Abstract
Coated urethral catheters were introduced in clinical practice to reduce the risk of catheter-acquired urinary tract infection (CAUTI). We aimed to systematically review the incidence of CAUTI and adverse effects in randomized clinical trials of patients requiring indwelling bladder catheterization by comparing coated vs. non-coated catheters. This review was performed according to the 2020 PRISMA framework. The incidence of CAUTI and catheter-related adverse events was evaluated using the Cochran–Mantel–Haenszel method with a random-effects model and reported as the risk ratio (RR), 95% CI, and p-values. Significance was set at p < 0.05 and a 95% CI. Twelve studies including 36,783 patients were included for meta-analysis. There was no significant difference in the CAUTI rate between coated and non-coated catheters (RR 0.87 95% CI 0.75–1.00, p = 0.06). Subgroup analysis demonstrated that the risk of CAUTI was significantly lower in the coated group compared with the non-coated group among patients requiring long-term catheterization (>14 days) (RR 0.82 95% CI 0.68–0.99, p = 0.04). There was no difference between the two groups in the incidence of the need for catheter exchange or the incidence of lower urinary tract symptoms after catheter removal. The benefit of coated catheters in reducing CAUTI risk among patients requiring long-term catheterization should be balanced against the increased direct costs to health care systems when compared to non-coated catheters.
1. Introduction
The word catheter is derived from the ancient Greek kathiénai, literally meaning “to thrust into” or “to send down” [1]. In use for more than 3500 years, urethral catheters are a bane and boon for patients and urologists alike as they may pose a risk to patients requiring long-term catheterization. The most common problems include hematuria, catheter encrustation requiring frequent catheter exchange, and catheter-acquired urinary tract infection (CAUTI).
With technical advancements in bioengineering and materials science, several types of indwelling catheters were developed to prevent CAUTI and improve patient tolerance. Coating agents were added to catheter surfaces to improve antimicrobial proprieties and to provide robust antibiofilm/antimicrobial activity, without causing an increase in patient discomfort [2,3]. Coated catheters can be divided into two types: those coated in antifouling materials, and those impregnated with bactericidal molecules.
Antifouling substances do not kill the bacteria but rather perturb their ability to colonize surfaces, preventing the formation of biofilms in the bladder or on the catheter surface. The most common antifouling materials are hydrogel and polytetrafluoroethylene (PTFE). Hydrogel catheters may reduce encrustation via forming hydration layers on the catheter surface; however, studies have demonstrated a similar incidence of nosocomial CAUTI and a higher rate of blockage when compared to standard silicone catheters [4]. PTFE-coated catheters seem to be more suitable candidates to inhibit biofilm formation because of their low coefficient of friction. Unfortunately, studies have demonstrated that PTFE-coated catheters are not superior to hydrogel or standard silicone catheters in preventing CAUTI [2].
Catheters can also be coated with antimicrobial agents such as metal ions (i.e., silver, gold, and/or palladium), antibiotics, and nitrofurazone. Among bactericidal-coated catheters, silver-coated catheters are the most popular and widely tested catheters. The release of silver ions into the bladder induces oxidative stress and disrupts bacteria membrane and proteins, but antimicrobial efficacy may vary with the silver-coated substance used. Although in vitro and in vivo studies have shown great efficacy in preventing infections [5], these have not necessarily translated to clear benefits in clinical trials [6].
Antibiotic-coated catheters are less frequently used, especially with the increased frequency of having multi-drug-resistant bacteria [2]. Nitrofurazone was a promising coating agent in in vivo and in vitro studies, but it was not efficient in preventing infections in clinical studies and caused patient discomfort [7].
This study aimed to systematically review the incidence of CAUTI and its adverse effects in randomized clinical trials of patients requiring indwelling bladder catheterization (transurethral or suprapubic) by comparing coated vs. non-coated catheters.
2. Materials and Methods
2.1. Aim of This Review
The present study aims to systematically review the incidence of CAUTI in patients requiring indwelling bladder catheterization by comparing coated vs. non-coated catheters. The primary outcome was the CAUTI rate between the two types of catheters. The secondary outcomes were the CAUTI rate according to catheterization time (cut-off: 14 days) and the rate of catheter-related adverse events (i.e., hematuria, need for catheter exchange or catheter removal, urinary symptoms after catheter removal). Additionally a cost-effectiveness analysis was performed.
