The Impact of Mental Stress on Cardiovascular Health—Part II
Abstract
1. Introduction
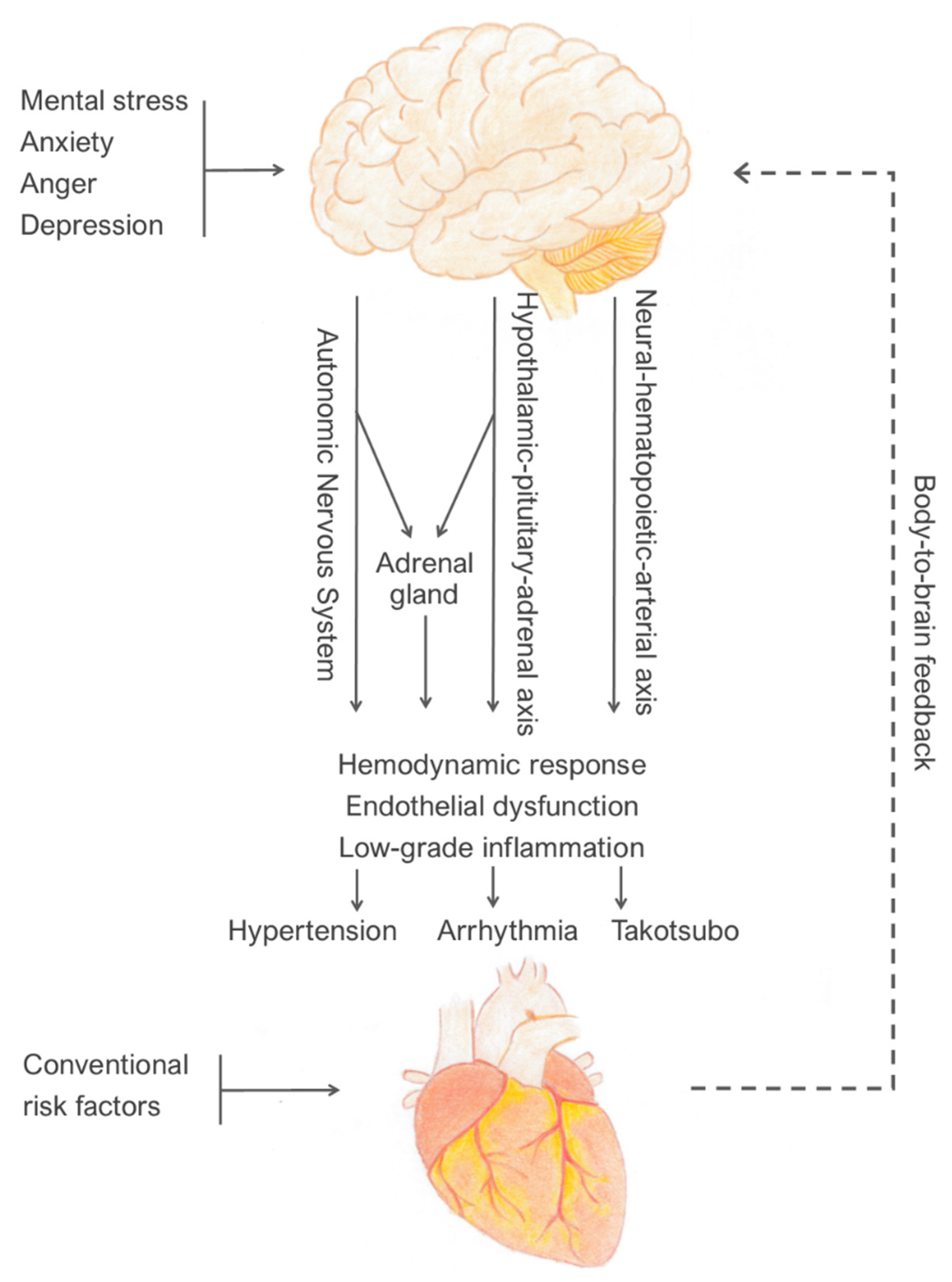
2. Recognised Underlying Mechanisms That Can Be Measured in Laboratory
3. Clinical Conditions Associated with Mental Stress
4. Gender Differences in Response to Mental Stress
5. Prevention and Treatment
6. Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-Control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Rosengren, A.; Hawken, S.; Ôunpuu, S.; Sliwa, K.; Zubaid, M.; Almahmeed, W.A.; Blackett, K.N.; Sitthi-amorn, C.; Sato, H.; Yusuf, S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11,119 cases and 13,648 controls from 52 countries (the INTERHEART study): Case-Control study. Lancet 2004, 364, 953–962. [Google Scholar] [CrossRef]
- Mostofsky, E.; Penner, E.A.; Mittleman, M.A. Outbursts of anger as a trigger of acute cardiovascular events: A systematic review and meta-analysis. Eur. Heart J. 2014, 35, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, A. Behavioral Cardiology: Current Advances and Future Directions. J. Am. Coll. Cardiol. 2014, 64, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, F.; Longo, G.; Vancheri, E.; Henein, M.Y. Mental Stress and Cardiovascular Health. J. Clin. Med. 2022, 11, 3353. [Google Scholar] [CrossRef] [PubMed]
- Brutsaert, D.L. Cardiac Endothelial-Myocardial Signaling: Its Role in Cardiac Growth, Contractile Performance, and Rhythmicity. Physiol. Rev. 2003, 83, 59–115. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109, III-27–III-32. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.-T.; Tan, Q.-w.; Li, P.; Mou, S.-f.; Liu, S.-j.; Bao, Y.; Jiao, H.-c.; Su, W.-G. Investigating the role of acute mental stress on endothelial dysfunction: A systematic review and meta-analysis. Clin. Res. Cardiol. 2015, 104, 310–319. [Google Scholar] [CrossRef]
- Camici, P.G.; Crea, F. Coronary Microvascular Dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Flammer, A.J.; Lerman, L.O.; Elízaga, J.; Lerman, A.; Fernández-Avilés, F. Endothelial dysfunction over the course of coronary artery disease. Eur. Heart J. 2013, 34, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Nakanishi-Toda, M. How mental stress affects endothelial function. Pflügers Arch. Eur. J. Physiol. 2011, 462, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Gokce, N.; Keaney, J.F.; Vita, J.A. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 42, 1149–1160. [Google Scholar] [CrossRef]
- Spicer, J.; Shimbo, D.; Johnston, N.; Harlapur, M.; Purdie-Vaughns, V.; Cook, J.; Fu, J.; Burg, M.M.; Wager, T.D. Prevention of Stress-Provoked Endothelial Injury by Values Affirmation: A Proof of Principle Study. Ann. Behav. Med. 2015, 50, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Shimbo, D.; Rosenberg, L.B.; Chaplin, W.; Zhao, S.; Goldensohn, E.R.; Cholankeril, M.; Fu, J.; Hong, S.B.; Jelic, S.; Burg, M.M. Endothelial cell activation, reduced endothelial cell reparative capacity, and impaired endothelial-dependent vasodilation after anger provocation. Int. J. Cardiol. 2013, 167, 1064–1065. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Vallance, P.; Vita, J.; Vogel, R.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Poitras, V.J.; Pyke, K.E. The impact of acute mental stress on vascular endothelial function: Evidence, mechanisms and importance. Int. J. Psychophysiol. 2013, 88, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Spieker, L.E.; Hürlimann, D.; Ruschitzka, F.; Corti, R.; Enseleit, F.; Shaw, S.; Hayoz, D.; Deanfield, J.E.; Lüscher, T.F.; Noll, G. Mental Stress Induces Prolonged Endothelial Dysfunction via Endothelin—A Receptors. Circulation 2002, 105, 2817–2820. [Google Scholar] [CrossRef]
- Broadley, A.J.M.; Korszun, A.; Abdelaal, E.; Moskvina, V.; Jones, C.J.H.; Nash, G.B.; Ray, C.; Deanfield, J.; Frenneaux, M.P. Inhibition of Cortisol Production with Metyrapone Prevents Mental Stress-Induced Endothelial Dysfunction and Baroreflex Impairment. J. Am. Coll. Cardiol. 2005, 46, 344–350. [Google Scholar] [CrossRef]
- Kivimäki, M.; Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 2018, 15, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Ghiadoni, L.; Donald, A.E.; Cropley, M.; Mullen, M.J.; Oakley, G.; Taylor, M.; O’Connor, G.; Betteridge, J.; Klein, N.; Steptoe, A.; et al. Mental Stress Induces Transient Endothelial Dysfunction in Humans. Circulation 2000, 102, 2473–2478. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.B.; Hammadah, M.; Kim, J.H.; Uphoff, I.; Shah, A.; Levantsevych, O.; Almuwaqqat, Z.; Moazzami, K.; Sullivan, S.; Ward, L.; et al. Association of Transient Endothelial Dysfunction Induced by Mental Stress with Major Adverse Cardiovascular Events in Men and Women with Coronary Artery Disease. JAMA Cardiol. 2019, 4, 988–996. [Google Scholar] [CrossRef]
