Increased T-bet/GATA-3 and ROR-γt /Foxp3 Ratios in Cerebrospinal Fluid as Potential Criteria for Definite Neuro-Behçet’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood and CSF Samples
2.3. CSF Albumin and Immunoglobulin Analysis
2.4. RNA Isolation and cDNA Synthesis
2.5. Quantitative Real-Time PCR
2.6. Statistical Analysis
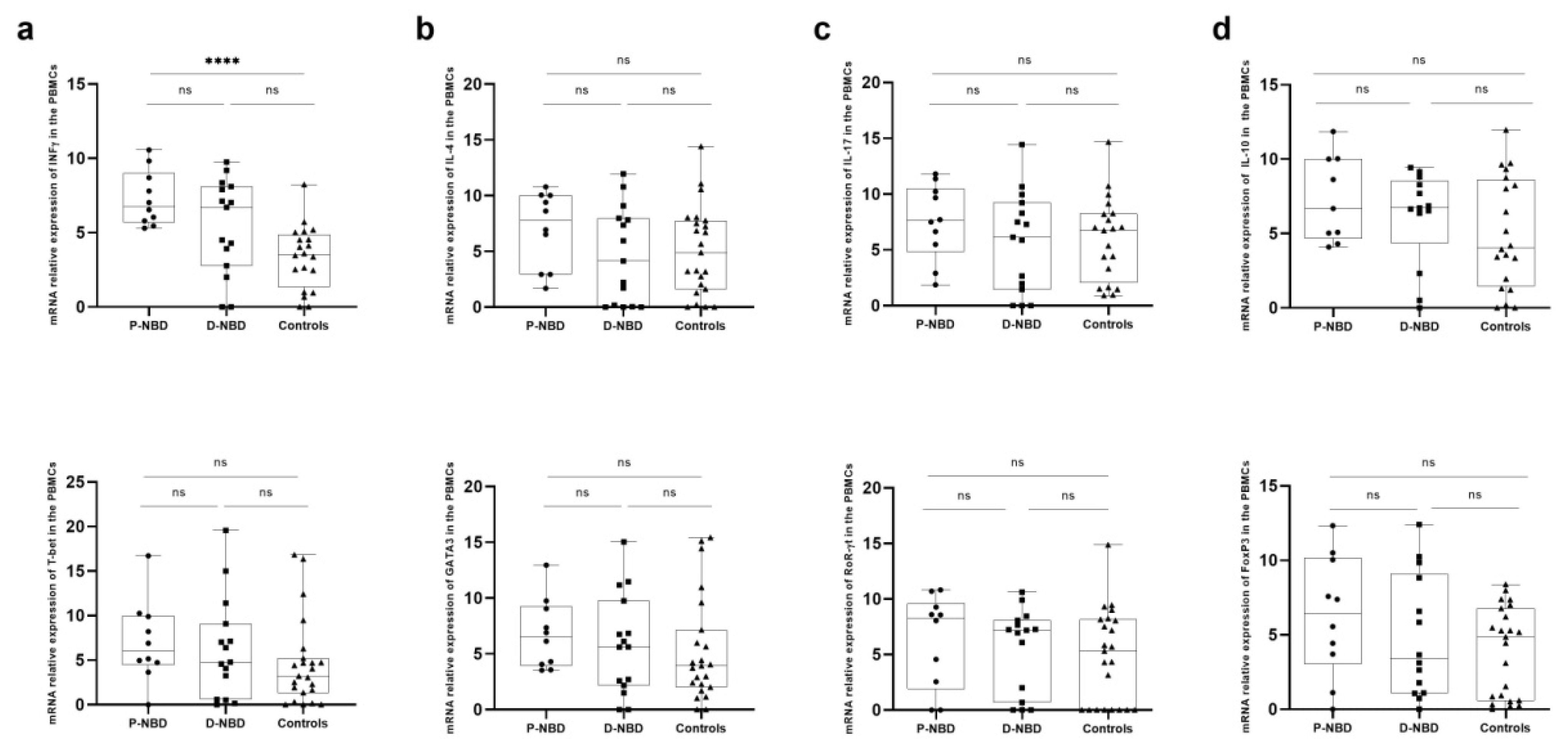
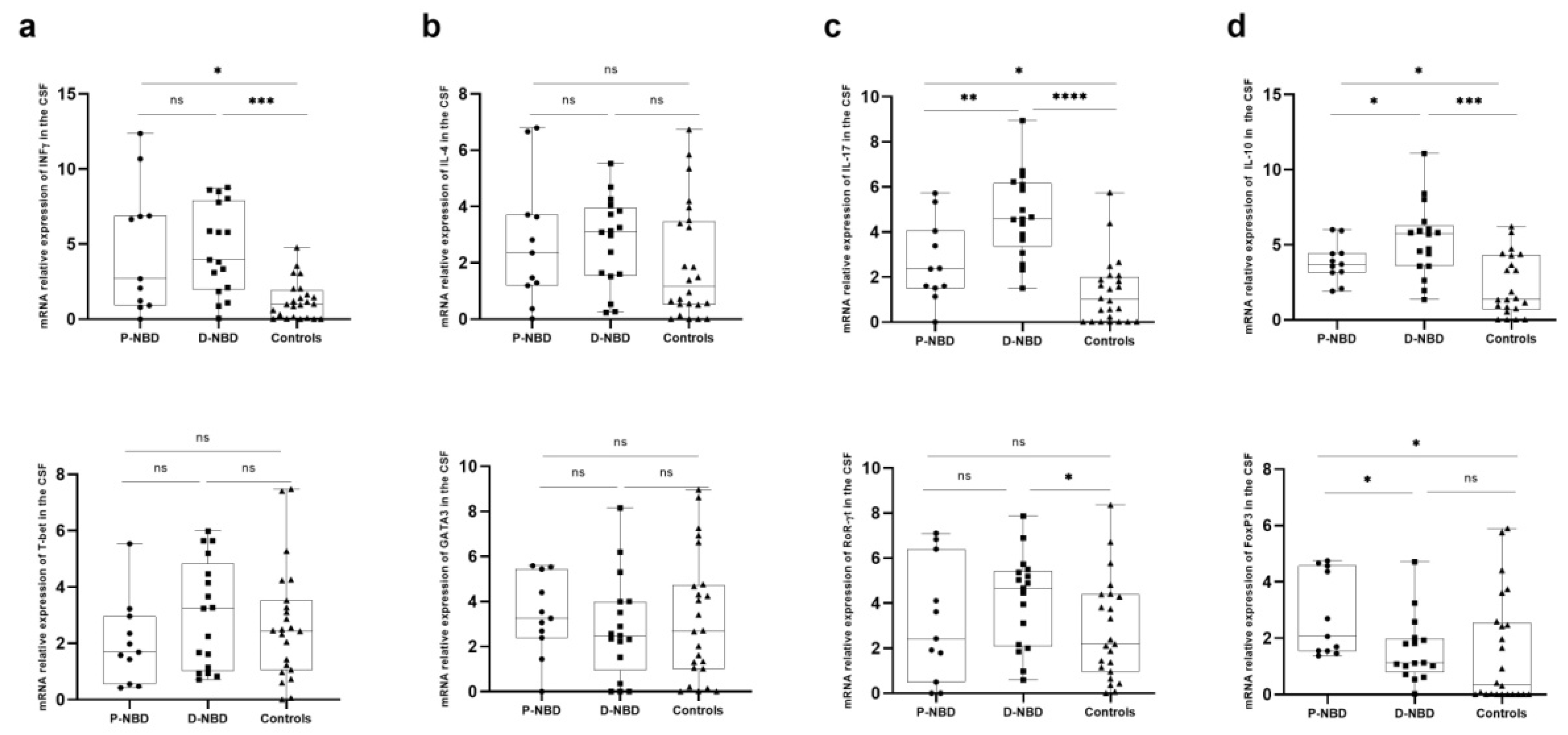
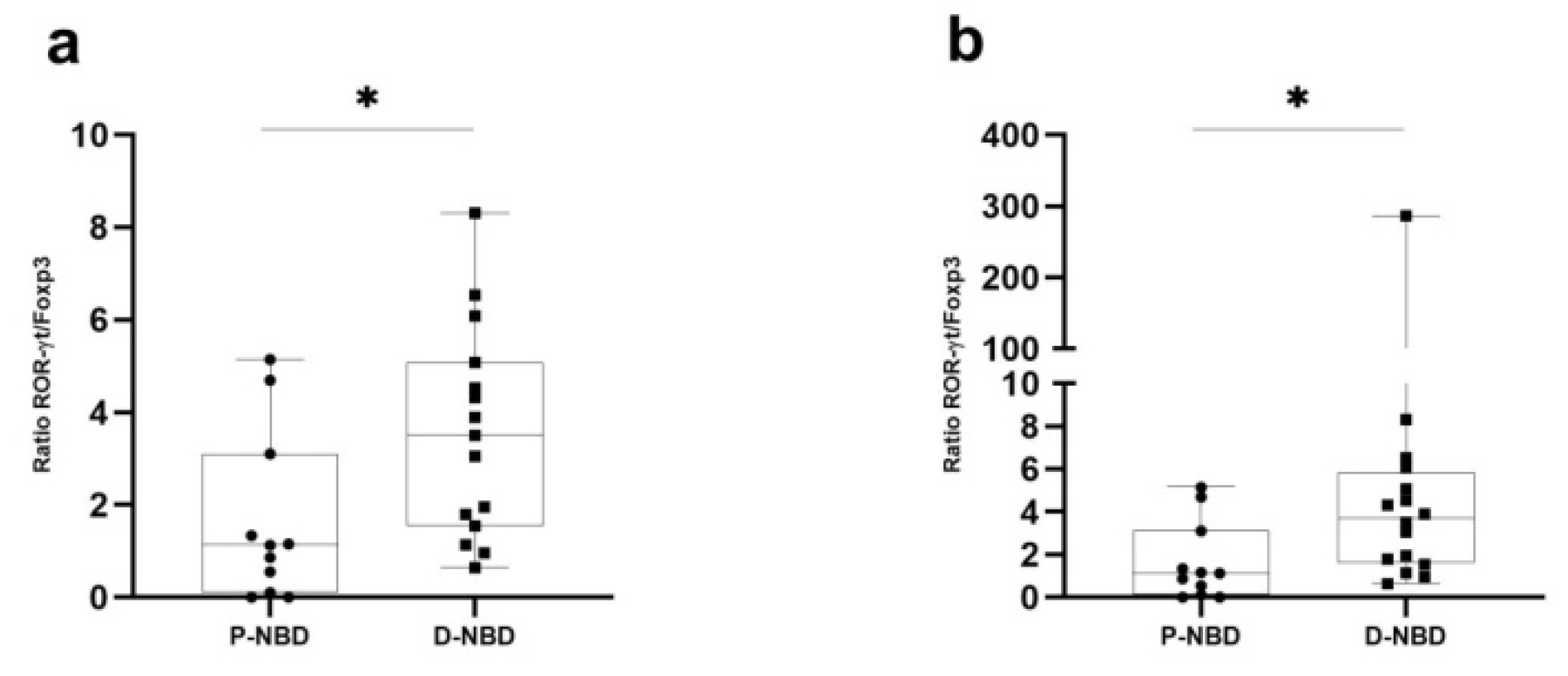
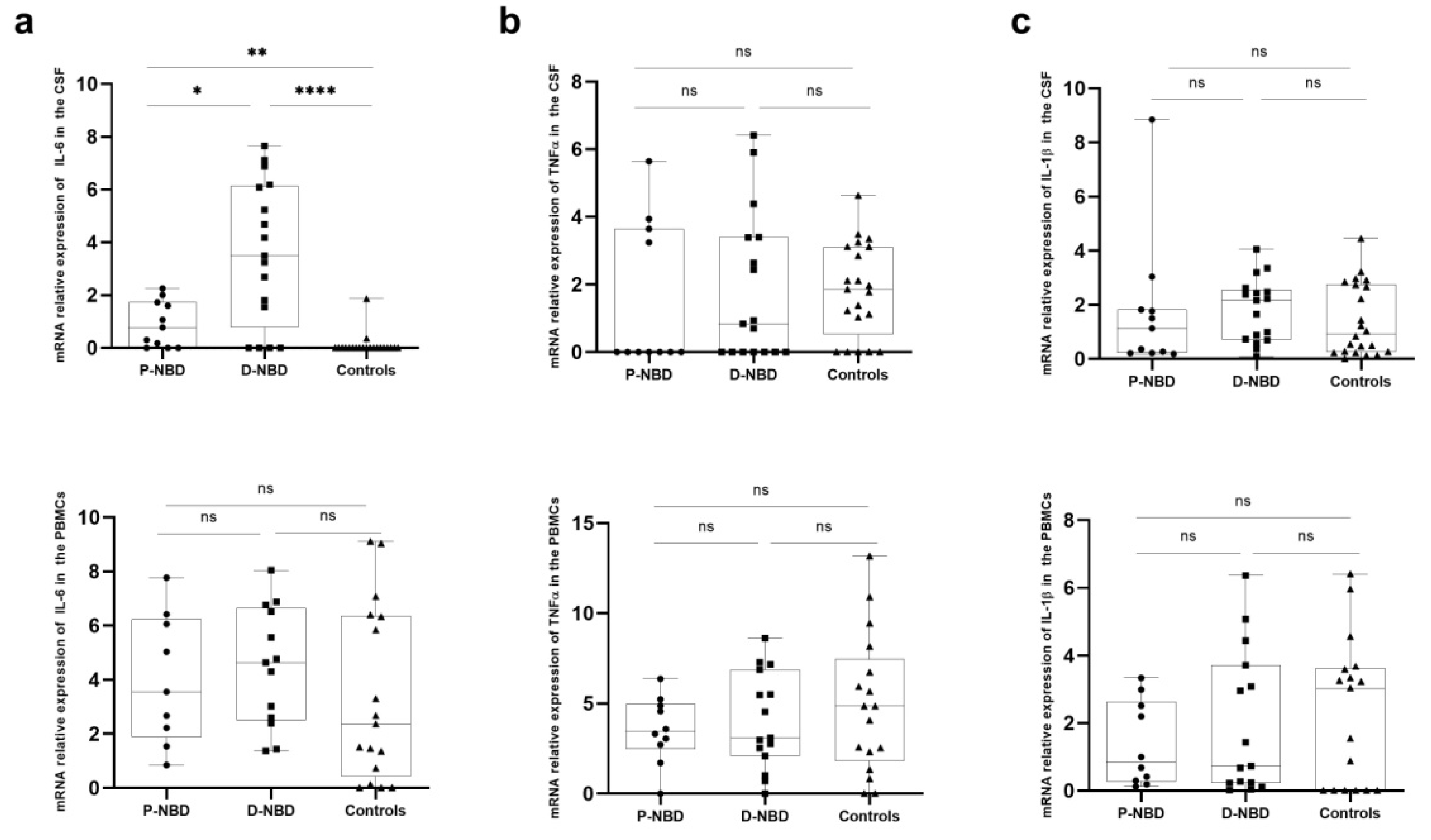
3. Results
3.1. Demographic Features
3.2. Neurological Characteristics, MRI Findings, and CSF Analysis
3.3. Cytokines Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borhani Haghighi, A.; Ittehadi, H.; Nikseresht, A.R.; Rahmati, J.; Ghaffari Poorjahromi, S.; Pourabbas, B.; Nazarinia, M.A.; Habibagahi, Z.; Fattahi, M.J.; Ghaderi, A. CSF Levels of Cytokines in Neuro-Behçet’s Disease. Clin. Neurol. Neurosurg. 2009, 111, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Al-Araji, A.; Kidd, D.P. Neuro-Behçet’s Disease: Epidemiology, Clinical Characteristics, and Management. Lancet Neurol. 2009, 8, 192–204. [Google Scholar] [CrossRef]
- Akman-Demir, G.; Serdaroglu, P.; Tasçi, B. Clinical Patterns of Neurological Involvement in Behçet’s Disease: Evaluation of 200 Patients. Brain 1999, 122, 2171–2182. [Google Scholar] [CrossRef] [Green Version]
- Saip, S.; Akman-Demir, G.; Siva, A. Neuro-Behçet Syndrome. Handb. Clin. Neurol. 2014, 121, 1703–1723. [Google Scholar]
- Albayram, S.; Saip, S.; Hasiloglu, Z.I.; Teke, M.; Ceyhan, E.; Tutuncu, M.; Selcuk, H.; Kina, A.; Siva, A. Evaluation of Parenchymal Neuro-Behçet Disease by Using Susceptibility-Weighted Imaging. Am. J. Neuroradiol. 2011, 32, 1050–1055. [Google Scholar] [CrossRef] [Green Version]