2.2. Literature Search
This review was performed according to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) framework. A broad literature search was performed on 1 May 2022, using MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials. Medical Subject Heading (MeSH) terms and keywords such as (urinary tract infection OR infections OR sepsis) AND (short term OR long OR indwelling) AND (standard urethral catheter OR impregnated urethral catheter OR silicone OR hydrogel OR antibiotic coated OR silver-impregnated) were used. The search was restricted to English papers only. No date limits were imposed. Pediatric and animal studies were excluded. The review protocol was submitted for registration in PROSPERO (receipt #332889).
2.3. Selection Criteria
The Patient Intervention Comparison Outcome Study (PICOS) model was used to frame and answer the clinical question. P: adults requiring bladder catheterization; Intervention: coated catheters; Comparison: non-coated catheters; Outcome: CAUTI and catheter-related adverse effects; Study type: prospective and randomized studies. Patients were assigned to two groups according to the type of catheter (coated vs. non-coated catheters).
2.4. Study Screening and Selection
Two independent authors screened all retrieved records through Covidence Systematic Review Management® (Veritas Health Innovation, Melbourne, Australia). Discrepancies were solved by a third author. Studies were included based on PICOS eligibility criteria. Only prospective and randomized studies were accepted. Meeting abstracts, retrospective, and prospective nonrandomized studies were excluded. Case reports, reviews, letters to the editor, and editorials were excluded. The full text of the screened papers was selected if found relevant to the purpose of this study.
2.5. Statistical Analysis
The incidence of CAUTI and catheter-related adverse effects was evaluated using the Cochran–Mantel–Haenszel method with a random-effects model and reported as the risk ratio (RR), 95% CI, and p-values. For studies with 3 groups of patients, intervention groups were combined to create a single pair-wise comparison [8]. Analyses were two tailed and significance was set at p < 0.05 and a 95% CI. Study heterogeneity was assessed utilizing the I2 value. Substantial heterogeneity was defined as an I2 value > 50%. Meta-analysis was performed using Review Manager (RevMan) 5.4 software by Cochrane Collaboration. The quality assessment of the included studies was performed using RoB 2 [9].
3. Results
The literature search retrieved 2689 studies. After eliminating 297 duplicates, 2392 studies were left for screening. Another 2326 papers were further excluded against the title and abstract screening because they were unrelated to the purpose of this review. The full texts of the remaining 66 studies were screened and 54 papers were further excluded. Finally, 12 studies were accepted and included for meta-analysis. Figure 1 shows the PRISMA flow diagram.
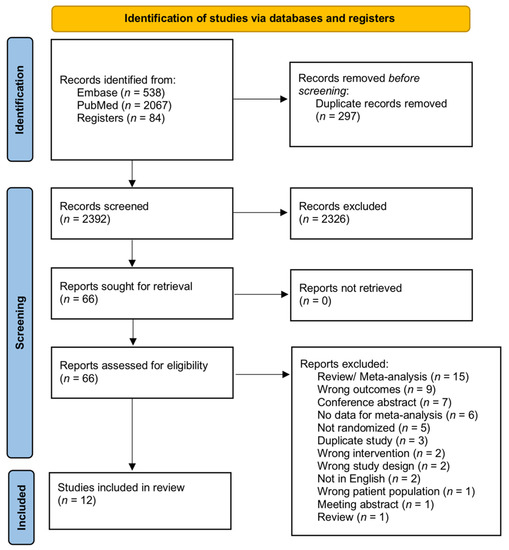
Figure 1.
PRISMA diagram of this study.
3.1. Study Characteristics and Quality Assessment
Twelve prospective, randomized studies compared coated vs. non-coated catheters in patients requiring an indwelling catheter [7,10,11,12,13,14,15,16,17,18,19,20]. No study with a suprapubic catheter was retrieved. Study characteristics are summarized in Table 1. Only one study had catheters with antibacterial/antifouling coating (i.e., hydrogel) [16] and the other 11 had catheters coated with bactericidal molecules, i.e., pure silver ions [7,10,12,13,18], noble ions (silver, gold, palladium) [14], or silver ions mixed with hydrogel [19], nitrofurazone [7,11,15,17], and a polymer of zinc oxide bonded carbon nanotube [20]. There were 36,783 patients included in 12 studies: 19,404 patients in the coated catheter group and 17,379 in the non-coated catheter group.