- Waclawovsky, A.J.; de Brito, E.; Smith, L.; Vancampfort, D.; da Silva, A.M.V.; Schuch, F.B. Endothelial dysfunction in people with depressive disorders: A systematic review and meta-analysis. J. Psychiatr. Res. 2021, 141, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Aird, C.W. Endothelium as an organ system. Crit. Care Med. 2004, 32, S271–S279. [Google Scholar] [CrossRef]
- Lavi, S.; Bae, J.-H.; Rihal, C.S.; Prasad, A.; Barsness, G.W.; Lennon, R.J.; Holmes, D.R.; Lerman, A. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart 2009, 95, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From Vulnerable Plaque to Vulnerable Patient: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef]
- Choi, B.-J.; Prasad, A.; Gulati, R.; Best, P.J.; Lennon, R.J.; Barsness, G.W.; Lerman, L.O.; Lerman, A. Coronary endothelial dysfunction in patients with early coronary artery disease is associated with the increase in intravascular lipid core plaque. Eur. Heart J. 2013, 34, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Gössl, M.; Yoon, M.-H.; Choi, B.-J.; Rihal, C.; Tilford, J.M.; Reriani, M.; Gulati, R.; Sandhu, G.; Eeckhout, E.; Lennon, R.; et al. Accelerated Coronary Plaque Progression and Endothelial Dysfunction: Serial Volumetric Evaluation by IVUS. JACC Cardiovasc. Imaging 2014, 7, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, J.; Zhang, L.; Xu, W.; He, D.; Wei, W.; Ge, Y.; Dandu, C. An evidence of brain-heart disorder: Mental stress-induced myocardial ischemia regulated by inflammatory cytokines. Neurol. Res. 2020, 42, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Paine, N.J.; Bosch, J.A.; Veldhuijzen Van Zanten, J.J.C.S. Inflammation and Vascular Responses to Acute Mental Stress: Implications for the Triggering of Myocardial Infarction. Curr. Pharm. Des. 2012, 18, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Hammadah, M.; Sullivan, S.; Pearce, B.; Al Mheid, I.; Wilmot, K.; Ramadan, R.; Tahhan, A.S.; O’Neal, W.T.; Obideen, M.; Alkhoder, A.; et al. Inflammatory response to mental stress and mental stress induced myocardial ischemia. Brain Behav. Immun. 2018, 68, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, A.; Georgiopoulou, V.; Psaty, B.M.; Rodondi, N.; Smith, A.L.; Harrison, D.G.; Liu, Y.; Hoffmann, U.; Bauer, D.C.; Newman, A.B.; et al. Inflammatory Markers and Incident Heart Failure Risk in Older Adults: The Health ABC (Health, Aging, and Body Composition) Study. J. Am. Coll. Cardiol. 2010, 55, 2129–2137. [Google Scholar] [CrossRef]
- Kang, D.O.; Eo, J.S.; Park, E.J.; Nam, H.S.; Song, J.W.; Park, Y.H.; Park, S.Y.; Na, J.O.; Choi, C.U.; Kim, E.J.; et al. Stress-associated neurobiological activity is linked with acute plaque instability via enhanced macrophage activity: A prospective serial 18F-FDG-PET/CT imaging assessment. Eur. Heart J. 2021, 42, 1883–1895. [Google Scholar] [CrossRef]
- Moriya, J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 2019, 73, 22–27. [Google Scholar] [CrossRef]
- Libby, P.; Tabas, I.; Fredman, G.; Fisher, E.A. Inflammation and its Resolution as Determinants of Acute Coronary Syndromes. Circ. Res. 2014, 114, 1867–1879. [Google Scholar] [CrossRef]
- Rohleder, N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom. Med. 2014, 76, 181–189. [Google Scholar] [CrossRef]
- Tawakol, A.; Ishai, A.; Takx, R.A.P.; Figueroa, A.L.; Ali, A.; Kaiser, Y.; Truong, Q.A.; Solomon, C.J.E.; Calcagno, C.; Mani, V.; et al. Relation between resting amygdalar activity and cardiovascular events: A longitudinal and cohort study. Lancet 2017, 389, 834–845. [Google Scholar] [CrossRef]
- Goyal, A.; Dey, A.K.; Chaturvedi, A.; Elnabawi, Y.A.; Aberra, T.M.; Chung, J.H.; Belur, A.D.; Groenendyk, J.W.; Lerman, J.B.; Rivers, J.P.; et al. Chronic Stress-Related Neural Activity Associates with Subclinical Cardiovascular Disease in Psoriasis: A Prospective Cohort Study. JACC Cardiovasc. Imaging 2020, 13, 465–477. [Google Scholar] [CrossRef]
- Gianaros, P.J.; Hariri, A.R.; Sheu, L.K.; Muldoon, M.F.; Sutton-Tyrrell, K.; Manuck, S.B. Preclinical Atherosclerosis Covaries with Individual Differences in Reactivity and Functional Connectivity of the Amygdala. Biol. Psychiatry 2009, 65, 943–950. [Google Scholar] [CrossRef]
