Heart Rate-Dependent Degree of Motion Artifacts in Coronary CT Angiography Acquired by a Novel Purpose-Built Cardiac CT Scanner
Abstract
:1. Introduction
2. Materials and Methods
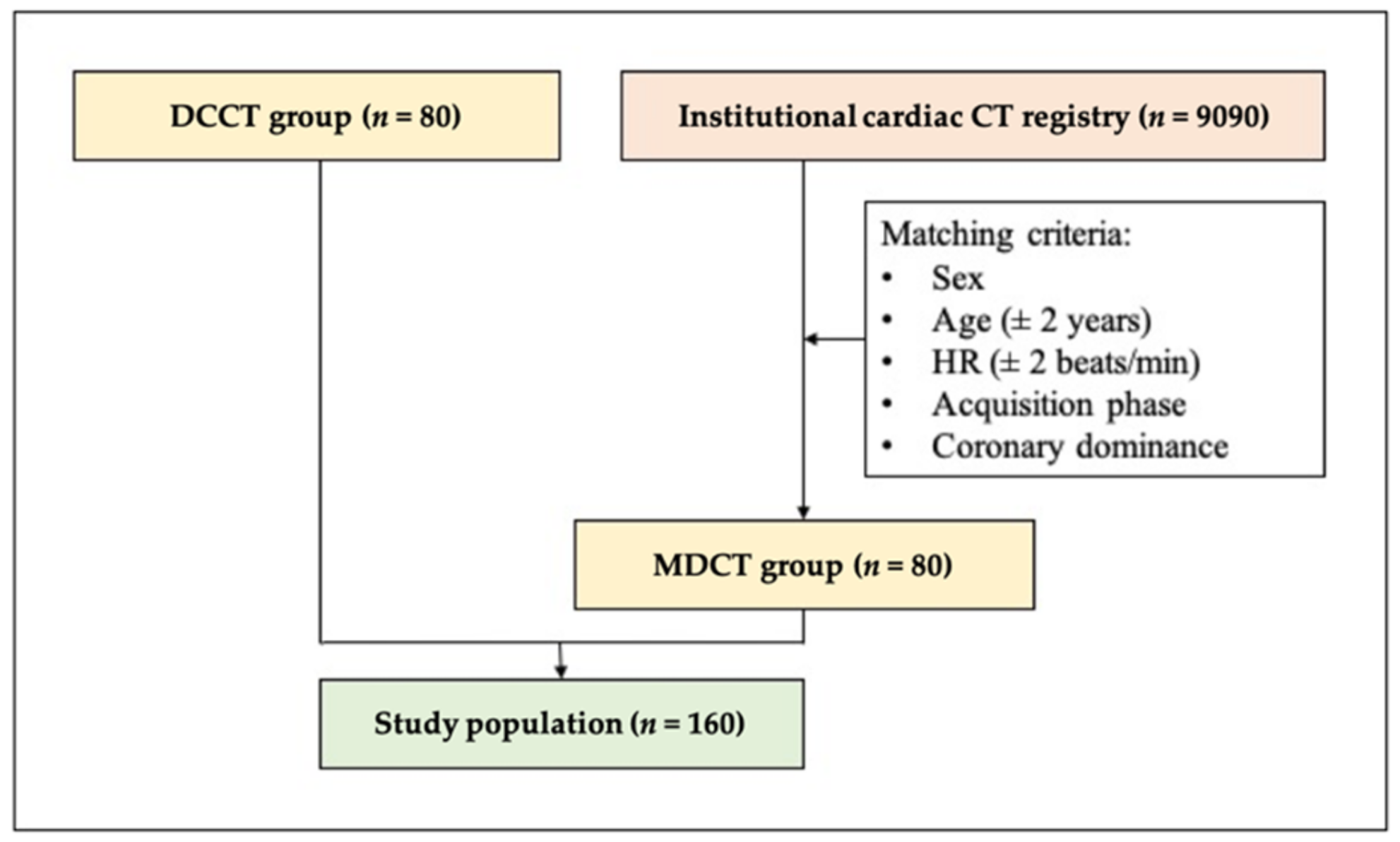
2.1. Study Population
2.2. CT Acquisition
2.3. CTA Image Reconstruction
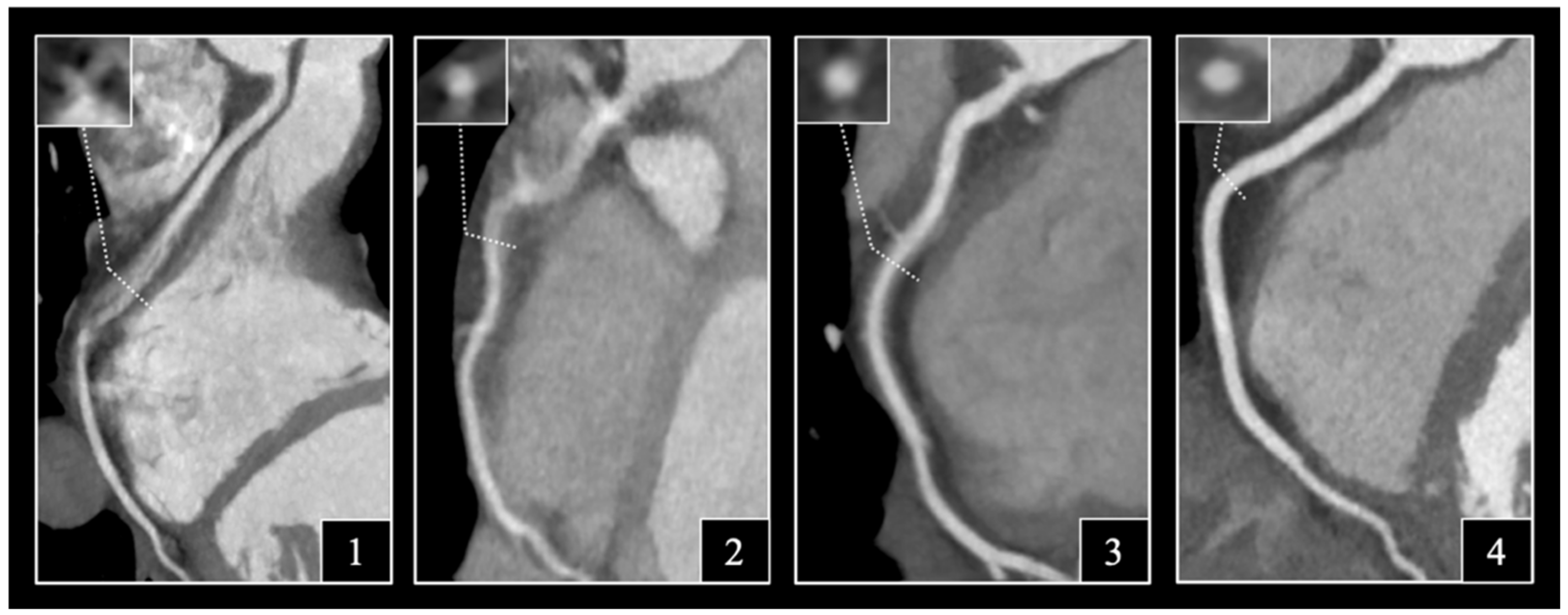
2.4. Assessment of Image Quality
2.5. Statistical Analysis
3. Results
3.1. Demographic and Scanning Parameters
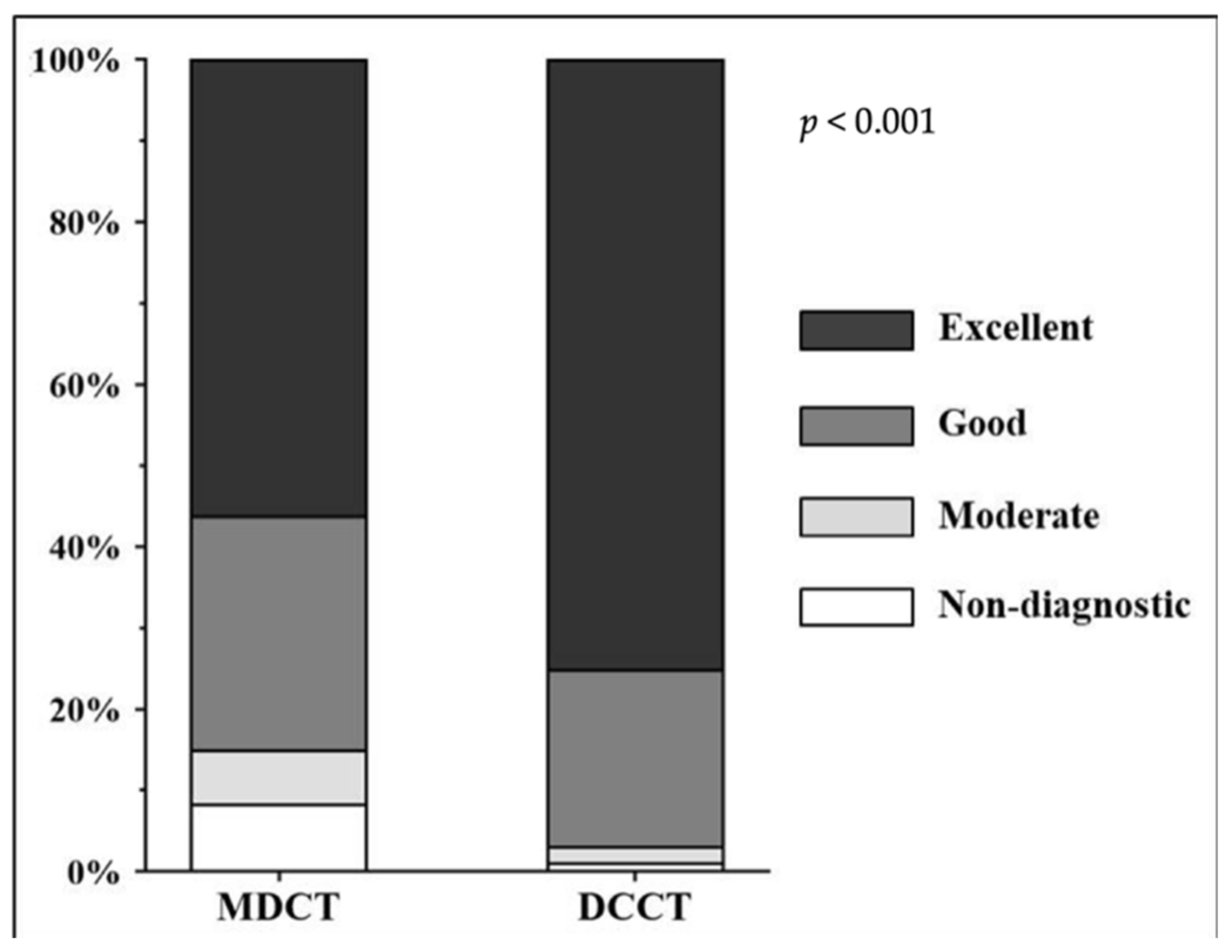
3.2. Distribution of Likert Scores
3.3. Image Interpretability
3.4. Subjective Image Quality on Per-Patient, Per-Vessel and Per-Segment Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| DCCT (n = 80) | MDCT (n = 80) | |
|---|---|---|
| Collimation (mm) | 280 × 0.5 | 128 × 0.625 |
| Gantry rotation time (ms) | 240 | 270 |
| Temporal resolution (ms) | 120 | 135 |
| Detector row width (mm) | 0.5 | 0.625 |
| Number of detector rows | 192 (280 slices per rotation) | 128 |
| Z-axis coverage (mm) | 14 | 8 |
| Tube voltage (kV) | 100/120 | 100/120 |
| Tube current (mAs) | 164–280 | 286–300 |
| Matrix size | 512 × 512 | 512 × 512 |
| Maximum field of view (mm) | 250 | 500 |
| Slice thickness | 0.8 | 0.5 |
| Number | 160 |
|---|---|
| Intravenous beta blocker (mg) | |
| 0, n (%) | 73 (45.6) |
| 5, n (%) | 54 (33.8) |
| 10, n (%) | 24 (15.0) |
| 15, n (%) | 8 (5.0) |
| 20, n (%) | 1 (0.6) |
References
- Celeng, C.; Takx, R.A.; Ferencik, M.; Maurovich-Horvat, P. Non-invasive and invasive imaging of vulnerable coronary plaque. Trends Cardiovasc. Med. 2016, 26, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Eisenberg, E.; Friedman, J.; Cheng, V.; Hayes, S.; Tamarappoo, B.; Thomson, L.; Berman, D.S. Impact of heart rate on coronary computed tomographic angiography interpretability with a third-generation dual-source scanner. Int. J. Cardiol. 2019, 295, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [Green Version]