Table 1.
Characteristics of the included studies. NA: not available. UTI: urinary tract infections. SCI: spinal cord injury.
Table 2 shows data on pathogen species isolated in urine culture. The most common detected pathogens were Escheria coli, Enterococcus, Pseudomonas spp., Klebsiella spp., Gram-positive cocci, including Staphylococcus aureus, followed by Candida spp. and Yeasts. Polymicrobial infections were uncommon.

Table 2.
Pathogens isolated in urine cultures.
Figure 2 shows the details of quality assessment in the included studies. Six studies showed a low overall risk of bias and the remaining six demonstrated some concerns. The most common reason for bias arose from the randomization process, followed by bias due to missing outcome data.
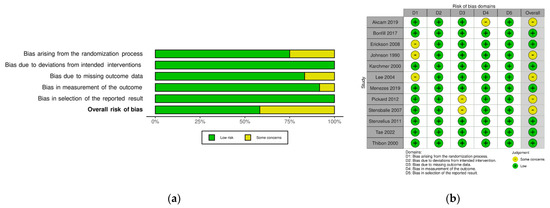
Figure 2.
Risk of bias of the included study (Rob2): (a) review authors’ judgments about each risk of bias item presented as percentages across all included studies; (b) review authors’ judgments about each risk of bias item for each included study.
3.2. Meta-Analysis of CAUTI
Meta-analysis from 12 studies (19,328 cases in the coated and 17,287 cases in the non-coated group) showed that the risk of CAUTI did not differ significantly between the groups (RR 0.87 95% CI 0.75–1.00, p = 0.06) (Figure 3). There was no significant heterogeneity among the studies (I2 = 22%). Subgroup analysis for catheter dwelling time demonstrated that the risk of CAUTI was significantly lower in the coated group compared with the non-coated group (RR 0.82 95% CI 0.68–0.99, p = 0.04). Only one study reported the rate of sepsis and another the rate of cystitis, making meta-analysis not feasible.
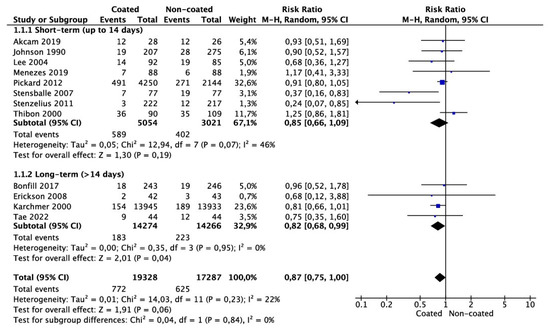
Figure 3.
Meta-analysis of CAUTI incidence.
3.3. Meta-Analysis of Need for Catheter Removal or Catheter Exchange
Meta-analysis from three studies (499 cases in the coated and 502 cases in the non-coated group) showed no significant risk in the need for catheter removal or exchange (OR 0.93 95% CI 0.52–1.65, p = 0.80) (Figure 4). There was no significant heterogeneity among the studies (I2 = 0%).
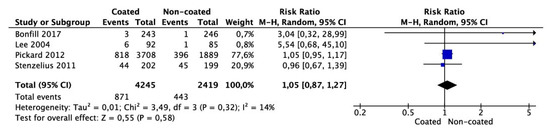
Figure 4.
Meta-analysis of need for removal/change of catheter.
3.4. Meta-Analysis of Lower Urinary Tract Symptoms at Follow-Up after Removal of Catheter
Meta-analysis from four studies (4245 cases in the coated and 2419 cases in the non-coated group) showed that the number of patients complaining of lower urinary tract symptoms after catheter removal did not differ between the groups (OR 1.05 95% CI 0.87–1.17, p = 0.58) (Figure 5). There was no significant heterogeneity among the studies (I2 = 14%).
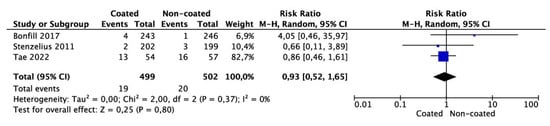
Figure 5.