- Farah, S.; Al-Shubaili, A.; Montaser, A.; Hussein, J.M.; Malaviya, A.N.; Mukhtar, M.; Al-Shayeb, A.; Khuraibet, A.J.; Khan, R.; Trontelj, J.V. Behcet’s Syndrome: A Report of 41 Patients with Emphasis on Neurological Manifestations. J. Neurol. Neurosurg. Psychiatry 1998, 64, 382–384. [Google Scholar] [CrossRef]
- Tursen, U.; Gurler, A.; Boyvat, A. Evaluation of Clinical Findings according to Sex in 2313 Turkish Patients with Behcet’s Disease. Int. J. Dermatol. 2003, 42, 346–351. [Google Scholar] [CrossRef]
- Hamzaoui, S.B.; B’chir Hamzaoui, S.; Harmel, A.; Bouslama, K.; Abdallah, M.; Ennafaa, M.; M’rad, S.; Ben Dridi, M. La Maladie de Behçet En Tunisie. Étude Clinique de 519 Cas. La Rev. De Médecine Interne 2006, 27, 742–750. [Google Scholar]
- Houman, M.-H.; Bellakhal, S.; Ben Salem, T.; Hamzaoui, A.; Braham, A.; Lamloum, M.; Monia, S.-K.; Ben Ghorbel, I. Characteristics of Neurological Manifestations of Behçet’s Disease: A Retrospective Monocentric Study in Tunisia. Clin. Neurol. Neurosurg. 2013, 115, 2015–2018. [Google Scholar] [CrossRef]
- Sidhom, Y.; Maillart, E.; du Montcel, S.T.; Kacem, I.; Lubetzki, C.; Gouider, R.; Papeix, C. Fast Multiple Sclerosis Progression in North Africans. Neurology 2017, 88, 1218–1225. [Google Scholar] [CrossRef]
- Kalra, S.; Silman, A.; Akman-Demir, G.; Bohlega, S.; Borhani-Haghighi, A.; Constantinescu, C.S.; Houman, H.; Mahr, A.; Salvarani, C.; Sfikakis, P.P.; et al. Diagnosis and Management of Neuro-Behçet’s Disease: International Consensus Recommendations. J. Neurol. 2014, 261, 1662–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saruhan-Direskeneli, G.; Yentür, S.P.; Akman-Demir, G.; Işik, N.; Serdaroğlu, P. Cytokines and Chemokines in Neuro-Behçet’s Disease Compared to Multiple Sclerosis and Other Neurological Diseases. J. Neuroimmunol. 