Thyroid Hormones and Health-Related Quality of Life in Normal Pressure Hydrocephalus Patients before and after the Ventriculoperitoneal Shunt Surgery: A Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Procedure
2.2. Self-Reported Health-Related Quality of Life
2.3. Blood Samples
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients
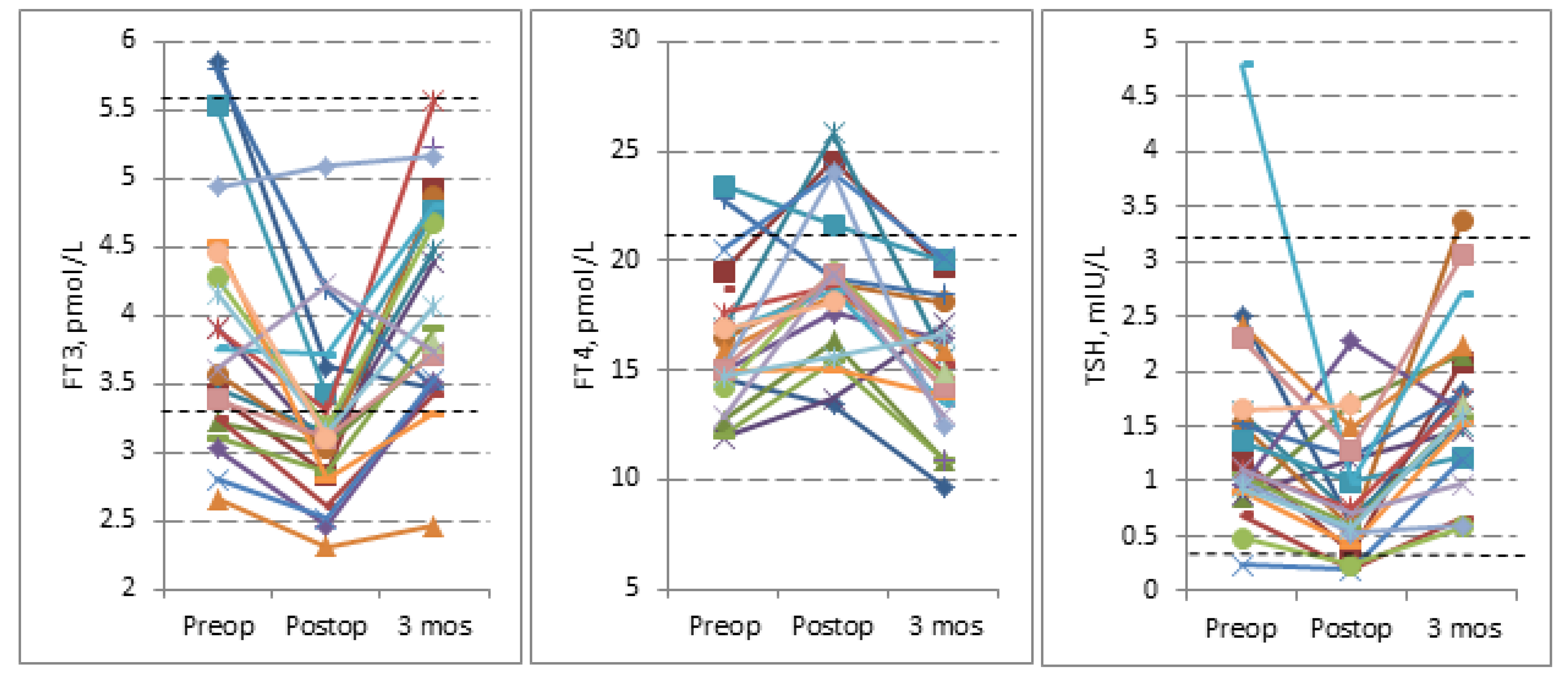
3.2. Evans Index and Thyroid Hormones Levels
3.3. Low T3 Syndrome
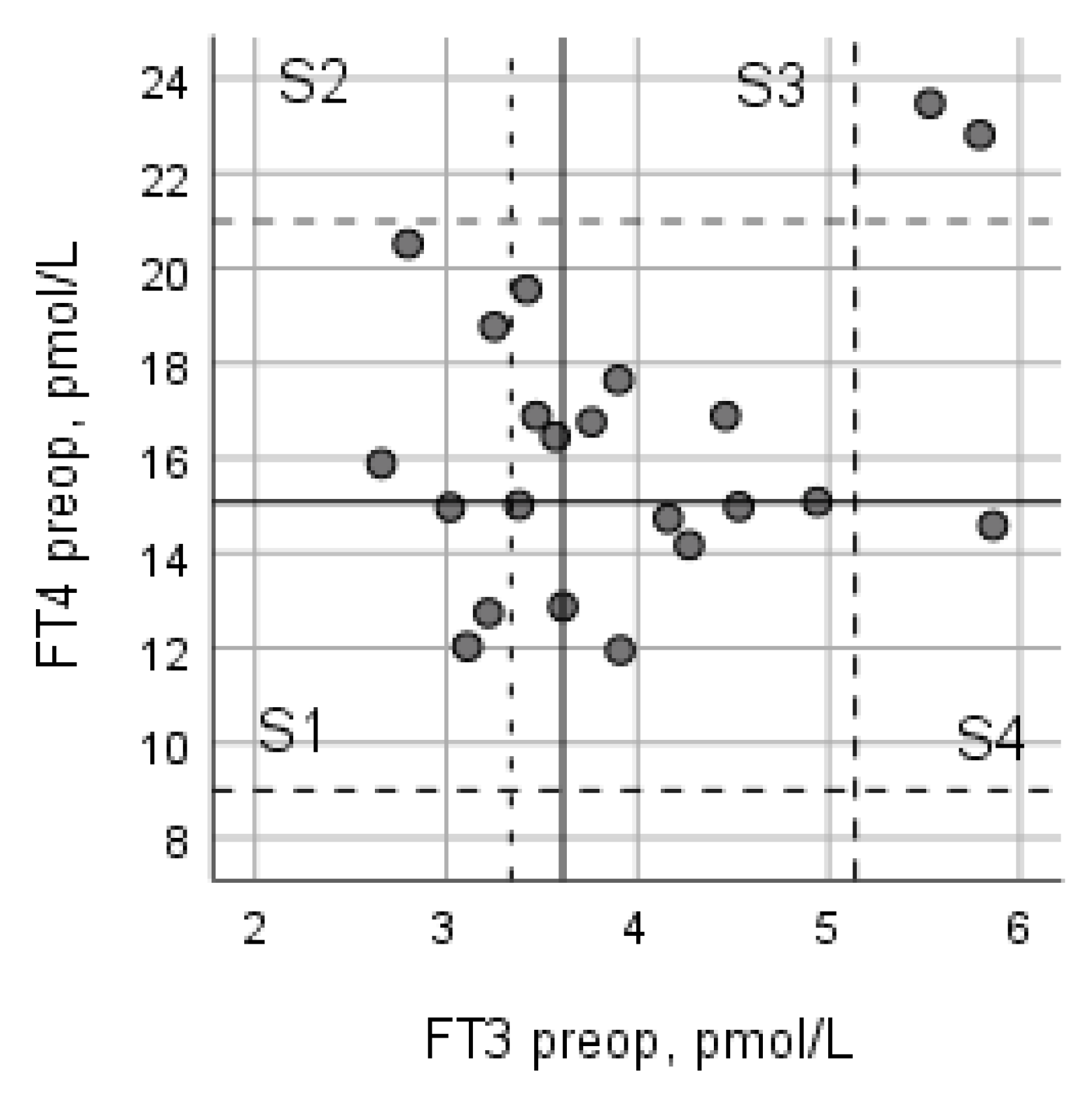
3.4. Relationship between Preoperative FT3 and FT4
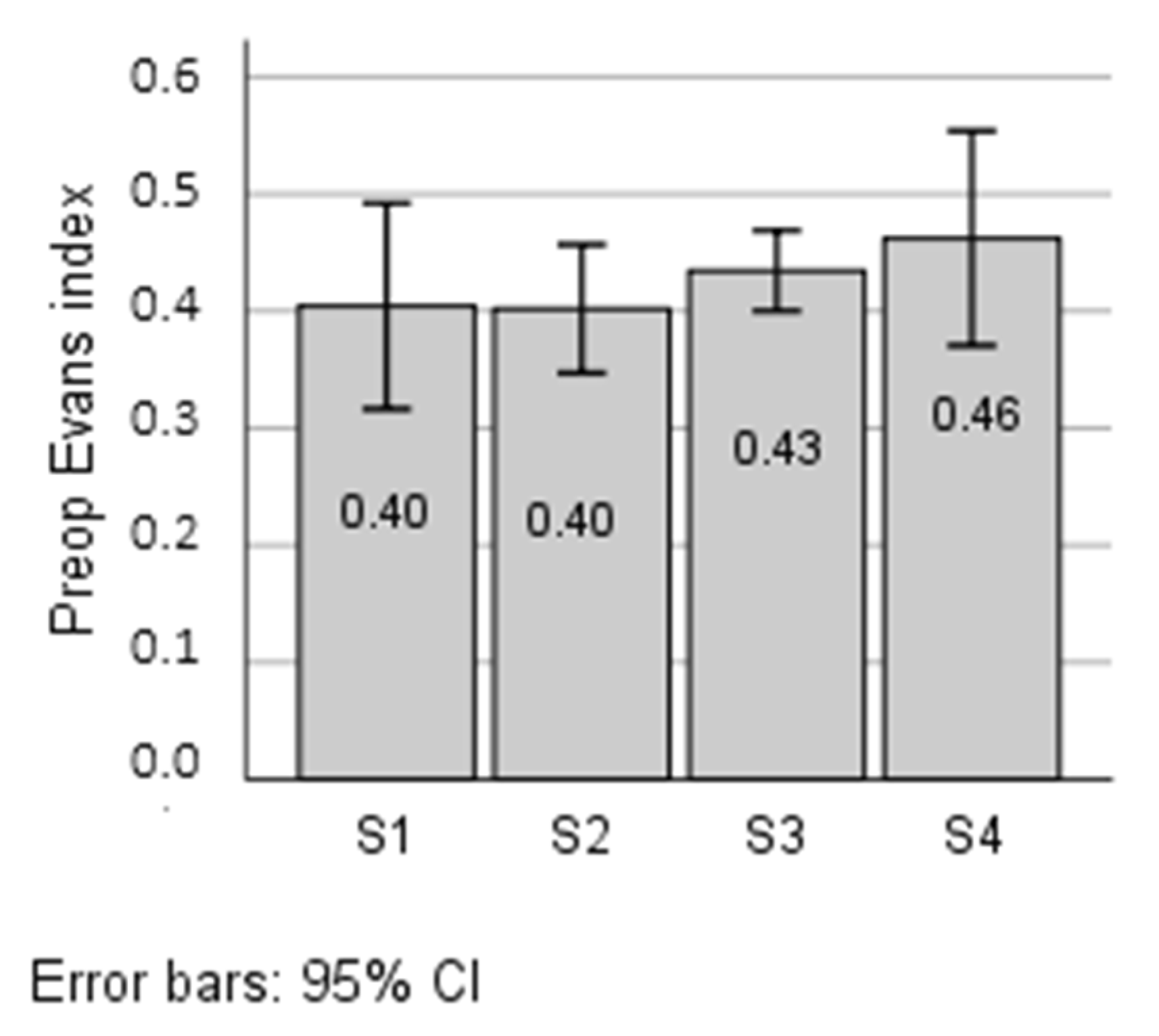
3.5. Correlation of the Thyroid Hormones with Evans Index
3.6. Outcomes with Respect to Health-Related Quality of Life
3.7. Preoperative Low T3 Syndrome and Its Effect on Outcome
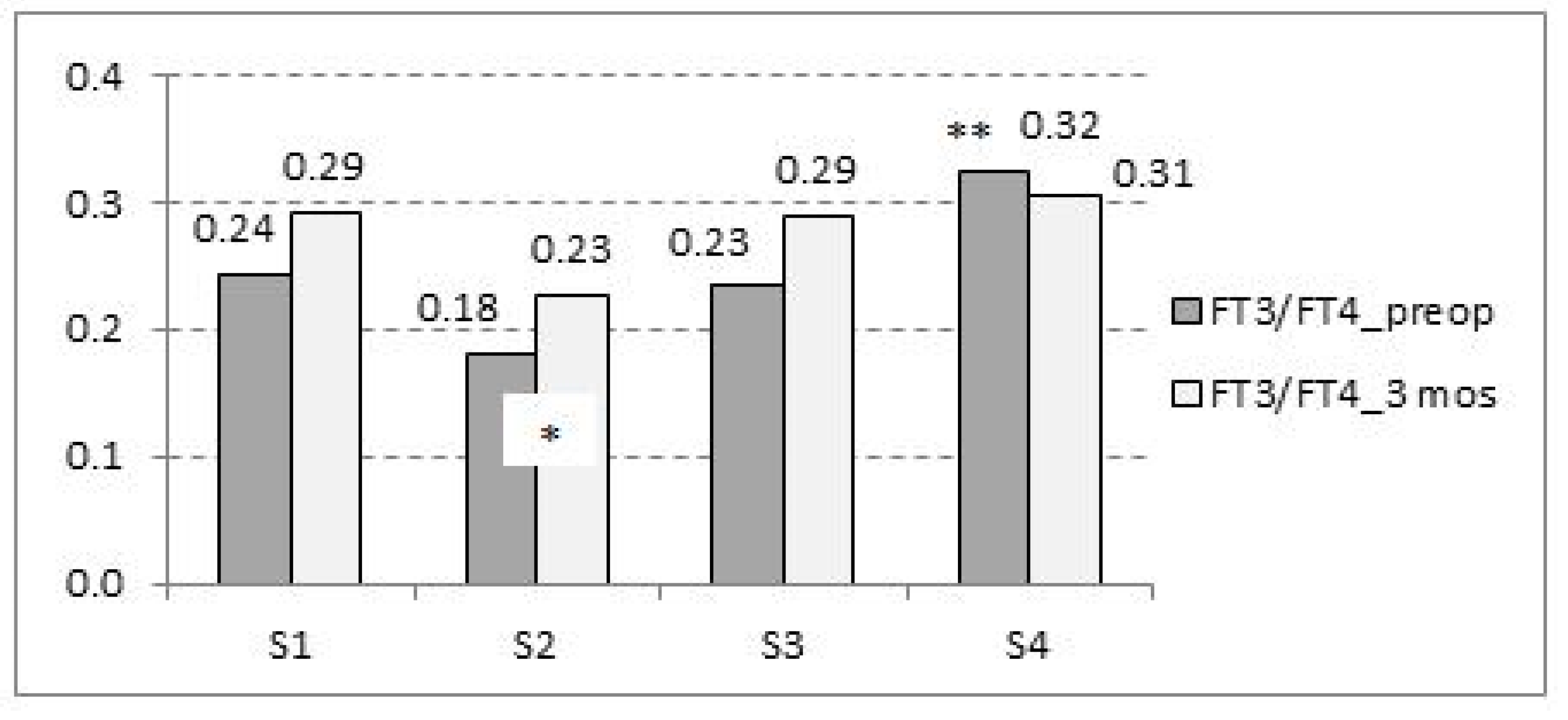
3.8. Preoperative FT3/FT4 Ratio and Its Effect on Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mori, E.; Ishikawa, M.; Kato, T.; Kazui, H.; Miyake, H.; Miyajima, M.; Nakajima, M.; Hashimoto, M.; Kuriyama, N.; Tokuda, T.; et al. Guidelines for Management of Idiopathic Normal Pressure Hydrocephalus: Second Edition. Neurol. Med. Chir. 2012, 52, 775–809. [Google Scholar] [CrossRef] [PubMed]
- Hebb, A.O.; Cusimano, M.D. Idiopathic Normal Pressure Hydrocephalus: A Systematic Review of Diagnosis and Outcome. Neurosurgery 2001, 49, 1166–1184, discussion 1184–1186. [Google Scholar] [CrossRef] [PubMed]
- Ziegelitz, D.; Starck, G.; Kristiansen, D.; Jakobsson, M.; Hultenmo, M.; Mikkelsen, I.K.; Hellström, P.; Tullberg, M.; Wikkelsø, C. Cerebral perfusion measured by dynamic susceptibility contrast MRI is reduced in patients with idiopathic normal pressure hydrocephalus. J. Magn. Reson. Imaging 2014, 39, 1533–1542. [Google Scholar] [CrossRef]