- Carrascosa, P.; Capuñay, C.; Deviggiano, A.; Goldsmit, A.; Tajer, C.; Bettinotti, M.; Carrascosa, J.; Ivanc, T.B.; Fallahi, A.; García, M.J. Accuracy of low-dose prospectively gated axial coronary CT angiography for the assessment of coronary artery stenosis in patients with stable heart rate. J. Cardiovasc. Comput. Tomogr. 2010, 4, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Bastarrika, G.; Lee, Y.S.; Huda, W.; Ruzsics, B.; Costello, P.; Schoepf, U.J. CT of coronary artery disease. Radiology 2009, 253, 317–338. [Google Scholar] [CrossRef] [Green Version]
- Maffei, E.; Palumbo, A.A.; Martini, C.; Tedeschi, C.; Tarantini, G.; Seitun, S.; Ruffini, L.; Aldrovandi, A.; Weustink, A.C.; Meijboom, W.B.; et al. “In-house” pharmacological management for computed tomography coronary angiography: Heart rate reduction, timing and safety of different drugs used during patient preparation. Eur. Radiol. 2009, 19, 2931–2940. [Google Scholar] [CrossRef]
- Maurovich-Horvat, P.; Károlyi, M.; Horváth, T.; Szilveszter, B.; Bartykowszki, A.; Jermendy, L.; Panajotu, A.; Celeng, C.; Suhai, F.I.; Major, G.P.; et al. Esmolol is noninferior to metoprolol in achieving a target heart rate of 65 beats/min in patients referred to coronary CT angiography: A randomized controlled clinical trial. J. Cardiovasc. Comput. Tomogr. 2015, 9, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Maggiore, P.; Huang, A.L.; Anastasius, M.; Brown, R.; Boroditsky, J.; Ariel, E.; Ezekiel, J.; Merkur, J.; Blanke, P.; Leipsic, J. A comparative assessment of the performance of a state-of-the art small footprint dedicated cardiovascular CT scanner. J. Cardiovasc. Comput. Tomogr. 2021, 15, 85–87. [Google Scholar] [CrossRef]
