Anatomical and Functional Effects of Oral Administration of Curcuma Longa and Boswellia Serrata Combination in Patients with Treatment-Naïve Diabetic Macular Edema
Abstract
:1. Introduction
2. Methods
Statistical Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bandello, F.; Battaglia Parodi, M.; Lanzetta, P.; Loewenstein, A.; Massin, P.; Menchini, F.; Veritti, D. Diabetic Macular Edema. Dev. Ophthalmol. 2017, 58, 102–138. [Google Scholar] [CrossRef] [PubMed]
- Starace, V.; Battista, M.; Brambati, M.; Cavalleri, M.; Bertuzzi, F.; Amato, A.; Lattanzio, R.; Bandello, F.; Cicinelli, M.V. The Role of Inflammation and Neurodegeneration in Diabetic Macular Edema. Ther. Adv. Ophthalmol. 2021, 13, 251584142110559. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Yasuda, K.; Shimura, M. Involvement of Cytokines in the Pathogenesis of Diabetic Macular Edema. Int. J. Mol. Sci. 2021, 22, 3427. [Google Scholar] [CrossRef]
- Romero-Aroca, P.; Baget-Bernaldiz, M.; Pareja-Rios, A.; Lopez-Galvez, M.; Navarro-Gil, R.; Verges, R. Diabetic Macular Edema Pathophysiology: Vasogenic versus Inflammatory. J. Diabetes Res. 2016, 2016, 2156273. [Google Scholar] [CrossRef] [Green Version]
- Georgiadou, E.; Moschos, M.M.; Margetis, I.; Chalkiadakis, J.; Markomichelakis, N.N. Structural and Functional Outcomes after Treatment of Uveitic Macular Oedema: An Optical Coherence Tomography and Multifocal Electroretinogram Study. Clin. Exp. Optom. 2012, 95, 89–93. [Google Scholar] [CrossRef]
- Elsharkawy, M.; Elrazzaz, M.; Sharafeldeen, A.; Alhalabi, M.; Khalifa, F.; Soliman, A.; Elnakib, A.; Mahmoud, A.; Ghazal, M.; El-Daydamony, E.; et al. The Role of Different Retinal Imaging Modalities in Predicting Progression of Diabetic Retinopathy: A Survey. Sensors 2022, 22, 3490. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.W.; Glassman, A.R.; Beaulieu, W.T.; Antoszyk, A.N.; Browning, D.J.; Chalam, K.V.; Grover, S.; Jampol, L.M.; Jhaveri, C.D.; Melia, M.; et al. Effect of Initial Management with Aflibercept vs Laser Photocoagulation vs. Observation on Vision Loss Among Patients with Diabetic Macular Edema Involving the Center of the Macula and Good Visual Acuity. JAMA 2019, 321, 1880. [Google Scholar] [CrossRef] [Green Version]
- Busch, C.; Fraser-Bell, S.; Zur, D.; Rodríguez-Valdés, P.J.; Cebeci, Z.; Lupidi, M.; Fung, A.T.; Gabrielle, P.-H.; Giancipoli, E.; Chaikitmongkol, V.; et al. Real-World Outcomes of Observation and Treatment in Diabetic Macular Edema with Very Good Visual Acuity: The OBTAIN Study. Acta Diabetol. 2019, 56, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Huynh, T.-P.; Mann, S.N.; Mandal, N.A. Botanical Compounds: Effects on Major Eye Diseases. Evid. Based Complement. Altern. Med. 2013, 2013, 549174. [Google Scholar] [CrossRef] [Green Version]
- Pescosolido, N.; Giannotti, R.; Plateroti, A.; Pascarella, A.; Nebbioso, M. Curcumin: Therapeutical Potential in Ophthalmology. Planta Med. 2013, 80, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peddada, K.V.; Brown, A.; Verma, V.; Nebbioso, M. Therapeutic Potential of Curcumin in Major Retinal Pathologies. Int. Ophthalmol. 2019, 39, 725–734. [Google Scholar] [CrossRef]