2003, 145, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hirohata, S.; Kikuchi, H. Changes in Biomarkers Focused on Differences in Disease Course or Treatment in Patients with Neuro-Behçet’s Disease. Intern. Med. 2012, 51, 3359–3365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamzaoui, K.; Borhani-Haghighi, A.; Kaabachi, W.; Hamzaoui, A. Increased Interleukin 33 in Patients with Neuro-Behcet’s Disease: Correlation with MCP-1 and IP-10 Chemokines. Cell. Mol. Immunol. 2014, 11, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Aridogan, B.C.; Yildirim, M.; Baysal, V.; Inaloz, H.S.; Baz, K.; Kaya, S. Serum Levels of IL-4, IL-10, IL-12, IL-13 and IFN-Gamma in Behçet’s Disease. J. Dermatol. 2003, 30, 602–607. [Google Scholar] [CrossRef]
- Wang, C.R.; Chuang, C.Y.; Chen, C.Y. Anticardiolipin Antibodies and Interleukin-6 in Cerebrospinal Fluid and Blood of Chinese Patients with Neuro-Behçet’s Syndrome. Clin. Exp. Rheumatol. 1992, 10, 599–602. [Google Scholar]
- Nanke, Y.; Yago, T.; Kotake, S. The Role of Th17 Cells in the Pathogenesis of Behcet’s Disease. J. Clin. Med. Res. 2017, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Hirohata, S.; Kikuchi, H.; Sawada, T.; Nagafuchi, H.; Kuwana, M.; Takeno, M.; Ishigatsubo, Y. Clinical Characteristics of Neuro-Behcet’s Disease in Japan: A Multicenter Retrospective Analysis. Mod. Rheumatol. 2012, 22, 405–413. [Google Scholar] [CrossRef]
- Andersson, M.; Alvarez-Cermeño, J.; Bernardi, G.; Cogato, I.; Fredman, P.; Frederiksen, J.; Fredrikson, S.; Gallo, P.; Grimaldi, L.M.; Grønning, M. Cerebrospinal Fluid in the Diagnosis of Multiple Sclerosis: A Consensus Report. J. Neurol. Neurosurg. Psychiatry 1994, 57, 897–902. [Google Scholar] [CrossRef] [Green Version]