- Muscatell, K.A.; Dedovic, K.; Slavich, G.M.; Jarcho, M.R.; Breen, E.C.; Bower, J.E.; Irwin, M.R.; Eisenberger, N.I. Greater amygdala activity and dorsomedial prefrontal–amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav. Immun. 2015, 43, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Kop, W.J.; Weissman, N.J.; Zhu, J.; Bonsall, R.W.; Doyle, M.; Stretch, M.R.; Glaes, S.B.; Krantz, D.S.; Gottdiener, J.S.; Tracy, R.P. Effects of Acute Mental Stress and Exercise on Inflammatory Markers in Patients with Coronary Artery Disease and Healthy Controls. Am. J. Cardiol. 2008, 101, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Tracey, K.J. The vagus nerve and the inflammatory reflex—Linking immunity and metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef]
- Olshansky, B. Vagus nerve modulation of inflammation: Cardiovascular implications. Trends Cardiovasc. Med. 2016, 26, 1–11. [Google Scholar] [CrossRef]
- Wirtz, P.H.; von Känel, R. Psychological Stress, Inflammation, and Coronary Heart Disease. Curr. Cardiol. Rep. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Steptoe, A.; Hamer, M.; Chida, Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav. Immun. 2007, 21, 901–912. [Google Scholar] [CrossRef]
- Burg, M.M.; Soufer, A.; Lampert, R.; Collins, D.; Soufer, R. Autonomic Contribution to Endothelin-1 Increase during Laboratory Anger-Recall Stress in Patients with Coronary Artery Disease. Mol. Med. 2011, 17, 495–501. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Zhang, W.; Speiser, J.L.; Ye, F.; Tsai, M.Y.; Cainzos-Achirica, M.; Nasir, K.; Herrington, D.M.; Shapiro, M.D. High-Sensitivity C-Reactive Protein Modifies the Cardiovascular Risk of Lipoprotein(a): Multi-Ethnic Study of Atherosclerosis. J. Am. Coll. Cardiol. 2021, 78, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W. C-Reactive Protein and Cardiovascular Risk: Will the Controversy End after CANTOS? Clin. Chem. 2017, 63, 1897–1898. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W. High-sensitivity C-reactive protein and atherosclerotic disease: From improved risk prediction to risk-guided therapy. Int. J. Cardiol. 2013, 168, 5126–5134. [Google Scholar] [CrossRef]
- Heidt, T.; Sager, H.B.; Courties, G.; Dutta, P.; Iwamoto, Y.; Zaltsman, A.; von Zur Muhlen, C.; Bode, C.; Fricchione, G.L.; Denninger, J.; et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 2014, 20, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Hinterdobler, J.; Schott, S.; Jin, H.; Meesmann, A.; Steinsiek, A.-L.; Zimmermann, A.-S.; Wobst, J.; Müller, P.; Mauersberger, C.; Vilne, B.; et al. Acute mental stress drives vascular inflammation and promotes plaque destabilization in mouse atherosclerosis. Eur. Heart J. 2021, 42, 4077–4088. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.N.; Hedrick, C.C. Stressing out stem cells: Linking stress and hematopoiesis in cardiovascular disease. Nat. Med. 2014, 20, 707–708. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden Charlotte, D.C.C.; Groh, L.; Keating, S.T.; Kaffa, C.; Noz, M.P.; Kersten, S.; Herwaarden, A.E.v.; Hoischen, A.; Joosten, L.A.B.; Timmers, H.J.L.M.; et al. Catecholamines Induce Trained Immunity in Monocytes In Vitro and In Vivo. Circ. Res. 2020, 127, 269–283. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M. Leukocyte Behavior in Atherosclerosis, Myocardial Infarction, and Heart Failure. Science 2013, 339, 161–166. [Google Scholar] [CrossRef]
- Moore, K.J.; Tabas, I. Macrophages in the Pathogenesis of Atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Mechanisms of Acute Coronary Syndromes and Their Implications for Therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef]
- Lagraauw, H.M.; Wezel, A.; van der Velden, D.; Kuiper, J.; Bot, I. Stress-induced mast cell activation contributes to atherosclerotic plaque destabilization. Sci. Rep. 2019, 9, 2134. [Google Scholar] [CrossRef] [PubMed]
- Eryd, S.A.; Smith, J.G.; Melander, O.; Hedblad, B.; Engström, G. Incidence of Coronary Events and Case Fatality Rate in Relation to Blood Lymphocyte and Neutrophil Counts. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Lagraauw, H.M.; Kuiper, J.; Bot, I. Acute and chronic psychological stress as risk factors for cardiovascular disease: Insights gained from epidemiological, clinical and experimental studies. Brain Behav. Immun. 2015, 50, 18–30. [Google Scholar] [CrossRef]
- Walker, B.R. Glucocorticoids and Cardiovascular Disease. Eur. J. Endocrinol. 2007, 157, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Endrighi, R.; Venuraju, S.M.; Lahiri, A.; Steptoe, A. Cortisol Responses to Mental Stress and the Progression of Coronary Artery Calcification in Healthy Men and Women. PLoS ONE 2012, 7, e31356. [Google Scholar] [CrossRef] [PubMed]
- Black, P.H.; Garbutt, L.D. Stress, inflammation and cardiovascular disease. J. Psychosom. Res. 2002, 52, 1–23. [Google Scholar] [CrossRef]
- Nijm, J.; Kristenson, M.; Olsson, A.G.; Jonasson, L. Impaired cortisol response to acute stressors in patients with coronary disease. Implications for inflammatory activity. J. Intern. Med. 2007, 262, 375–384. [Google Scholar] [CrossRef]