- Momjian, S.; Owler, B.K.; Czosnyka, Z.; Czosnyka, M.; Pena, A.; Pickard, J.D. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain 2004, 127, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Graf-Radford, N.R. Alzheimer CSF biomarkers may be misleading in normal-pressure hydrocephalus. Neurology 2014, 83 (Suppl. 17), 1573–1575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Hu, F.; Ding, J.; Wang, X. Pathogenesis and pathophysiology of idiopathic normal pressure hydrocephalus. CNS Neurosci. Ther. 2020, 26 (Suppl. 12), 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Wikkelsö, C.; Andersson, H.; Blomstrand, C.; Lindqvist, G.; Svendsen, P. Normal pressure hydrocephalus. Predictive value of the cerebrospinal fluid tap-test. Acta Neurol. Scand. 1986, 73, 566–573. [Google Scholar] [CrossRef]
- Tullberg, M.; Hellström, P.; Piechnik, S.K.; Starmark, J.-E.; Wikkelsö, C. Impaired wakefulness is associated with reduced anterior cingulate CBF in patients with normal pressure hydrocephalus. Acta Neurol. Scand. 2004, 110, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Goto, H.; Izaki, K.; Tamura, S.; Tomii, M.; Sasanuma, J.; Maeno, K.; Kikuchi, Y.; Koizumi, J.; Watanabe, Z.; et al. Pathophysiology of Cerebral Circulatory Disorders in Idiopathic Normal Pressure Hydrocephalus. Neurol. Med. Chir. 2007, 47, 299–306. [Google Scholar] [CrossRef]
- Larsson, A.; Bergh, A.-C.; Bilting, M.; Jacobsson, L.; Stephensen, H. Regional cerebral blood flow in normal pressure hydrocephalus: Diagnostic and prognostic aspects. Eur. J. Nucl. Med. 1994, 21, 118–123. [Google Scholar] [CrossRef]