- Freedman, M.S.; Thompson, E.J.; Deisenhammer, F.; Giovannoni, G.; Grimsley, G.; Keir, G.; Ohman, S.; Racke, M.K.; Sharief, M.; Sindic, C.J.M.; et al. Recommended Standard of Cerebrospinal Fluid Analysis in the Diagnosis of Multiple Sclerosis: A Consensus Statement. Arch. Neurol. 2005, 62, 865–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belghith, M.; Bahrini, K.; Kchaou, M.; Maghrebi, O.; Belal, S.; Barbouche, M.R. Cerebrospinal Fluid IL-10 as an Early Stage Discriminative Marker between Multiple Sclerosis and Neuro-Behçet Disease. Cytokine 2018, 108, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Bahrini, K.; Belghith, M.; Maghrebi, O.; Bekir, J.; Kchaou, M.; Jeridi, C.; Amouri, R.; Hentati, F.; Belal, S.; Ben Sassi, S.; et al. Discriminative Expression of CD39 and CD73 in Cerebrospinal Fluid of Patients with Multiple Sclerosis and Neuro-Behçet’s Disease. Cytokine 2020, 130, 155054. [Google Scholar] [CrossRef]
- Akman-Demir, G.; Tüzün, E.; Içöz, S.; Yeşilot, N.; Yentür, S.P.; Kürtüncü, M.; Mutlu, M.; Saruhan-Direskeneli, G. Interleukin-6 in Neuro-Behçet’s Disease: Association with Disease Subsets and Long-Term Outcome. Cytokine 2008, 44, 373–376. [Google Scholar] [CrossRef]
- Hirohata, S.; Isshi, K.; Oguchi, H.; Ohse, T.; Haraoka, H.; Takeuchi, A.; Hashimoto, T. Cerebrospinal Fluid Interleukin-6 in Progressive Neuro-Behçet’s Syndrome. Clin. Immunol. Immunopathol. 1997, 82, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Al-Araji, A.; Sharquie, K.; Al-Rawi, Z. Prevalence and Patterns of Neurological Involvement in Behcet’s Disease: A Prospective Study from Iraq. J. Neurol. Neurosurg. Psychiatry 2003, 74, 608–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serdaroğlu, P.; Yazici, H.; Özdemir, C.; Yurdakul, S.; Bahar, S.; Aktin, E. Neurologic Involvement in Behçet’s Syndrome: A Prospective Study. Arch. Neurol. 1989, 46, 265–269. [Google Scholar] [CrossRef]
