - | - |
| Diabetes + Hypertension | 0.53 | 0.19–1.45 | 0.219 | - | - | - |
| Complications of COVID-19 | ||||||
| Sepsis | 24.34 | 12.89–45.95 | <0.001 | 2.86 | 1.26–6.43 | 0.012 |
| Shock | 18.41 | 10.45–32.47 | <0.001 | 2.49 | 1.28–4.86 | 0.007 |
| Mechanical ventilation | 15.84 | 4.96–50.62 | <0.001 | 9.11 | 1.23–67.57 | 0.031 |
| Drugs used in the treatment of COVID-19 * | ||||||
| Aminoglycosides | 0.65 | 0.95–4.39 | 0.656 | - | - | - |
| Vancomycin | 21.91 | 10.64–45.11 | <0.001 | 3.15 | 1.19–8.27 | 0.020 |
| Ivermectin | 1.74 | 0.82–3.70 | 0.147 | - | - | - |
| Azithromycin | 2.55 | 1.39–4.70 | 0.003 | 3.10 | 0.23–42.31 | 0.396 |
| Corticosteroids | 0.47 | 0.20–1.08 | 0.075 | - | - | - |
| Ivermectin + Azithromycin | 2.69 | 1.30–5.54 | 0.007 | 1.97 | 0.74–5.25 | 0.177 |
| Ivermectin + Azithromycin + Corticosteroids | 2.15 | 0.93–4.98 | 0.075 | - | - | - |
| Azithromycin + Corticosteroids | 2.18 | 1.15–4.13 | 0.017 | 0.39 | 0.03–5.02 | 0.475 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romaní, L.; León-Figueroa, D.A.; Rafael-Navarro, D.; Barboza, J.J.; Rodriguez-Morales, A.J. Association between the Use of Antibiotics and the Development of Acute Renal Injury in Patients Hospitalized for COVID-19 in a Hospital in the Peruvian Amazon. J. Clin. Med. 2022, 11, 4493. https://doi.org/10.3390/jcm11154493
Romaní L, León-Figueroa DA, Rafael-Navarro D, Barboza JJ, Rodriguez-Morales AJ. Association between the Use of Antibiotics and the Development of Acute Renal Injury in Patients Hospitalized for COVID-19 in a Hospital in the Peruvian Amazon. Journal of Clinical Medicine. 2022; 11(15):4493. https://doi.org/10.3390/jcm11154493
Chicago/Turabian StyleRomaní, Luccio, Darwin A. León-Figueroa, David Rafael-Navarro, Joshuan J. Barboza, and Alfonso J. Rodriguez-Morales. 2022. "Association between the Use of Antibiotics and the Development of Acute Renal Injury in Patients Hospitalized for COVID-19 in a Hospital in the Peruvian Amazon" Journal of Clinical Medicine 11, no. 15: 4493. https://doi.org/10.3390/jcm11154493
APA StyleRomaní, L., León-Figueroa, D. A., Rafael-Navarro, D., Barboza, J. J., & Rodriguez-Morales, A. J. (2022). Association between the Use of Antibiotics and the Development of Acute Renal Injury in Patients Hospitalized for COVID-19 in a Hospital in the Peruvian Amazon. Journal of Clinical Medicine, 11(15), 4493. https://doi.org/10.3390/jcm11154493