- Barber, S.G.; Garvan, N. Hypopituitarism in normal-pressure hydrocephalus. Br. Med. J. 1979, 1, 1039–1041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moin, T.; Bergsneider, M.; Vespa, P.; Heaney, A.P. Pituitary Function in Patients with Normal Pressure Hydrocephalus before and after Neurosurgical Correction. J. Clin. Endocrinol. Metab. 2012, 97, 3545–3549. [Google Scholar] [CrossRef] [PubMed]
- Ucler, N.; Erol, F.S.; Ozturk, S.; Akgun, B.; Kaplan, M.; Sen, Y. Does Ventriculoperitoneal Shunting Improve Thyroid Hormone Levels in Hydrocephalic Newborns? Pediatr. Neurosurg. 2017, 52, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Lamba, N.; Liu, C.; Zaidi, H.; Broekman, M.; Simjian, T.; Shi, C.; Doucette, J.; Ren, S.; Smith, T.R.; Mekary, R.A.; et al. A prognostic role for Low tri-iodothyronine syndrome in acute stroke patients: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2018, 169, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Bunevicius, A.; Laws, E.R.; Deltuva, V.; Tamasauskas, A. Association of thyroid hormone concentrations with quality of life of primary brain tumor patients: A pilot study. J. Neuro-Oncol. 2017, 131, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.J. Clinical review 86: Euthyroid Sick Syndrome: Is It a Misnomer? J. Clin. Endocrinol. Metab. 1997, 82, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Relkin, N.; Marmarou, A.; Klinge, P.; Bergsneider, M.; Black, P.M. Diagnosing Idiopathic Normal-pressure Hydrocephalus. Neurosurgery 2005, 57 (Suppl. 3), S4–S16. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Kazui, H.; Yoshida, T.; Kito, Y.; Kimura, N.; Tokunaga, H.; Ogino, A.; Miyake, H.; Ishikawa, M.; Takeda, M. Validation of Grading Scale for Evaluating Symptoms of Idiopathic Normal-Pressure Hydrocephalus. Dement. Geriatr. Cogn. Disord. 