- Ashjazadeh, N.; Borhani Haghighi, A.; Samangooie, S.; Moosavi, H. Neuro-Behcet’s Disease: A Masquerader of Multiple Sclerosis. A Prospective Study of Neurologic Manifestations of Behcet’s Disease in 96 Iranian Patients. Exp. Mol. Pathol. 2003, 74, 17–22. [Google Scholar] [CrossRef]
- Al-Fahad, S.A.; Al-Araji, A.H. Neuro-Behcet’s Disease in Iraq: A Study of 40 Patients. J. Neurol. Sci. 1999, 170, 105–111. [Google Scholar] [CrossRef]
- Joseph, F.G.; Scolding, N.J. Neuro-Behçet’s Disease in Caucasians: A Study of 22 Patients. Eur. J. Neurol. 2007. [Google Scholar] [CrossRef]
- Kidd, D.; Steuer, A.; Denman, A.M.; Rudge, P. Neurological Complications in Behçet’s Syndrome. Brain 1999, 122 Pt 11, 2183–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koçer, N.; Islak, C.; Siva, A.; Saip, S.; Akman, C.; Kantarci, O.; Hamuryudan, V. CNS Involvement in Neuro-Behçet Syndrome: An MR Study. AJNR Am. J. Neuroradiol. 1999, 20, 1015–1024. [Google Scholar] [PubMed]
- Uygunoglu, U.; Zeydan, B.; Ozguler, Y.; Ugurlu, S.; Seyahi, E.; Kocer, N.; Islak, C.; Kantarci, K.; Saip, S.; Siva, A.; et al. Myelopathy in Behçet’s Disease: The Bagel Sign. Ann. Neurol. 2017, 82, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Takase, S.; Itahara, K. Cytological Examination of Cerebrospinal Fluid in Eight Patients with Neuro-Behcet’s Disease. Tohoku J. Exp. Med. 1980, 132, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Saruhan-Direskeneli, G.; Yentür, S.P.; Mutlu, M.; Shugaiv, E.; Yesilot, N.; Kürtüncü, M.; Akman-Demir, G. Intrathecal Oligoclonal IgG Bands Are Infrequently Found in Neuro-Behçet’s Disease. Clin. Exp. Rheumatol. 2013, 31, 25–27. [Google Scholar]
- Kapsimali, V.D.; Kanakis, M.A.; Vaiopoulos, G.A.; Kaklamanis, P.G. Etiopathogenesis of Behçet’s Disease with Emphasison the Role of Immunological Aberrations. Clin. Rheumatol. 2010, 29, 1211–1216. [Google Scholar] [CrossRef]