Meta-analysis of the number of patients reporting lower urinary tract symptoms at follow-up after removal of catheter.
3.5. Meta-Analysis of Hematuria Incidence
There was only one study reporting hematuria, making meta-analysis not feasible.
4. Discussion
In our meta-analysis, we found no difference in the incidence of CAUTI in patients with coated and non-coated catheters even though subgroup analysis regarding dwelling time (short- vs. long-term catheterization) showed a significantly lower risk for CAUTI in patients using coated catheters (p = 0.04). The interest in developing catheters that can decrease the risk of CAUTI started in 1979 with Akyama and Okamoto, who were the first to describe a decreased risk for bacteria associated with coated urinary catheters [21]. Other studies reported only a “protective effect” of coated urinary tract catheters but these trials were performed with a small number of patients [19,22,23]. Thibon et al. evaluated the effects of coated catheters with hydrogel and silver salts on the incidence of hospital-acquired urinary tract infection and showed no protective effect of coated catheters [19]. With regard to studies that reported a significant reduction in CAUTI in patients on silver-alloy catheters [12,22,23], some methodological critiques were made to these studies as they were performed by randomizing the hospital unit instead of the individual patients, which could lead to bias since hospital units can differ significantly in terms of catheter placement technique, indwelling time, and patient comorbidities.
Another confounding factor in considering indwelling catheters and CAUTI risk is the surgical procedure performed. Ideally, catheters should be removed at the earliest possible time. The misconception that the use of antibiotic- or silver-coated catheters has better outcomes in patients undergoing urological procedures needing a short duration of catheterization was refuted in a study by Pickard et al. [7]. Likewise, Erickson et al. compared silicone- and hydrogel-coated latex catheters in men needing short-term postoperative bladder drainage after urethral surgeries and showed no absolute advantage for either type [16]. Menzies et al. compared nitrofurazone-coated and non-coated urinary catheters in kidney transplant recipients and did not find any difference in the rate of urinary tract infection (8% and 6.8%, p = 0.99) among the two groups [11]. Instead, the incidence of adverse events was more frequent in the nitrofurazone-impregnated silicone urinary catheter group (46.6% and 26.1%, p = 0.007) [11]. Tae et al. studied the incidence of CAUTI in patients who underwent radical cystectomy with an orthotopic neobladder for bladder cancer and received either a coated or conventional non-coated catheter for 2 weeks [20]. The incidence of CAUTI 2 weeks after radical cystectomy and orthotopic neobladder was 21.95% (case) and 27.27% (control), with no significant difference between the two groups. However, asymptomatic bacteriuria was significantly lower in the antibiotic-coated catheter group [20]. The authors concluded that the prevention of biofilm formation on coated catheters has the potential to prevent CAUTI. One explanation for why the CAUTI rate was similar between the groups is that the duration of catheterization was short for this cohort (2 weeks); as we demonstrated in our meta-analysis, coated catheters may only be of benefit during longer catheterization durations. When taken together, the results of the present meta-analysis (Figure 3) support the safety of using non-coated catheters in patients undergoing surgical procedures in which catheter duration is expected to be less than 14 days. For patients requiring long-term catheters, the use of coated catheters may lower the risk of CAUTI together with routine catheter and/or drainage bag changes [24].
In a randomized trial of 17 patients, Priefer et al. observed that the practice of monthly catheter exchange resulted in fewer symptomatic urinary tract infections when compared to patients in whom catheters were exchanged at the time of either obstruction or infection [25]. In contrast, White et al. found that when patients were divided into short- versus long-term catheter exchange intervals, the incidence of infection was greater in those whose catheters were changed in 2 weeks or less [26]. Only 15.4% remained free of infection after one month in this group, whereas 80% of those whose catheters were changed between 4 and 6 weeks remained free of infection after 6 weeks. The number of exchange and the number of nurses who performed the catheter exchange might have influenced the CAUTI risk. Indeed, there is insufficient evidence to assess the value of different policies for replacing long-term urinary catheters on patient outcomes [24]. We found that the incidence of CAUTI was decreased when maintained well even for a long duration (RR 0.82 95% CI 0.68–0.99, p = 0.04). Thus, maybe the implementation of protocols using coated catheters could be of interest to prevent encrustation, obstruction, and infection, and increase the intervals between changes.