2008, 25, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”: A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Keller, S.D. SF-36 Physical and Mental Health Summary Scales: A User’s Manual; The Health Institute: Boston, MA, USA, 1994. [Google Scholar]
- Bunevicius, A. Reliability and validity of the SF-36 Health Survey Questionnaire in patients with brain tumors: A cross-sectional study. Health Qual. Life Outcomes 2017, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.R.; Sloan, J.A.; Wyrwich, K.W. Interpretation of Changes in Health-related Quality of Life. Med. Care 2003, 41, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Landin-Wilhelmsen, K.; Zetterberg, H.; Oleröd, G.; Isgaard, J.; Wikkelsö, C. Serum IGF-1 is higher in patients with idiopathic normal pressure hydrocephalus than in the population. Growth Horm. IGF Res. 2015, 25, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.K.; Suri, A.; Sharma, B.S.; Shamim, S.A.; Bal, C.S. Changes in cerebral perfusion hormone profile and cerebrospinal fluid flow across the third ventriculostomy after endoscopic third ventriculostomy in patients with aqueductal stenosis: A prospective study. J. Neurosurg. Pediatr. 2009, 3, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, M.J.; Bauer, M.; Partsch, C.J.; Sippell, W.G.; Mehdorn, H.M. Endocrine evaluation after endoscopic third ventriculostomy (ETV) in children. Child’s Nerv. Syst. 2007, 23, 627–631. [Google Scholar] [CrossRef]
- Al-Sofyani, K.A.; Uddin, M.S.; Qulisy, E.A.; Al-Radi, O.O. Patterns and Determinants of Change in Cortisol Levels and Thyroid Function as a Function of Cardiac Risk in Children Undergoing Cardiac Surgery. Int. J. Pediatrics 2022, 2022, 6730666. [Google Scholar] [CrossRef] [PubMed]