- Zhou, C.; Hao, G.; Thomas, P.; Liu, J.; Yu, M.; Sun, S.; Öz, O.K.; Sun, X.; Zheng, J. Near-Infrared Emitting Radioactive Gold Nanoparticles with Molecular Pharmacokinetics. Angew. Chem. Int. Ed. Engl. 2012, 51, 10118–10122. [Google Scholar] [CrossRef]
- Chi, W.; Zhu, X.; Yang, P.; Liu, X.; Lin, X.; Zhou, H.; Huang, X.; Kijlstra, A. Upregulated IL-23 and IL-17 in Behçet Patients with Active Uveitis. Investig. Opthalmology Vis. Sci. 2008, 49, 3058. [Google Scholar] [CrossRef]
- Hamzaoui, A.; Maalmi, H.; Berraïes, A.; Abid, H.; Ammar, J.; Hamzaoui, K. Transcriptional Characteristics of CD4 T Cells in Young Asthmatic Children: RORC and FOXP3 Axis. J. Inflamm. Res. 2011, 4, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Hamzaoui, K.; Hamzaoui, A.; Ghorbel, I.; Khanfir, M.; Houman, H. Levels of IL-15 in Serum and Cerebrospinal Fluid of Patients with Behçet’s Disease. Scand. J. Immunol. 2006, 64, 655–660. [Google Scholar] [CrossRef]
- Aldinucci, A.; Bonechi, E.; Biagioli, T.; Repice, A.M.; D’Elios, M.M.; Emmi, L.; Emmi, G.; Silvestri, E.; Barilaro, A.; Ballerini, C. CSF/serum Matrix Metallopeptidase-9 Ratio Discriminates Neuro Behçet from Multiple Sclerosis. Ann. Clin. Transl. Neurol. 2018, 5, 493–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirohata, S.; Suda, H.; Hashimoto, T. Low-Dose Weekly Methotrexate for Progressive Neuropsychiatric Manifestations in Behcet’s Disease. J. Neurol. Sci. 1998, 159, 181–185. [Google Scholar] [CrossRef]