- Brydon, L.; Magid, K.; Steptoe, A. Platelets, coronary heart disease, and stress. Brain Behav. Immun. 2006, 20, 113–119. [Google Scholar] [CrossRef]
- Bytyçi, I.; Bajraktari, G.; Penson, P.E.; Henein, M.Y.; Banach, M. Efficacy and safety of colchicine in patients with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Br. J. Clin. Pharmacol. 2021, 88, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Andreis, A.; Imazio, M.; Piroli, F.; Avondo, S.; Casula, M.; Paneva, E.; De Ferrari, G.M. Efficacy and safety of colchicine for the prevention of major cardiovascular and cerebrovascular events in patients with coronary artery disease: A systematic review and meta-analysis on 12,869 patients. Eur. J. Prev. Cardiol. 2021, 28, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D. Antiinflammatory therapy with canakinumab for atherosclerotic disease. New Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Kivimäki, M. Stress and Cardiovascular Disease: An Update on Current Knowledge. Annu. Rev. Public Health 2013, 34, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Esler, M. Mental stress and human cardiovascular disease. Neurosci. Biobehav. Rev. 2017, 74, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Jokela, M.; Nyberg, S.T.; Singh-Manoux, A.; Fransson, E.I.; Alfredsson, L.; Bjorner, J.B.; Borritz, M.; Burr, H.; Casini, A.; et al. Long working hours and risk of coronary heart disease and stroke: A systematic review and meta-analysis of published and unpublished data for 603,838 individuals. Lancet 2015, 386, 1739–1746. [Google Scholar] [CrossRef]
- Bergmann, N.; Gyntelberg, F.; Faber, J. The appraisal of chronic stress and the development of the metabolic syndrome: A systematic review of prospective cohort studies. Endocr. Connect. 2014, 3, R55–R80. [Google Scholar] [CrossRef] [PubMed]
- Spruill, T.M. Chronic Psychosocial Stress and Hypertension. Curr. Hypertens. Rep. 2010, 12, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-Y.; Li, N.; Li, W.A.; Khan, H. Association between psychosocial stress and hypertension: A systematic review and meta-analysis. Neurol. Res. 2017, 39, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.; Eikelis, N.; Schlaich, M.; Lambert, G.; Alvarenga, M.; Dawood, T.; Kaye, D.; Barton, D.; Pier, C.; Guo, L.; et al. Chronic mental stress is a cause of essential hypertension: Presence of biological markers of stress. Clin. Exp. Pharmacol. Physiol. 2008, 35, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Zaman, M.A.; Calhoun, D.A. Pathogenesis of hypertension. Ann. Intern. Med. 2003, 139, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Steptoe, A. Cortisol Responses to Mental Stress and Incident Hypertension in Healthy Men and Women. J. Clin. Endocrinol. Metab. 2012, 97, E29–E34. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.; Ginty, A.T.; Der, G.; Hunt, K.; Benzeval, M.; Phillips, A.C. Increased blood pressure reactions to acute mental stress are associated with 16-year cardiovascular disease mortality. Psychophysiology 2012, 49, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Katholi, C.R.; McCreath, H.; Whooley, M.A.; Williams, D.R.; Zhu, S.; Markovitz, J.H. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation 2004, 110, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Kivimäki, M.; Lowe, G.; Rumley, A.; Hamer, M. Blood Pressure and Fibrinogen Responses to Mental Stress as Predictors of Incident Hypertension over an 8-Year Period. Ann. Behav. Med. 2016, 50, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.R.; Kamarck, T.W.; Everson-Rose, S.A.; Kaplan, G.A.; Manuck, S.B.; Salonen, J.T. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation 2004, 110, 2198–2203. [Google Scholar] [CrossRef]
- Chida, Y.; Steptoe, A. Greater Cardiovascular Responses to Laboratory Mental Stress Are Associated with Poor Subsequent Cardiovascular Risk Status: A Meta-Analysis of Prospective Evidence. Hypertension 2010, 55, 1026–1032. [Google Scholar] [CrossRef]
- Barnett, P.A.; Spence, J.D.; Manuck, S.B.; Jennings, J.R. Psychological stress and the progression of carotid artery disease. J. Hypertens. 1997, 15, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, S.; Zureik, M.; Ducimetière, P.; Touboul, P.-J.; Fève, J.-M.; Alpérovitch, A. Sustained Anxiety and 4-Year Progression of Carotid Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, H.; Malik, M.; Marmot, M. Social and psychosocial influences on sudden cardiac death, ventricular arrhythmia and cardiac autonomic function. Eur. Heart J. 2001, 22, 1082–1101. [Google Scholar] [CrossRef][Green Version]