- Haas, N.A.; Camphausen, C.K.; Kececioglu, D. Clinical review: Thyroid hormone replacement in children after cardiac surgery—Is it worth a try? Crit. Care 2006, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, E.A.; Gabriel, S.A. Thyroid Hormones and Cardiovascular Surgery; Springer: London, UK, 2013. [Google Scholar]
- DeGroot, L.J. The Non-Thyroidal Illness Syndrome. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK285570/ (accessed on 1 December 2021).
- Wajner, S.M.; Maia, A.L. New Insights toward the Acute Non-Thyroidal Illness Syndrome. Front. Endocrinol. 2012, 3, 8. [Google Scholar] [CrossRef]
- Strich, D.; Karavani, G.; Edri, S.; Gillis, D. TSH enhancement of FT4 to FT3 conversion is age dependent. Eur. J. Endocrinol. 2016, 175, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Chiaravalloti, A.; Ursini, F.; Fiorentini, A.; Barbagallo, G.; Martorana, A.; Koch, G.; Tavolozza, M.; Schillaci, O. Functional correlates of TSH, fT3 and fT4 in Alzheimer disease: A F-18 FDG PET/CT study. Sci. Rep. 2017, 7, 6220. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Byun, M.S.; Yi, D.; Sohn, B.K.; Lee, J.H.; Lee, J.-Y.; Kim, Y.K.; Lee, D.Y.; For the KBASE Research Group. Associations of thyroid hormone serum levels with in-vivo Alzheimer’s disease pathologies. Alzheimer’s Res. Ther. 2017, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Junkkari, A.; Häyrinen, A.; Rauramaa, T.; Sintonen, H.; Nerg, O.; Koivisto, A.M.; Roine, R.P.; Viinamäki, H.; Soininen, H.; Luikku, A.; et al. Health-related quality-of-life outcome in patients with idiopathic normal-pressure hydrocephalus—A 1-year follow-up study. Eur. J. Neurol. 2017, 24, 58–66. [Google Scholar] [CrossRef] [PubMed]