- Lell, M.M.; Kachelrieß, M. Recent and upcoming technological developments in computed tomography: High speed, low dose, deep learning, multienergy. Investig. Radiol. 2020, 55, 8–19. [Google Scholar] [CrossRef]
- Karády, J.; Panajotu, A.; Kolossváry, M.; Szilveszter, B.; Jermendy, L.; Bartykowszki, A.; Károlyi, M.; Celeng, C.; Merkely, B.; Maurovich-Horvat, P. The effect of four-phasic versus three-phasic contrast media injection protocols on extravasation rate in coronary CT angiography: A randomized controlled trial. Eur. Radiol. 2017, 27, 4538–4543. [Google Scholar] [CrossRef] [Green Version]
- Deak, P.D.; Smal, Y.; Kalender, W.A. Multisection CT protocols: Sex- and age-specific conversion dose from dose-length product. Radiology 2010, 257, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Leipsic, J.; Abbara, S.; Achenbach, S.; Cury, R.; Earls, J.P.; Mancini, G.J.; Nieman, K.; Pontone, G.; Raff, G.L. SCCT guidelines for the interpretation and reporting of coronary CT angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 2014, 8, 342–358. [Google Scholar] [CrossRef]
- Moss, A.J.; Williams, M.C.; Newby, D.E.; Nicol, E.D. The updated NICE guidelines: Cardiac CT as the first-line test for coronary artery disease. Curr. Cardiovasc. Imaging Rep. 2017, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Carrabba, N.; Migliorini, A.; Pradella, S.; Acquafresca, M.; Guglielmo, M.; Baggiano, A.; Moscogiuri, G.; Valenti, R. Old and new NICE guidelines for the evaluation of new onset stable chest pain: A real world perspective. BioMed Res. Int. 2018, 2018, 3762305. [Google Scholar] [CrossRef]