- Batelaan, N.M.; Seldenrijk, A.; van den Heuvel, O.A.; van Balkom, A.J.L.M.; Kaiser, A.; Reneman, L.; Tan, H.L. Anxiety, Mental Stress, and Sudden Cardiac Arrest: Epidemiology, Possible Mechanisms and Future Research. Front. Psychiatry 2022, 12, 813518. [Google Scholar] [CrossRef]
- Lampert, R. Mental Stress and Ventricular Arrhythmias. Curr. Cardiol. Rep. 2016, 18, 118. [Google Scholar] [CrossRef] [PubMed]
- Lampert, R.; Joska, T.; Burg, M.M.; Batsford, W.P.; McPherson, C.A.; Jain, D. Emotional and physical precipitants of ventricular arrhythmia. Circulation 2002, 106, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Rosman, L.; Lampert, R.; Ramsey, C.M.; Dziura, J.; Chui, P.W.; Brandt, C.; Haskell, S.; Burg, M.M. Posttraumatic Stress Disorder and Risk for Early Incident Atrial Fibrillation: A Prospective Cohort Study of 1.1 Million Young Adults. J. Am. Heart Assoc. 2019, 8, e013741. [Google Scholar] [CrossRef]
- Cheng, Y.-F.; Leu, H.-B.; Su, C.-C.; Huang, C.-C.; Chiang, C.-H.; Huang, P.-H.; Chung, C.-M.; Lin, S.-J.; Chen, J.-W.; Chan, W.-L. Association between panic disorder and risk of atrial fibrillation:a nationwide study. Psychosom. Med. 2013, 75, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-X.; Sun, Y.-M.; Gu, H.-H.; Zhang, Y.; Shen, Z.-W.; Liang, X.-N.; Ding, D.; Wang, J. Association between anxiety symptoms and atrial fibrillation in a community cohort of Chinese older adults: A case-control study. BMC Cardiovasc. Disord. 2021, 21, 471. [Google Scholar] [CrossRef] [PubMed]
- Winbo, A.; Paterson, D.J. The Brain-Heart Connection in Sympathetically Triggered Inherited Arrhythmia Syndromes. Heart Lung Circ. 2020, 29, 529–537. [Google Scholar] [CrossRef]
- Lankaputhra, M.; Voskoboinik, A. Congenital long QT syndrome: A clinician’s guide. Intern. Med. J. 2021, 51, 1999–2011. [Google Scholar] [CrossRef]
- Ylänen, K.; Poutanen, T.; Hiippala, A.; Swan, H.; Korppi, M. Catecholaminergic polymorphic ventricular tachycardia. Eur. J. Pediatrics 2010, 169, 535–542. [Google Scholar] [CrossRef]
- Paavonen, K.J.; Swan, H.; Piippo, K.; Hokkanen, L.; Laitinen, P.; Viitasalo, M.; Toivonen, L.; Kontula, K. Response of the QT interval to mental and physical stress in types LQT1 and LQT2 of the long QT syndrome. Heart 2001, 86, 39–44. [Google Scholar] [CrossRef]
- Etienne, P.; Huchet, F.; Gaborit, N.; Barc, J.; Thollet, A.; Kyndt, F.; Guyomarch, B.; Le Marec, H.; Charpentier, F.; Schott, J.-J.; et al. Mental stress test: A rapid, simple, and efficient test to unmask long QT syndrome. Europace 2018, 20, 2014–2020. [Google Scholar] [CrossRef]
- Skinner, J.R.; Winbo, A.; Abrams, D.; Vohra, J.; Wilde, A.A. Channelopathies That Lead to Sudden Cardiac Death: Clinical and Genetic Aspects. Heart Lung Circ. 2019, 28, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Finlay, M.C.M.P.; Lambiase, P.D.P.F.F.; Ben-Simon, R.M.D.F.; Taggart, P.D.F. Effect of mental stress on dynamic electrophysiological properties of the endocardium and epicardium in humans. Heart Rhythm. 2016, 13, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Herring, N.; Kalla, M.; Paterson, D.J. The autonomic nervous system and cardiac arrhythmias: Current concepts and emerging therapies. Nat. Rev. Cardiol. 2019, 16, 707–726. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Xie, L.-H.; Olcese, R.; Karagueuzian, H.S.; Chen, P.-S.; Garfinkel, A.; Weiss, J.N. Early afterdepolarizations in cardiac myocytes: Beyond reduced repolarization reserve. Cardiovasc. Res. 2013, 99, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Song, Z.; Qu, Z. Determinants of early afterdepolarization properties in ventricular myocyte models. PLoS Comput. Biol. 2018, 14, e1006382. [Google Scholar] [CrossRef]
- Leite, L.R.; Henz, B.D.; Macedo, P.G.; Santos, S.N.; Barreto, J.R.; Zanatta, A.; Fenelon, G.; Cruz Filho, F.E.S. Catecholaminergic polymorphic ventricular tachycardia: A current overview. Future Cardiol. 2009, 5, 191–199. [Google Scholar] [CrossRef]
- Tse, G.M.A.M.P. Mechanisms of cardiac arrhythmias. J. Arrhythmia 2015, 32, 75–81. [Google Scholar] [CrossRef]
- Ackerman, M.J.M.D.P.; Priori, S.G.M.D.P.; Dubin, A.M.D.F.; Kowey, P.M.D.; Linker, N.J.M.D.F.; Slotwiner, D.M.D.F.; Triedman, J.M.D.F.C.C.; Van Hare, G.F.M.D.F.C.C.; Gold, M.R.M.D.P.F. Beta Blocker Therapy for Long QT Syndrome and Catecholaminergic Polymorphic Ventricular Tachycardia: Are all beta blockers equivalent? Heart Rhythm. 2016, 14, e41–e44. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef]