| Preop a | 3 Months a | z | Sig b | Decrease of 1 or More Points | |
|---|---|---|---|---|---|
| iNPH grading scale: | |||||
| Gait | 2 (2–2.5) | 1 (0–1) | −4.49 | <0.001 | 23 (96%) |
| Cognition | 2 (1–2) | 1 (0–1) | −3.94 | <0.001 | 17 (68%) |
| Urinary function | 2 (1–3) | 1 (1–2) | −3.05 | 0.002 | 10 (40%) |
| Total | 5 (4–7.5) | 3 (2–4.5) | −4.41 | <0.001 | |
| TUG, secs (N = 24) | 20 (18.25–23.5) | 15 (13–18.5) | −4.31 | <0.001 | |
| MMSE, score | 23 (21.5–24) | 25 (24–28) | −4.39 | <0.001 |
| Preop | Postop | 3 Months | p-Value | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 vs. 2 | 2 vs. 3 | 1 vs. 3 | |
| Evans index | 0.427 ± 0.063 | 0.411 ± 0.067 | 0.402 ± 0.007 | 0.002 | 0.028 | <0.001 |
| Thyroid hormones | ||||||
| FT3, pmol/L | 3.93 ± 0.92 | 3.21 ± 0.64 | 4.09 ± 0.76 | <0.001 | <0.001 | 0.454 |
| Median (range) | 3.68 (2.66–5.86) | 3.11 (2.30–5.10) | 3.90 (2.47–5.58) | |||
| FT4, pmol/L | 16.18 ± 3.18 | 18.93 ± 3.50 | 15.29 ± 3.01 | 0.001 | <0.001 | 0.077 |
| Median (range) | 15.40 (11.95–23.47) | 18.82 (13.39–25.83) | 14.93 (9.66–20.08) | |||
| TSH, mIU/L | 1.41 ± 0.95 | 0.87 ± 0.55 | 1.69 ± 0.74 | 0.019 | <0.001 | 0.119 |
| Median (range) | 1.10 (0.24–4.79) | 0.69 (0.19–2.27) | 1.59 (0.58–3.38) | |||
| Low T3 syndrome a (n = 6) | ||||||
| Low T3, n (%) | 6 (24) | 16 (64) | 2 (8) | 0.002 b | <0.001 b | 0.219 b |
| Low T3, pmol/L | 3.01 ± 0.23 | 2.63 ± 0.27 | 3.43 ± 0.50 | 0.004 | 0.002 | 0.038 |
| Change (3 Months–Preop) | ||||||
|---|---|---|---|---|---|---|
| SF-36 Subscale | Preop | 3 Months | Wilcoxon-Z | p | Mean, Score | Effect Size |