- Commandeur, F.; Goeller, M.; Dey, D. Cardiac CT: Technological advances in hardware, software, and machine learning applications. Curr. Cardiovasc. Imaging Rep. 2018, 11, 19. [Google Scholar] [CrossRef]
- Leipsic, J.; Labounty, T.M.; Hague, C.J.; Mancini, G.J.; O’Brien, J.M.; Wood, D.A.; Taylor, C.M.; Cury, R.C.; Earls, J.P.; Heilbron, B.G.; et al. Effect of a novel vendor-specific motion-correction algorithm on image quality and diagnostic accuracy in persons undergoing coronary CT angiography without rate-control medications. J. Cardiovasc. Comput. Tomogr. 2012, 6, 164–171. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.A.; Lee, J.S.; Suh, J.; Paik, S.H.; Park, J.S. Impact of a vendor-specific motion-correction algorithm on image quality, interpretability, and diagnostic performance of daily routine coronary CT angiography: Influence of heart rate on the effect of motion-correction. Int. J. Cardiovasc. Imaging 2014, 30, 1603–1612. [Google Scholar] [CrossRef]
- Muenzel, D.; Noel, P.B.; Dorn, F.; Dobritz, M.; Rummeny, E.J.; Huber, A. Step and shoot coronary CT angiography using 256-slice CT: Effect of heart rate and heart rate variability on image quality. Eur. Radiol. 2011, 21, 2277–2284. [Google Scholar] [CrossRef]
- Abbara, S.; Blanke, P.; Maroules, C.D.; Cheezum, M.; Choi, A.D.; Han, B.K.; Marwan, M.; Naoum, C.; Nørgaard, B.; Rubinshtein, R.; et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 2016, 10, 435–449. [Google Scholar] [CrossRef]
- Androshchuk, V.; Sabharwal, N.; Noble, V.S.; Kelion, A. Speeding up beta-blockade prior to coronary CT angiography: Can we predict the dose of intravenous metoprolol required to achieve target heart rate in a given patient? Clin. Radiol. 2020, 76, 236.e21–236.e25. [Google Scholar] [CrossRef]