- Sanghai, S.; Abbott, N.J.; Dewland, T.A.; Henrikson, C.A.; Elman, M.R.; Wollenberg, M.; Ivie, R.; Gonzalez-Sotomayor, J.; Nazer, B. Stellate Ganglion Blockade with Continuous Infusion Versus Single Injection for Treatment of Ventricular Arrhythmia Storm. Clin. Electrophysiol. 2021, 7, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Lampert, R.; Shusterman, V.; Burg, M.; McPherson, C.; Batsford, W.; Goldberg, A.; Soufer, R. Anger-Induced T-Wave Alternans Predicts Future Ventricular Arrhythmias in Patients with Implantable Cardioverter-Defibrillators. J. Am. Coll. Cardiol. 2009, 53, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.D.; Jennings, J.R. Hemispheric asymmetry, autonomic asymmetry and the problem of sudden cardiac death. In Brain asymmetry; Davidson, R.J.H.K., Ed.; MIT Press: Cambridge, MA, USA, 1995; pp. 271–304. [Google Scholar]
- Critchley, H.D.; Taggart, P.; Sutton, P.M.; Holdright, D.R.; Batchvarov, V.; Hnatkova, K.; Malik, M.; Dolan, R.J. Mental stress and sudden cardiac death: Asymmetric midbrain activity as a linking mechanism. Brain 2005, 128, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Vargas, E.; Sörös, P.; Shoemaker, J.K.; Hachinski, V. Human cerebral circuitry related to cardiac control: A neuroimaging meta-analysis. Ann. Neurol. 2016, 79, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Chouchou, F.; Mauguière, F.; Vallayer, O.; Catenoix, H.; Isnard, J.; Montavont, A.; Jung, J.; Pichot, V.; Rheims, S.; Mazzola, L. How the insula speaks to the heart: Cardiac responses to insular stimulation in humans. Hum. Brain Mapp. 2019, 40, 2611–2622. [Google Scholar] [CrossRef] [PubMed]
- Taggart, P.; Critchley, H.; Lambiase, P.D. Heart–brain interactions in cardiac arrhythmia. Heart 2011, 97, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.; Whang, W. Psychological Distress and Arrhythmia: Risk Prediction and Potential Modifiers. Prog. Cardiovasc. Dis. 2013, 55, 582–589. [Google Scholar] [CrossRef]
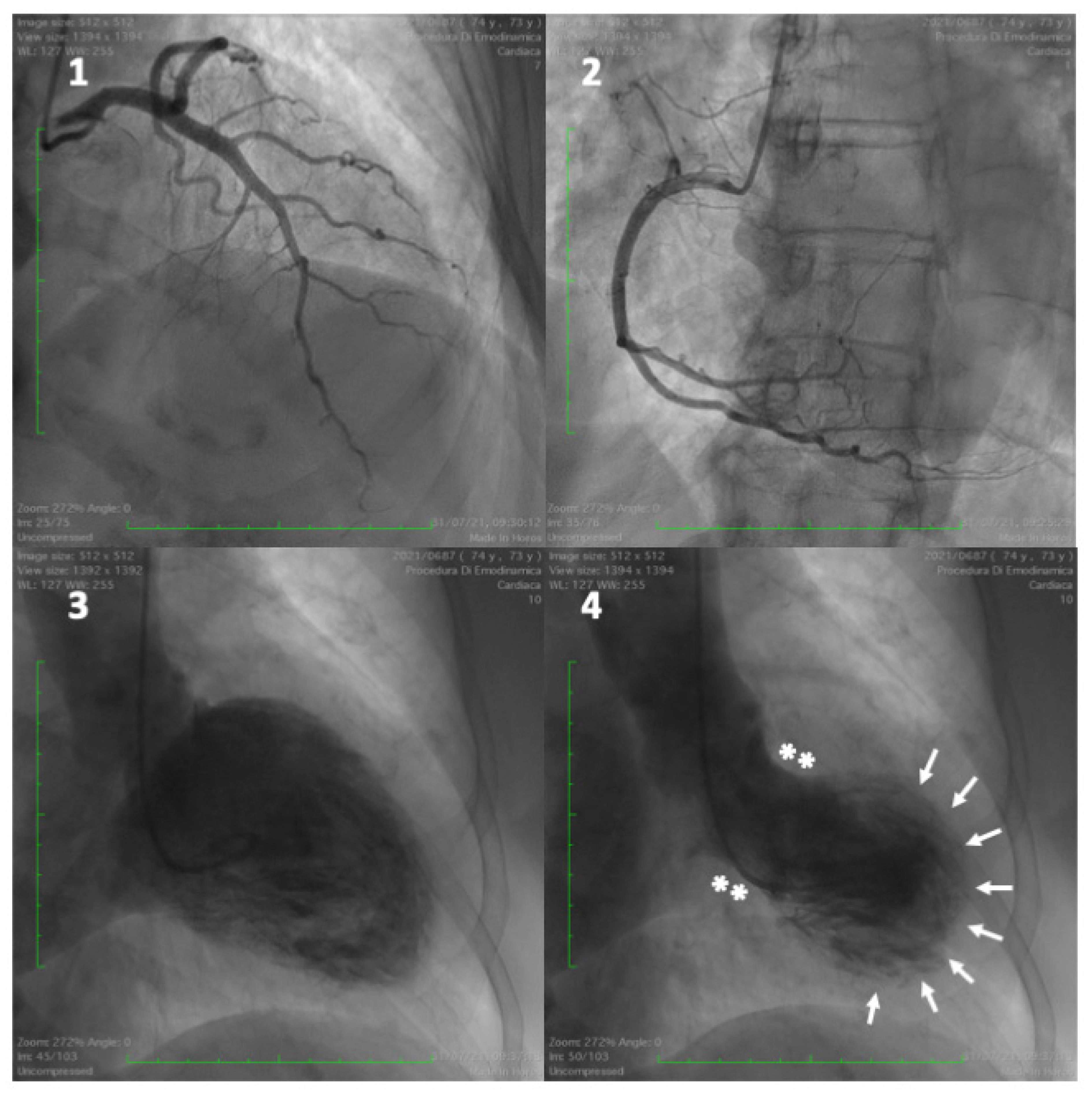
- Ghadri, J.-R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef]
- Ako, J.; Sudhir, K.; Farouque, H.M.O.; Honda, Y.; Fitzgerald, P.J. Transient Left Ventricular Dysfunction under Severe Stress: Brain-Heart Relationship Revisited. Am. J. Med. 2006, 119, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Osawa, A.; Nagai, M.; Dote, K.; Kato, M.; Oda, N.; Kunita, E.; Kagawa, E.; Yamane, A.; Kobatake, H.; Shiota, H.; et al. A mid-ventricular variant of Takotsubo syndrome: Was it triggered by insular cortex damage? ESC Heart Fail. 2021, 8, 3408–3412. [Google Scholar] [CrossRef]
- Akashi, Y.J.; Nef, H.M.; Lyon, A.R. Epidemiology and pathophysiology of Takotsubo syndrome. Nat. Rev. Cardiol. 2015, 12, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, S.W.; Lesser, J.R.; Maron, M.S.; Maron, B.J. Why Not Just Call It Tako-Tsubo Cardiomyopathy: A Discussion of Nomenclature. J. Am. Coll. Cardiol. 2011, 57, 1496–1497. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, R.; Ayya, S.S. Neurogenic stress cardiomyopathy: What do we need to know. Ann. Card. Anaesth. 2018, 21, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Summers, M.R.; Lennon, R.J.; Prasad, A. Pre-Morbid Psychiatric and Cardiovascular Diseases in Apical Ballooning Syndrome (Tako-Tsubo/Stress-Induced Cardiomyopathy): Potential Pre-Disposing Factors? J. Am. Coll. Cardiol. 2010, 55, 700–701. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Sarcon, A.; Diekmann, J.; Bataiosu, D.R.; Cammann, V.L.; Jurisic, S.; Napp, L.C.; Jaguszewski, M.; Scherff, F.; Brugger, P.; et al. Happy heart syndrome: Role of positive emotional stress in takotsubo syndrome. Eur. Heart J. 2016, 37, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Kobayashi, Y.; Kobatake, H.; Dote, K.; Kato, M.; Oda, N.; Kunita, E.; Kagawa, E.; Yamane, A.; Osawa, A.; et al. Happy heart syndrome: A case of Takotsubo syndrome with left internal carotid artery occlusion. Clin. Auton. Res. 2020, 30, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Esslen, M.; Pascual-Marqui, R.D.; Hell, D.; Kochi, K.; Lehmann, D. Brain areas and time course of emotional processing. NeuroImage 2004, 21, 1189–1203. [Google Scholar] [CrossRef] [PubMed]
- Kaski, J.C. Cardiac syndrome X in women: The role of oestrogen deficiency. Heart 2006, 92, iii5–iii9. [Google Scholar] [CrossRef] [PubMed]
- Sader, M.A.; Celermajer, D.S. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc. Res. 2002, 53, 597–604. [Google Scholar] [CrossRef]
- Sung, B.H.; Ching, M.; Izzo, J.L.; Dandona, P.; Wilson, M.F. Estrogen improves abnormal norepinephrine-induced vasoconstriction in postmenopausal women. J. Hypertens. 1999, 17, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Mendelsohn, M.E.; Rosano, G.M.C. Gender differences in the cardiovascular effect of sex hormones. Nat. Rev. Cardiol. 2009, 6, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; Kaski, J.C.; Crea, F.; Camici, P.G. Pathophysiology of Takotsubo Syndrome. Circulation 2017, 135, 2426–2441. [Google Scholar] [CrossRef]