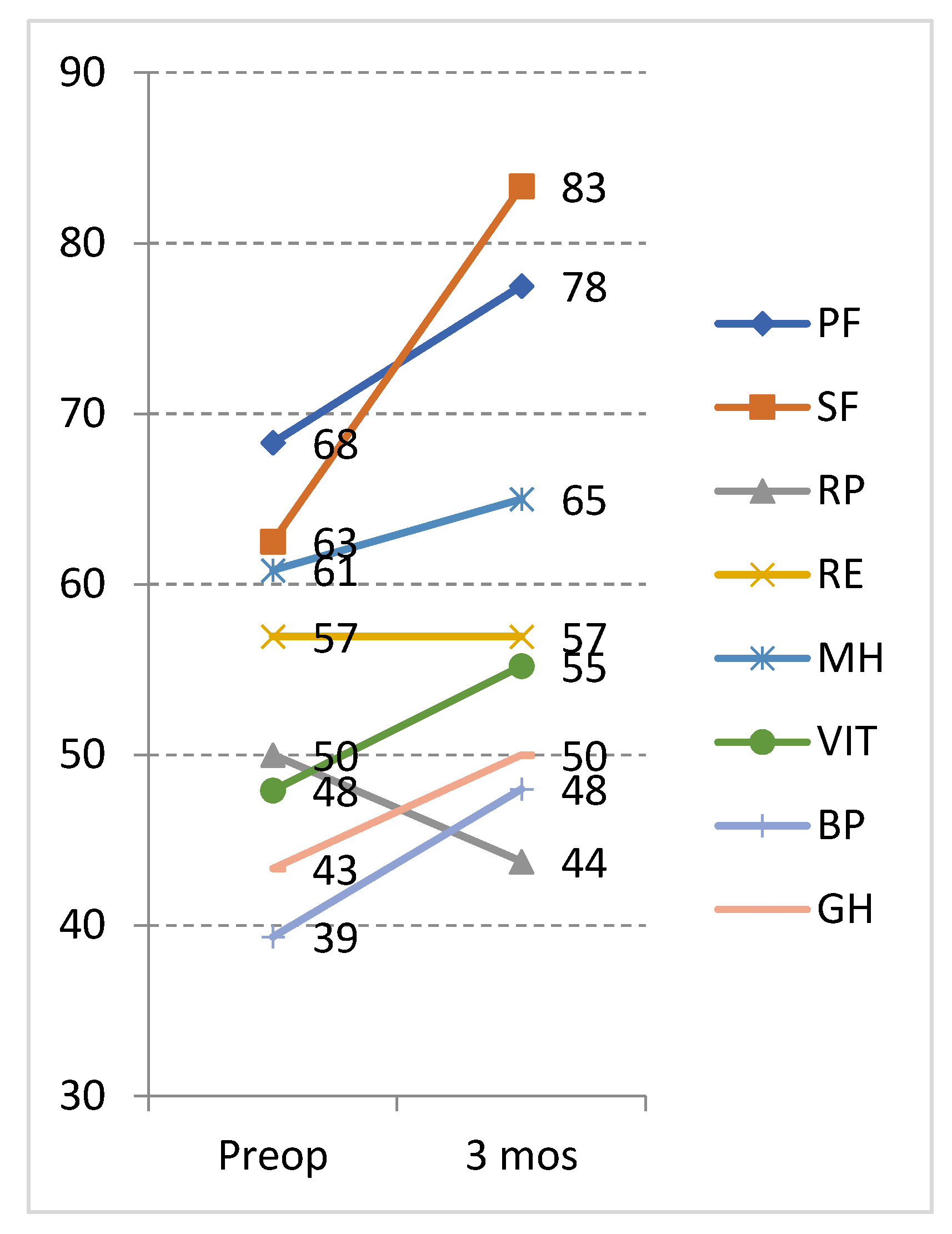
| Physical functioning | 43.54 ± 30.52 | 56.04 ± 29.70 | −2.19 | 0.028 | 12.50 | 0.4096 |
| Social functioning | 42.18 ± 27.28 | 55.72 ± 25.53 | −2.45 | 0.014 | 13.54 | 0.4962 |
| Role physical | 34.63 ± 23.31 | 34.89 ± 25.19 | −0.74 | 0.454 | 0.26 | 0.0112 |
| Role emotional | 40.62 ± 30.02 | 39.93 ± 28.96 | −0.24 | 0.808 | −0.69 | −0.0231 |
| Mental health | 48.95 ± 21.05 | 53.87 ± 16.24 | −2.09 | 0.036 | 4.91 | 0.2335 |
| Vitality | 40.10 ± 21.24 | 48.38 ± 19.24 | −2.52 | 0.012 | 8.28 | 0.3903 |
| Bodily pain | 44.00 ± 22.35 | 56.79 ± 23.28 | −2.31 | 0.021 | 12.79 | 0.5722 |
| General health | 37.91 ± 15.52 | 46.25 ± 14.68 | −2.32 | 0.020 | 8.33 | 0.5367 |
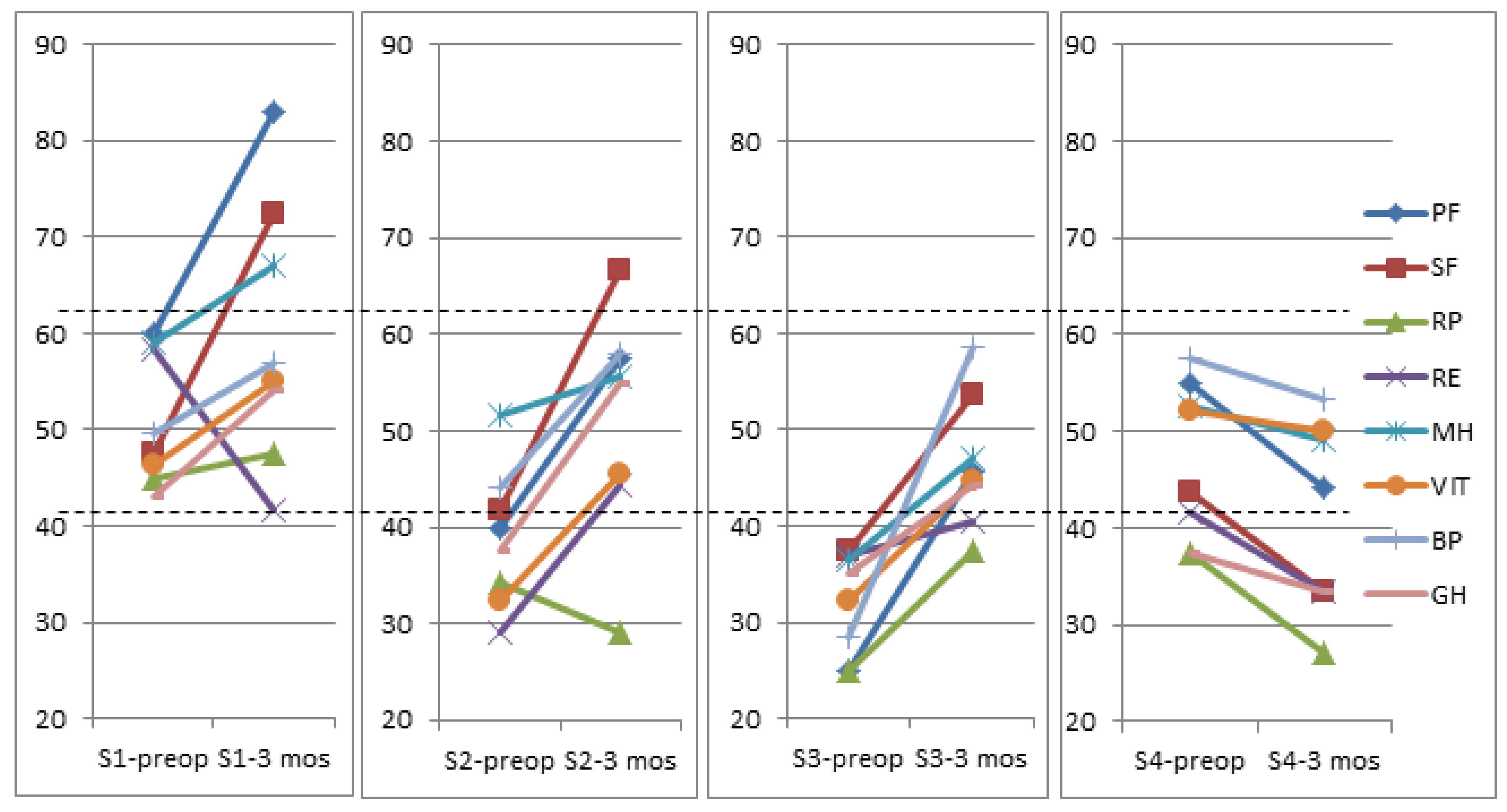
| S1 Low FT3/Low FT4 | S2 Low FT3/High FT4 | S3 High FT3/High FT4 | S4 High FT3/Low FT4 | |
|---|---|---|---|---|
| FT3/FT4 Preop Mean (95% CI) | 0.243 (0.205–0.281) | 0.179 (0.148–0.209) | 0.233 (0.2166–0.263) | 0.322 (0.279–0.367) * |
| Physical functioning | 0.753 | 0.573 | 0.753 | −0.35 |
| Social functioning | 0.916 | 0.916 | 0.916 | −0.39 |
| Role physical | 0.107 | −0.223 | 0.107 | −0.45 |
| Role emotional | −0.555 | 0.508 | −0.555 | −0.27 |
| Mental health | 0.379 | 0.182 | 0.379 | −0.16 |
| Vitality | 0.412 | 0.628 | 0.412 | −0.09 |
| Bodily pain | 0.331 | 0.626 | 0.331 | −0.18 |
| General health | 0.708 | 1.127 | 0.708 | −0.26 |
| Dependent Variable a | Independent Variable | β | t | p | R2 | Model |
|---|---|---|---|---|---|---|
| PF | PF preop | 0.544 | 2.429 | 0.029 | 0.246 | <0.001 |
| SF | SF preop | 0.665 | 4.415 | <0.001 | 0.542 | <0.001 |
| FT3/FT4 preop | –0.531 | –3.52 | 0.002 | |||