- Hirohata, S.; Kikuchi, H.; Sawada, T.; Nagafuchi, H.; Kuwana, M.; Takeno, M.; Ishigatsubo, Y. Retrospective Analysis of Long-Term Outcome of Chronic Progressive Neurological Manifestations in Behcet’s Disease. J. Neurol. Sci. 2015, 349, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Maghrebi, O.; Hanachi, M.; Bahrini, K.; Kchaou, M. Differential Gene Expression Patterns in Blood and Cerebrospinal Fluid of Multiple Sclerosis and Neuro-Behçet Disease. Frontiers in 2021. Front. Genet. 2021, 12, 638236. [Google Scholar] [CrossRef]

| Overall (Patients) | Definite NBD | Probable NBD | Controls | p Value | |
|---|---|---|---|---|---|
| n = 48 | n = 28 | n = 17 | n = 11 | n = 20 | |
| Gender (F/M) | 12/16 | 8/9 | 4/7 | 16/4 | 0.0312 |
| Age (mean ± SD) | 40.66 (±10.39) | 41 (±11.14) | 40.2 (±9.80) | 42.52 (±20.96) | 0.8941 |
| Patients with BD first (ISG+) | n = 14 | n = 10 | n = 4 | 0.0754 | |
| Interval between BD and onset of neurological signs (months) | 26.14 (±22.02) | 31.2 (±16.21) | 13.5 (±12.54) | ||
| Patients with neurological signs first | n = 14 | n = 7 | n = 7 | ||
| Interval between neurological signs and BD (months) | - | 5.57 (±3.95) | - | ||
| Age at onset of neurological signs (mean ± SD) | 35.25 (9.74) | 35.76 (9.62) | 37.45 (9.00) | 0.5716 | |
| Follow-up period (months) (mean ± SD) | 49.5 (±13.4) | 50.46 (±14.2) | 49 (±13.2) | 0.7870 |
| Overall Patients (n = 28) | Definite NBD (n = 17) | Probable NBD (n = 11) | Controls (n = 20) | p Value | |
|---|---|---|---|---|---|
| Neurological characteristics: | - | ||||
| - Headaches | 8 | 4 | 4 | ||
| - Hemiparesis/pyramidal signs | 22 | 14 | 8 | ||
| - Sensory symptoms | 12 | 6 | 6 | ||
| - Cranial nerve involvement | 9 | 5 | 4 | ||
| - Ataxia | 6 | 5 | 1 | ||
| - Psychiatric signs | 5 | 1 | 4 | ||
| - Seizures | 1 | 1 | 0 | ||
| MRI findings | - | ||||
| - Isolated supratentorial location | 13 | 8 | 5 | ||
| - Basal ganglia | 9 | 5 | 4 | ||
| - Internal capsule | 7 | 2 | 5 | ||
| - Brainstem lesions | 14 | 9 | 4 | ||
| - Myelitis | 1 | 0 | 1 | ||