- Scally, C.; Abbas, H.; Ahearn, T.; Srinivasan, J.; Mezincescu, A.; Rudd, A.; Spath, N.; Yucel-Finn, A.; Yuecel, R.; Oldroyd, K.; et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2019, 139, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, T.F.; Templin, C. Is takotsubo syndrome a microvascular acute coronary syndrome? Towards of a new definition. Eur. Heart J. 2016, 37, 2816–2820. [Google Scholar] [CrossRef] [PubMed]
- Moloney, R.D.; Johnson, A.C.; O’Mahony, S.M.; Dinan, T.G.; Greenwood-Van Meerveld, B.; Cryan, J.F. Stress and the Microbiota–Gut–Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS Neurosci. Ther. 2016, 22, 102–117. [Google Scholar] [CrossRef]
- Templin, C.; Hänggi, J.; Klein, C.; Topka, M.S.; Hiestand, T.; Levinson, R.A.; Jurisic, S.; Lüscher, T.F.; Ghadri, J.-R.; Jäncke, L. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur. Heart J. 2019, 40, 1183–1187. [Google Scholar] [CrossRef]
- Hiestand, T.; Hänggi, J.; Klein, C.; Topka, M.S.; Jaguszewski, M.; Ghadri, J.R.; Lüscher, T.F.; Jäncke, L.; Templin, C. Takotsubo Syndrome Associated with Structural Brain Alterations of the Limbic System. J. Am. Coll. Cardiol. 2018, 71, 809–811. [Google Scholar] [CrossRef]
- Silva, A.R.; Magalhães, R.; Arantes, C.; Moreira, P.S.; Rodrigues, M.; Marques, P.; Marques, J.; Sousa, N.; Pereira, V.H. Brain functional connectivity is altered in patients with Takotsubo Syndrome. Sci. Rep. 2019, 9, 4187. [Google Scholar] [CrossRef]
- Dichtl, W.; Tuovinen, N.; Barbieri, F.; Adukauskaite, A.; Senoner, T.; Rubatscher, A.; Hintringer, F.; Siedentopf, C.; Bauer, A.; Gizewski, E.R.; et al. Functional neuroimaging in the acute phase of Takotsubo syndrome: Volumetric and functional changes of the right insular cortex. Clin. Res. Cardiol. 2020, 109, 1107–1113. [Google Scholar] [CrossRef]
- Klein, C.; Hiestand, T.; Ghadri, J.-R.; Templin, C.; Jäncke, L.; Hänggi, J. Takotsubo Syndrome—Predictable from brain imaging data. Sci. Rep. 2017, 7, 5434. [Google Scholar] [CrossRef] [PubMed]
- Sciagrà, R.; Parodi, G.; Del Pace, S.; Genovese, S.; Zampini, L.; Bellandi, B.; Gensini, G.F.; Pupi, A.; Antoniucci, D. Abnormal response to mental stress in patients with Takotsubo cardiomyopathy detected by gated single photon emission computed tomography. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Radfar, A.; Abohashem, S.; Osborne, M.T.; Wang, Y.; Dar, T.; Hassan, M.Z.O.; Ghoneem, A.; Naddaf, N.; Patrich, T.; Abbasi, T.; et al. Stress-associated neurobiological activity associates with the risk for and timing of subsequent Takotsubo syndrome. Eur. Heart J. 2021, 42, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Samad, Z.; Boyle, S.; Ersboll, M.; Vora, A.N.; Zhang, Y.; Becker, R.C.; Williams, R.; Kuhn, C.; Ortel, T.L.; Rogers, J.G.; et al. Sex Differences in Platelet Reactivity and Cardiovascular and Psychological Response to Mental Stress in Patients with Stable Ischemic Heart Disease: Insights from the REMIT Study. J. Am. Coll. Cardiol. 2014, 64, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, V.; Wilmot, K.; Mheid, I.A.; Ramadan, R.; Pimple, P.; Shah, A.J.; Garcia, E.V.; Nye, J.; Ward, L.; Hammadah, M.; et al. Sex Differences in Mental Stress-Induced Myocardial Ischemia in Patients with Coronary Heart Disease. J. Am. Heart Assoc. 2016, 5, e003630. [Google Scholar] [CrossRef]
- Wokhlu, A.; Pepine, C.J. Mental Stress and Myocardial Ischemia: Young Women at Risk. J. Am. Heart Assoc. 2016, 5, e004196. [Google Scholar] [CrossRef]
- Soufer, R.; Jain, H.; Yoon, A.J. Heart-brain interactions in mental stress-induced myocardial ischemia. Curr. Cardiol. Rep. 2009, 11, 133–140. [Google Scholar] [CrossRef]
- Kasher, N.; Wittbrodt, M.T.; Alam, Z.S.; Lima, B.B.; Nye, J.A.; Campanella, C.; Ladd, S.; Hammadah, M.; Shah, A.J.; Raggi, P.; et al. Sex differences in brain activation patterns with mental stress in patients with coronary artery disease. Biol. Sex Differ. 2019, 10, 35. [Google Scholar] [CrossRef]
- Sullivan, S.; Hammadah, M.; Al Mheid, I.; Wilmot, K.; Ramadan, R.; Alkhoder, A.; Isakadze, N.; Shah, A.; Levantsevych, O.; Pimple, P.M.; et al. Sex Differences in Hemodynamic and Microvascular Mechanisms of Myocardial Ischemia Induced by Mental Stress. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 473–480. [Google Scholar] [CrossRef]
- Martin, E.A.; Tan, S.-L.; MacBride, L.R.; Lavi, S.; Lerman, L.O.; Lerman, A. Sex differences in vascular and endothelial responses to acute mental stress. Clin. Auton. Res. 2008, 18, 339–345. [Google Scholar] [CrossRef]