- López-Malo, D.; Villarón-Casares, C.A.; Alarcón-Jiménez, J.; Miranda, M.; Díaz-Llopis, M.; Romero, F.J.; Villar, V.M. Curcumin as a Therapeutic Option in Retinal Diseases. Antioxidants 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammon, H. Boswellic Acids in Chronic Inflammatory Diseases. Planta Med. 2006, 72, 1100–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammon, H.P.T. Modulation of the Immune System by Boswellia Serrata Extracts and Boswellic Acids. Phytomedicine 2010, 17, 862–867. [Google Scholar] [CrossRef]
- Ammon, H.P.T.; Safayhi, H.; Mack, T.; Sabieraj, J. Mechanism of Antiinflammatory Actions of Curcumine and Boswellic Acids. J. Ethnopharmacol. 1993, 38, 105–112. [Google Scholar] [CrossRef]
- Haroyan, A.; Mukuchyan, V.; Mkrtchyan, N.; Minasyan, N.; Gasparyan, S.; Sargsyan, A.; Narimanyan, M.; Hovhannisyan, A. Efficacy and Safety of Curcumin and Its Combination with Boswellic Acid in Osteoarthritis: A Comparative, Randomized, Double-Blind, Placebo-Controlled Study. BMC Complement. Altern. Med. 2018, 18, 7. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.S.; Cheung, N.; Simó, R.; Cheung, G.C.M.; Wong, T.Y. Diabetic Macular Oedema. Lancet Diabetes Endocrinol. 2017, 5, 143–155. [Google Scholar] [CrossRef]
- Korobelnik, J.-F.; Loewenstein, A.; Eldem, B.; Joussen, A.M.; Koh, A.; Lambrou, G.N.; Lanzetta, P.; Li, X.; Lövestam-Adrian, M.; Navarro, R.; et al. Guidance for Anti-VEGF Intravitreal Injections during the COVID-19 Pandemic. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1149–1156. [Google Scholar] [CrossRef]
- Mitchell, P.; Bandello, F.; Schmidt-Erfurth, U.; Lang, G.E.; Massin, P.; Schlingemann, R.O.; Sutter, F.; Simader, C.; Burian, G.; Gerstner, O.; et al. The RESTORE Study. Ophthalmology 2011, 118, 615–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, Inflammation, and Chronic Diseases: How Are They Linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef]
- Loeser, K.; Seemann, S.; König, S.; Lenhardt, I.; Abdel-Tawab, M.; Koeberle, A.; Werz, O.; Lupp, A. Protective Effect of Casperome®, an Orally Bioavailable Frankincense Extract, on Lipopolysaccharide- Induced Systemic Inflammation in Mice. Front. Pharmacol. 2018, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Lulli, M.; Cammalleri, M.; Fornaciari, I.; Casini, G.; Dal Monte, M. Acetyl-11-Keto-β-Boswellic Acid Reduces Retinal Angiogenesis in a Mouse Model of Oxygen-Induced Retinopathy. Exp. Eye Res. 2015, 135, 67–80. [Google Scholar] [CrossRef]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative Absorption of a Standardized Curcuminoid Mixture and Its Lecithin Formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef]