Adverse events related to catheter use, such as hematuria, irritative lower urinary tract symptoms, or the need for catheter exchange or removal, were investigated as secondary endpoints in our study. Only one article classified the infections by differentiating into cystitis or urinary sepsis, preventing our analysis from evaluating these secondary outcomes. Furthermore, no studies comparing coated versus non-coated catheters evaluated rates of pyelonephritis. There were insufficient data to determine the relative influence of coated urinary catheters on hematuria. Hematuria, which was reported in only a single study, occurred in 18/243 (7.4%) patients in the silver alloy-coated catheter group and 20/246 (8.1%) patients using conventional catheters and this was not significantly different between groups [18]. Three studies involving a total of 1001 patients reported on the need for catheter removal or exchange. Overall, the need for urinary catheter exchange or removal was similar between non-coated and coated catheters [14,18,20]. In our analysis, four studies, which included 6664 patients, provided information on lower urinary tract symptoms (LUTS) after catheter removal [7,14,17,18]. LUTS ranged from 1.2% to 22% in the coated group and from 0.4% to 22.6% in the control group. Compared to standard urinary catheters, we found that the use of coated catheters did not significantly increase the risk of LUTS.
Salient to the discussion of comparing antibiotic- or alloy-coated catheters to conventional silicone/latex catheters is cost-effectiveness. Overall, four studies incorporate cost-effectiveness analyses [12,27,28,29]. Cost analyses can be further stratified into comparisons of cost among different catheters and their associated components as well as analyses incorporating both catheter costs as well as the estimated cost of consequent nosocomial urinary tract infections. The latter cost assessment can be challenging to perform as it may be difficult to delineate how much a CAUTI contributes to the length of hospital stay or utilization of hospital resources. Nonetheless, several studies have provided estimates of these costs.
In a 12-month randomized crossover trial comparing CAUTI rates in patients with silver alloy-coated versus non-coated catheters, the use of silver alloy-coated catheters was associated with a 2.5-fold higher direct material cost when compared to non-coated catheters [12]. However, when taking into account the estimated costs associated with CAUTI and associated sequela (i.e., bloodstream infection, upper tract involvement, need for intensive care unit stay) within their study population, the use of silver alloy-coated catheters yielded significant aggregate savings due to a reduction in CAUTI rates. The lower and higher estimate of cost savings were USD 14,000 and 500,000, respectively [12]. This finding was similarly demonstrated by Bologna et al., where the use of silver alloy-coated catheters was predicted to lead to superior cost savings over standard latex catheters [27]. However, this cost analysis was limited to a single institution, whose differential CAUTI rate between silver alloy-coated and standard silicone catheters significantly differed from that of the other four institutions included in the analysis. The authors also relied on estimates of cost savings by attributing CAUTI as a major driver of hospital and intensive care unit length of stay [27]. Importantly, a recent prospective crossover study comparing silver alloy-coated to standard silicone catheters demonstrated a 12% risk reduction against CAUTI with the use of silver alloy-coated catheters. This is contrary to a prior study that assumed a 30–40% relative reduction in the CAUTI rate with the use of silver alloy-coated catheters in their cost-effectiveness analyses [29]. Therefore, if the difference in the CAUTI rate between catheter types is modest, the cost savings with the use of silver alloy-coated catheters may be negated and may not outweigh the increased direct costs associated with these catheters [29].
In another large study involving 7102 patients admitted to NHS England hospitals, cost-effectiveness analysis demonstrated that nitrofurazone-coated catheters were the least costly [30]. When compared to nitrofurazone-coated catheters, PTFE and silver alloy-coated catheters cost on average USD 11 and 19 more, respectively. Based on their analysis, nitrofurazone-coated catheters had an approximately 70% chance of being a cost-saving and had an 84% chance of having an incremental cost per quality-adjusted life year [incremental cost-effectiveness ratio of < GBP 300,000 (USD 47,500), the willingness-to-pay threshold suggested by the UK National Institute of Health and Clinical Excellence] [30]. Conversely, silver alloy-coated catheters had a 0% chance of being cost-effective at all threshold values between GBP 0 and 50,000. Nonetheless, nitrofurazone-coated catheters were associated with greater patient discomfort and the cost-saving estimates were based on assumptions of large attribution of CAUTI as the main driver of the length of hospital stay. These results, therefore, do not provide robust evidence of cost-effectiveness for one catheter over another within a universal health care system [30].