| MH | MH preop | 0.882 | 9.27 | <0.001 | 0.810 | <0.001 |
| Age, year | –0.229 | –2.41 | 0.026 | |||
| V | V preop | 0.648 | 5.397 | <0.001 | 0.756 | <0.001 |
| Age | –0.404 | –3.123 | 0.006 | |||
| Gender (M0, W1) | 0.269 | 2.181 | 0.043 | |||
| BP | BP preop | 0.417 | 2.049 | 0.054 | 0.132 | 0.138 |
| GH | FT3 preop | –0.458 | –2.302 | 0.032 | 0.17 | 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbonas, M.; Raskauskiene, N.; Deltuva, V.P.; Bunevicius, A. Thyroid Hormones and Health-Related Quality of Life in Normal Pressure Hydrocephalus Patients before and after the Ventriculoperitoneal Shunt Surgery: A Longitudinal Study. J. Clin. Med. 2022, 11, 4438. https://doi.org/10.3390/jcm11154438
Urbonas M, Raskauskiene N, Deltuva VP, Bunevicius A. Thyroid Hormones and Health-Related Quality of Life in Normal Pressure Hydrocephalus Patients before and after the Ventriculoperitoneal Shunt Surgery: A Longitudinal Study. Journal of Clinical Medicine. 2022; 11(15):4438. https://doi.org/10.3390/jcm11154438
Chicago/Turabian StyleUrbonas, Mindaugas, Nijole Raskauskiene, Vytenis Pranas Deltuva, and Adomas Bunevicius. 2022. "Thyroid Hormones and Health-Related Quality of Life in Normal Pressure Hydrocephalus Patients before and after the Ventriculoperitoneal Shunt Surgery: A Longitudinal Study" Journal of Clinical Medicine 11, no. 15: 4438. https://doi.org/10.3390/jcm11154438
APA StyleUrbonas, M., Raskauskiene, N., Deltuva, V. P., & Bunevicius, A. (2022). Thyroid Hormones and Health-Related Quality of Life in Normal Pressure Hydrocephalus Patients before and after the Ventriculoperitoneal Shunt Surgery: A Longitudinal Study. Journal of Clinical Medicine, 11(15), 4438. https://doi.org/10.3390/jcm11154438