- Reis, S.E.; Holubkov, R.; Smith, A.J.C.; Kelsey, S.F.; Sharaf, B.L.; Reichek, N.; Rogers, W.J.; Merz, C.N.B.; Sopko, G.; Pepine, C.J. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: Results from the NHLBI WISE study. Am. Heart J. 2001, 141, 735–741. [Google Scholar] [CrossRef]
- Masi, C.M.; Chen, H.-Y.; Hawkley, L.C.; Cacioppo, J.T. A Meta-Analysis of Interventions to Reduce Loneliness. Personal. Soc. Psychol. Rev. 2011, 15, 219–266. [Google Scholar] [CrossRef] [PubMed]
- Gulliksson, M.; Burell, G.; Vessby, B.; Lundin, L.; Toss, H.; Svärdsudd, K. Randomized Controlled Trial of Cognitive Behavioral Therapy vs. Standard Treatment to Prevent Recurrent Cardiovascular Events in Patients with Coronary Heart Disease: Secondary Prevention in Uppsala Primary Health Care Project (SUPRIM). Arch. Intern. Med. 2011, 171, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Orth-Gomér, K.; Schneiderman, N.; Wang, H.-X.; Walldin, C.; Blom, M.; Jernberg, T. Stress reduction prolongs life in women with coronary disease: The Stockholm Women’s Intervention Trial for Coronary Heart Disease (SWITCHD). Circ. Cardiovasc. Qual. Outcomes 2009, 2, 25–32. [Google Scholar] [CrossRef]
- Kim, J.-M.; Stewart, R.; Lee, Y.-S.; Lee, H.-J.; Kim, M.C.; Kim, J.-W.; Kang, H.-J.; Bae, K.-Y.; Kim, S.-W.; Shin, I.-S.; et al. Effect of Escitalopram vs. Placebo Treatment for Depression on Long-Term Cardiac Outcomes in Patients with Acute Coronary Syndrome: A Randomized Clinical Trial. JAMA 2018, 320, 350–357. [Google Scholar] [CrossRef]
- Stewart, J.C.; Perkins, A.J.; Callahan, C.M. Effect of collaborative care for depression on risk of cardiovascular events: Data from the IMPACT randomized controlled trial. Psychosom. Med. 2014, 76, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Velazquez, E.J.; Kuchibhatla, M.; Samad, Z.; Boyle, S.H.; Kuhn, C.; Becker, R.C.; Ortel, T.L.; Williams, R.B.; Rogers, J.G.; et al. Effect of Escitalopram on Mental Stress–Induced Myocardial Ischemia: Results of the REMIT Trial. JAMA 2013, 309, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Glassman, A.H.; O’Connor, C.M.; Califf, R.M.; Swedberg, K.; Schwartz, P.; Bigger, J.J.T.; Krishnan, K.R.R.; van Zyl, L.T.; Swenson, J.R.; Finkel, M.S.; et al. Sertraline Treatment of Major Depression in Patients with Acute MI or Unstable Angina. JAMA 2002, 288, 701–709. [Google Scholar] [CrossRef]
- Berkman, L.F.; Blumenthal, J.; Burg, M.; Carney, R.M.; Catellier, D.; Cowan, M.J.; Czajkowski, S.M.; DeBusk, R.; Hosking, J.; Jaffe, A.; et al. Effects of Treating Depression and Low Perceived Social Support on Clinical Events after Myocardial Infarction: The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003, 289, 3106–3116. [Google Scholar] [CrossRef]
- Taylor, R.S.; Richards, S.H.; Anderson, L.; Jenkinson, C.E.; Whalley, B.; Rees, K.; Davies, P.; Bennett, P.; Liu, Z.; West, R.; et al. Psychological interventions for coronary heart disease. Cochrane Libr. 2017, 2021, CD002902. [Google Scholar] [CrossRef]
- van Halewijn, G.; Deckers, J.; Tay, H.Y.; van Domburg, R.; Kotseva, K.; Wood, D. Lessons from contemporary trials of cardiovascular prevention and rehabilitation: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 232, 294–303. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Sherwood, A.; Smith, P.J.; Watkins, L.; Mabe, S.; Kraus, W.E.; Ingle, K.; Miller, P.; Hinderliter, A. Enhancing Cardiac Rehabilitation with Stress Management Training: A Randomized, Clinical Efficacy Trial. Circulation 2016, 133, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.; Newman, J.D.; Whang, W.; Davidson, K.W. Emotional triggers in myocardial infarction: Do they matter? Eur. Heart J. 2012, 34, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Kraynak, T.E.; Marsland, A.L.; Gianaros, P.J. Neural Mechanisms Linking Emotion with Cardiovascular Disease. Curr. Cardiol. Rep. 2018, 20, 128. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Impact of Mental Stress on Cardiovascular Health—Part II. J. Clin. Med. 2022, 11, 4405. https://doi.org/10.3390/jcm11154405
Henein MY, Vancheri S, Longo G, Vancheri F. The Impact of Mental Stress on Cardiovascular Health—Part II. Journal of Clinical Medicine. 2022; 11(15):4405. https://doi.org/10.3390/jcm11154405
Chicago/Turabian StyleHenein, Michael Y., Sergio Vancheri, Giovanni Longo, and Federico Vancheri. 2022. "The Impact of Mental Stress on Cardiovascular Health—Part II" Journal of Clinical Medicine 11, no. 15: 4405. https://doi.org/10.3390/jcm11154405
APA StyleHenein, M. Y., Vancheri, S., Longo, G., & Vancheri, F. (2022). The Impact of Mental Stress on Cardiovascular Health—Part II. Journal of Clinical Medicine, 11(15), 4405. https://doi.org/10.3390/jcm11154405