When taken together, the use of metal alloy-coated or antibiotic-coated catheters may increase direct costs to health care systems when compared to standard silicone or latex catheters; however, it is unclear whether the risk reduction in the CAUTI rate (and associated health care utilization) outweighs this cost.
Our study has some limitations. This study precludes us from making absolute deductions on which coated catheters are better for minimizing CAUTI, and better clinical trials should address this in the future. We could deduce that patients with long-term indwelling catheters could be the ideal candidates for coated catheters and it is necessary to provide proper training to patients and caregivers for catheter maintenance. This could help optimize the cost-effectiveness for the patients as, from our results, due to paucity of information and likely variability in health care systems, it was difficult to make concrete conclusions on cost-effectiveness. Finally, there was no randomized clinical trial comparing coated vs. non-coated suprapubic catheters, considering that UTI incidence is not significantly different between urethral and suprapubic catheters in spinal cord injury and neurogenic bladder [31].
5. Conclusions
In this systematic review of randomized trials, we found that the use of indwelling coated catheters was not associated with a lower incidence of CAUTI and the need for removal/change of catheter compared to non-coated catheters. In addition, we also found no difference in lower urinary tract symptoms after catheter removal. However, the incidence of CUATI was significantly lower using silver alloy-coated catheters in patients who require more than 14 days of dwelling time. The utility of coated catheters to reduce CAUTI risk versus standard catheters must be balanced with differences in direct costs to patients and health care systems.
Author Contributions
Conceptualization, V.G., B.K.S. and J.d.l.R.; methodology, V.G. and D.C.; data gathering, V.G., D.C., C.N., G.C., A.T.G., R.D.d.S., F.L.H., J.Y.-C.T. and M.L.W.; validation, B.K.S., J.Y.-C.T., A.B.G. and J.d.l.R.; formal analysis, D.C.; writing—original draft preparation, V.G., D.C., C.N., G.C., A.T.G., R.D.d.S., F.L.H. and M.L.W.; writing—review and editing, D.C., V.G., B.K.S., A.B.G., J.Y.-C.T. and J.d.l.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be provide by the corresponding author upon a reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feneley, R.C.L.; Hopley, I.B.; Wells, P.N.T. Urinary Catheters: History, Current Status, Adverse Events and Research Agenda. J. Med. Eng. Technol. 2015, 39, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.J.; Flores-Mireles, A.L. Urinary Catheter Coating Modifications: The Race against Catheter-Associated Infections. Coatings 2020, 10, 23. [Google Scholar] [CrossRef]
- Henly, E.L.; Dowling, J.A.R.; Maingay, J.B.; Lacey, M.M.; Smith, T.J.; Forbes, S. Biocide Exposure Induces Changes in Susceptibility, Pathogenicity, and Biofilm Formation in Uropathogenic Escherichia Coli. Antimicrob. Agents Chemother. 2019, 63, e01892-18. [Google Scholar] [CrossRef]
- Kazmierska, K.A.; Thompson, R.; Morris, N.; Long, A.; Ciach, T. In Vitro Multicompartmental Bladder Model for Assessing Blockage of Urinary Catheters: Effect of Hydrogel Coating on Dynamics of Proteus Mirabilis Growth. Urology 2010, 76, 515.e15–515.e20. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.G.; Liao, K.S.; Cevallos, M.E.; Trautner, B.W. Silver or Nitrofurazone Impregnation of Urinary Catheters Has a Minimal Effect on Uropathogen Adherence. J. Urol. 2010, 184, 2565–2571. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.B.L.; Omar, M.I.; Fisher, E.; Gillies, K.; MacLennan, S. Types of Indwelling Urethral Catheters for Short-Term Catheterisation in Hospitalised Adults. Cochrane Database Syst. Rev. 2014, 9, CD004013. [Google Scholar] [CrossRef] [PubMed]