- Riva, A.; Morazzoni, P.; Artaria, C.; Allegrini, P.; Meins, J.; Savio, D.; Appendino, G.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. A Single-Dose, Randomized, Cross-over, Two-Way, Open-Label Study for Comparing the Absorption of Boswellic Acids and Its Lecithin Formulation. Phytomedicine 2016, 23, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- Hüsch, J.; Bohnet, J.; Fricker, G.; Skarke, C.; Artaria, C.; Appendino, G.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Enhanced Absorption of Boswellic Acids by a Lecithin Delivery Form (Phytosome®) of Boswellia Extract. Fitoterapia 2013, 84, 89–98. [Google Scholar] [CrossRef] [Green Version]
| Total N = 61 | Group A N = 12 | Group B N = 49 | p | ||
|---|---|---|---|---|---|
| Age (years) | 64.2 ± 14.13 | 65.8 ± 17.76 | 63.8 ± 13.23 | 0.44 | |
| Sex | Female | 31(50.8) | 7 (58.3) | 24 (49.0) | 0.56 |
| Male | 30 (49.2) | 5 (41.7) | 25 (51.0) | ||
| CMT (µm) at baseline | 276.3 ± 72.80 | 291.6 ± 47.63 | 272.6 (77.67) | 0.09 | |
| BCVA (ETDRS Letters) | 52.9 ± 14.60 | 51.7 ± 18.78 | 53.2 (13.61) | 0.82 | |
| Systemic hypertension | 32 (52.5) | 7 (58.3) | 25 (51.0) | 0.65 | |
| Dyslipidemia | 8 (13.1) | 2 (16.7) | 6 (12.2) | 0.68 | |
| Pseudophakia | 18 (29.5) | 3 (25.0) | 15 (30.6) | 0.70 | |
| Baseline | One Month | Six Months | Mixed-Model ANOVA | |
|---|---|---|---|---|
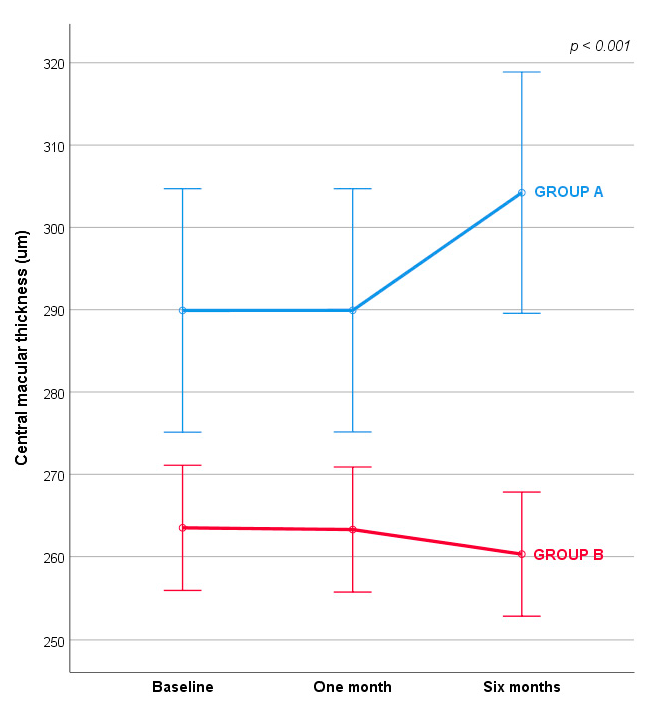
| Group A | 289.91 (14.79) | 289.92 (14.78) | 394.22 (14.66) | F (1.032,102.168) = 14.416; ƞ2 = 0.127; p < 0.001 |
| Group B | 263.50 (7.61) | 263.89 (7.60) | 260.30 (7.54) |
| Baseline | One Month | Six Months | Mixed-Model ANOVA | |
|---|---|---|---|---|
| Group A | 53.14 (3.60) | 53.14 (3.59) | 50.50 (3.69) | F (1.084, 108.386) = 12.514; ƞ2 = 0.111; p < 0.001 |
| Group B | 53.75 (1.85) | 53.70 (1.85) | 54.44 (1.90) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarino, O.; Iovino, C.; Di Iorio, V.; Rosolia, A.; Schiavetti, I.; Lanza, M.; Simonelli, F. Anatomical and Functional Effects of Oral Administration of Curcuma Longa and Boswellia Serrata Combination in Patients with Treatment-Naïve Diabetic Macular Edema. J. Clin. Med. 2022, 11, 4451. https://doi.org/10.3390/jcm11154451
Guarino O, Iovino C, Di Iorio V, Rosolia A, Schiavetti I, Lanza M, Simonelli F. Anatomical and Functional Effects of Oral Administration of Curcuma Longa and Boswellia Serrata Combination in Patients with Treatment-Naïve Diabetic Macular Edema. Journal of Clinical Medicine. 2022; 11(15):4451. https://doi.org/10.3390/jcm11154451
Chicago/Turabian StyleGuarino, Olimpia, Claudio Iovino, Valentina Di Iorio, Andrea Rosolia, Irene Schiavetti, Michele Lanza, and Francesca Simonelli. 2022. "Anatomical and Functional Effects of Oral Administration of Curcuma Longa and Boswellia Serrata Combination in Patients with Treatment-Naïve Diabetic Macular Edema" Journal of Clinical Medicine 11, no. 15: 4451. https://doi.org/10.3390/jcm11154451
APA StyleGuarino, O., Iovino, C., Di Iorio, V., Rosolia, A., Schiavetti, I., Lanza, M., & Simonelli, F. (2022). Anatomical and Functional Effects of Oral Administration of Curcuma Longa and Boswellia Serrata Combination in Patients with Treatment-Naïve Diabetic Macular Edema. Journal of Clinical Medicine, 11(15), 4451. https://doi.org/10.3390/jcm11154451