| - No abnormalities | 2 | 0 | 2 | ||
| CSF analysis | |||||
| - CSF protein concentration (g/L) | … | 0.60 (+0.21) | 0.63 (+0.19) | 0.39 (+0.13) | 0.0004 |
| - Cell count (/mm3) | … | 32.6 (+7.32) | 23.7 (+7.66) | 4.3 (+5.6) | <0.0001 |
| - IgG Index | … | 0.59 | 0.495 | 0.49 | 0.4979 |
| - IEPP | |||||
| Type 1 | 25 | 15 | 10 | 20 | |
| Type 2 and 3 | 1 | 0 | 1 | 0 | |
| Type 4 | 2 | 2 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belghith, M.; Maghrebi, O.; Cherif, A.; Bahrini, K.; Saied, Z.; Belal, S.; Sassi, S.B.; Barbouche, M.-R.; Kchaou, M. Increased T-bet/GATA-3 and ROR-γt /Foxp3 Ratios in Cerebrospinal Fluid as Potential Criteria for Definite Neuro-Behçet’s Disease. J. Clin. Med. 2022, 11, 4415. https://doi.org/10.3390/jcm11154415
Belghith M, Maghrebi O, Cherif A, Bahrini K, Saied Z, Belal S, Sassi SB, Barbouche M-R, Kchaou M. Increased T-bet/GATA-3 and ROR-γt /Foxp3 Ratios in Cerebrospinal Fluid as Potential Criteria for Definite Neuro-Behçet’s Disease. Journal of Clinical Medicine. 2022; 11(15):4415. https://doi.org/10.3390/jcm11154415
Chicago/Turabian StyleBelghith, Meriam, Olfa Maghrebi, Aroua Cherif, Khadija Bahrini, Zakaria Saied, Samir Belal, Samia Ben Sassi, Mohamed-Ridha Barbouche, and Mariem Kchaou. 2022. "Increased T-bet/GATA-3 and ROR-γt /Foxp3 Ratios in Cerebrospinal Fluid as Potential Criteria for Definite Neuro-Behçet’s Disease" Journal of Clinical Medicine 11, no. 15: 4415. https://doi.org/10.3390/jcm11154415
APA StyleBelghith, M., Maghrebi, O., Cherif, A., Bahrini, K., Saied, Z., Belal, S., Sassi, S. B., Barbouche, M.-R., & Kchaou, M. (2022). Increased T-bet/GATA-3 and ROR-γt /Foxp3 Ratios in Cerebrospinal Fluid as Potential Criteria for Definite Neuro-Behçet’s Disease. Journal of Clinical Medicine, 11(15), 4415. https://doi.org/10.3390/jcm11154415