- Pickard, R.; Lam, T.; MacLennan, G.; Starr, K.; Kilonzo, M.; McPherson, G.; Gillies, K.; McDonald, A.; Walton, K.; Buckley, B.; et al. Antimicrobial Catheters for Reduction of Symptomatic Urinary Tract Infection in Adults Requiring Short-Term Catheterisation in Hospital: A Multicentre Randomised Controlled Trial. Lancet 2012, 380, 1927–1935. [Google Scholar] [CrossRef]
- Higgins, J.P.; Eldridge, S.; Tianjing, L. How to Include Multiple Groups from One Study. Available online: https://training.cochrane.org/handbook/current/chapter-23#section-23-3 (accessed on 15 May 2022).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Akcam, F.Z.; Kaya, O.; Temel, E.N.; Buyuktuna, S.A.; Unal, O.; Yurekli, V.A. An Investigation of the Effectiveness against Bacteriuria of Silver-Coated Catheters in Short-Term Urinary Catheter Applications: A Randomized Controlled Study. J. Infect. Chemother. 2019, 25, 797–800. [Google Scholar] [CrossRef]
- Menezes, F.G.; Corrêa, L.; Medina-Pestana, J.O.; Aguiar, W.F.; Camargo, L.F.A. A Randomized Clinical Trial Comparing Nitrofurazone-Coated and Uncoated Urinary Catheters in Kidney Transplant Recipients: Results from a Pilot Study. Transpl. Infect. Dis. 2019, 21, e13031. [Google Scholar] [CrossRef] [PubMed]
- Karchmer, T.B.; Giannetta, E.T.; Muto, C.A.; Strain, B.A.; Farr, B.M. A Randomized Crossover Study of Silver-Coated Urinary Catheters in Hospitalized Patients. Arch. Intern. Med. 2000, 160, 3294–3298. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Roberts, P.L.; Olsen, R.J.; Moyer, K.A.; Stamm, W.E. Prevention of Catheter-Associated Urinary Tract Infection with a Silver Oxide-Coated Urinary Catheter: Clinical and Microbiologic Correlates. J. Infect. Dis. 1990, 162, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Stenzelius, K.; Persson, S.; Olsson, U.-B.; Stjärneblad, M. Noble Metal Alloy-Coated Latex versus Silicone Foley Catheter in Short-Term Catheterization: A Randomized Controlled Study. Scand. J. Urol. Nephrol. 2011, 45, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Stensballe, J.; Tvede, M.; Looms, D.; Lippert, F.K.; Dahl, B.; Tønnesen, E.; Rasmussen, L.S. Infection Risk with Nitrofurazone-Impregnated Urinary Catheters in Trauma Patients: A Randomized Trial. Ann. Intern. Med. 2007, 147, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Erickson, B.A.; Navai, N.; Patil, M.; Chang, A.; Gonzalez, C.M. A Prospective, Randomized Trial Evaluating the Use of Hydrogel Coated Latex versus All Silicone Urethral Catheters after Urethral Reconstructive Surgery. J. Urol. 2008, 179, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Kim, S.W.; Cho, Y.-H.; Shin, W.-S.; Lee, S.E.; Kim, C.-S.; Hong, S.J.; Chung, B.H.; Kim, J.J.; Yoon, M.S. A Comparative Multicentre Study on the Incidence of Catheter-Associated Urinary Tract Infection between Nitrofurazone-Coated and Silicone Catheters. Int. J. Antimicrob. Agents 2004, 24 (Suppl. S1), S65–S659. [Google Scholar] [CrossRef]
- Bonfill, X.; Rigau, D.; Esteban-Fuertes, M.; Barrera-Chacón, J.M.; Jáuregui-Abrisqueta, M.L.; Salvador, S.; Alemán-Sánchez, C.M.; Borau, A.; Bea-Muñoz, M.; Hidalgo, B.; et al. Efficacy and Safety of Urinary Catheters with Silver Alloy Coating in Patients with Spinal Cord Injury: A Multicentric Pragmatic Randomized Controlled Trial. The ESCALE Trial. Spine J. 2017, 17, 1650–1657. [Google Scholar] [CrossRef]
- Thibon, P.; Le Coutour, X.; Leroyer, R.; Fabry, J. Randomized Multi-Centre Trial of the Effects of a Catheter Coated with Hydrogel and Silver Salts on the Incidence of Hospital-Acquired Urinary Tract Infections. J. Hosp. Infect. 2000, 45, 117–124. [Google Scholar] [CrossRef]
- Tae, B.S.; Oh, J.J.; Jeong, B.C.; Ku, J.H. Catheter-Associated Urinary Tract Infections in Patients Who Have Undergone Radical Cystectomy for Bladder Cancer: A Prospective Randomized Clinical Study of Two Silicone Catheters (Clinical Benefit of Antibiotic Silicone Material). Investig. Clin. Urol. 2022, 63, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Okamoto, S. Prophylaxis of Indwelling Urethral Catheter Infection: Clinical Experience with a Modified Foley Catheter and Drainage System. J. Urol. 1979, 121, 40–42. [Google Scholar] [CrossRef]
- Liedberg, H.; Lundeberg, T. Silver Alloy Coated Catheters Reduce Catheter-Associated Bacteriuria. Br. J. Urol. 1990, 65, 379–381. [Google Scholar] [CrossRef]
- Liedberg, H.; Lundeberg, T.; Ekman, P. Refinements in the Coating of Urethral Catheters Reduces the Incidence of Catheter-Associated Bacteriuria. An Experimental and Clinical Study. Eur. Urol. 1990, 17, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Cooper, F.P.M.; Alexander, C.E.; Sinha, S.; Omar, M.I. Policies for Replacing Long-Term Indwelling Urinary Catheters in Adults. Cochrane Database Syst. Rev. 2016, 7, CD011115. [Google Scholar] [CrossRef] [PubMed]
- Priefer, B.A.; Duthie, E.H.J.; Gambert, S.R. Frequency of Urinary Catheter Change and Clinical Urinary Tract Infection. Study in Hospital-Based, Skilled Nursing Home. Urology 1982, 20, 141–142. [Google Scholar] [CrossRef]
- White, M.C.; Ragland, K.E. Urinary Catheter-Related Infections among Home Care Patients. J. Wound Ostomy Cont. Nurs. 1995, 22, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Bologna, R.A.; Tu, L.M.; Polansky, M.; Fraimow, H.D.; Gordon, D.A.; Whitmore, K.E. Hydrogel/Silver Ion-Coated Urinary Catheter Reduces Nosocomial Urinary Tract Infection Rates in Intensive Care Unit Patients: A Multicenter Study. Urology 1999, 54, 982–987. [Google Scholar] [CrossRef]
- Chung, P.H.; Wong, C.W.; Lai, C.K.; Siu, H.K.; Tsang, D.N.; Yeung, K.Y.; Ip, D.K.; Tam, P.K. A Prospective Interventional Study to Examine the Effect of a Silver Alloy and Hydrogel-Coated Catheter on the Incidence of Catheter-Associated Urinary Tract Infection. Hong Kong Med. J. 2017, 23, 239–245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Srinivasan, A.; Karchmer, T.; Richards, A.; Song, X.; Perl, T.M. A Prospective Trial of a Novel, Silicone-Based, Silver-Coated Foley Catheter for the Prevention of Nosocomial Urinary Tract Infections. Infect. Control Hosp. Epidemiol. 2006, 27, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Pickard, R.; Lam, T.; Maclennan, G.; Starr, K.; Kilonzo, M.; McPherson, G.; Gillies, K.; McDonald, A.; Walton, K.; Buckley, B.; et al. Types of Urethral Catheter for Reducing Symptomatic Urinary Tract Infections in Hospitalised Adults Requiring Short-Term Catheterisation: Multicentre Randomised Controlled Trial and Economic Evaluation of Antimicrobial- and Antiseptic-Impregnated Urethra. Health Technol. Assess. 2012, 16, 1–197. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, N.; Barnett, D.; O’Callaghan, M.; Horsell, K.; Gani, J.; Hennessey, D. The Impact of Catheter-Based Bladder Drainage Method on Urinary Tract Infection Risk in Spinal Cord Injury and Neurogenic Bladder: A Systematic Review. Neurourol. Urodyn. 2020, 39, 854–862. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).