- Cademartiri, F.; Garot, J.; Tendera, M.; Zamorano, J.L. Intravenous ivabradine for control of heart rate during coronary CT angiography: A randomized, double-blind, placebo-controlled trial. J. Cardiovasc. Comput. Tomogr. 2015, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-J.; Wu, S.-Y.; Wang, J.; Lu, Y.; Zhang, Z.-L.; Jiang, S.-S.; Zhou, C.-S.; Lu, G.-M. Diagnostic accuracy of dual-source CT coronary angiography: The effect of average heart rate, heart rate variability, and calcium score in a clinical perspective. Acta Radiol. 2010, 51, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Stehli, J.; Dougoud, S.; Fiechter, M.; Sah, B.-R.; Buechel, R.R.; Bull, S.; Gaemperli, O.; Kaufmann, P.A. Impact of a new motion-correction algorithm on image quality of low-dose coronary CT angiography in patients with insufficient heart rate control. Acad. Radiol. 2014, 21, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, P.; Su, Z.; Yao, X.; Wang, Y.; Wang, C.; Du, X.; Li, K. Effect of a novel motion correction algorithm (SSF) on the image quality of coronary CTA with intermediate heart rates: Segment-based and vessel-based analyses. Eur. J. Radiol. 2014, 83, 2024–2032. [Google Scholar] [CrossRef]

| DCCT (n = 80) | MDCT (n = 80) | p | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 60.0 (50.9–66.6) | 62.5 (50.3–67.7) | 0.08 |
| Female sex, n (%) | 32 (40.0) | 32 (40.0) | 1.00 |
| BSA (m2) | 2.0 (1.8–2.1) | 2.0 (1.8–2.2) | 0.24 |
| BMI (kg/m2) | 27.4 (25.1–30.1) | 27.8 (25.3–31.4) | 0.22 |
| Cardiovascular risk factors | |||
| Current smoker, n (%) | 15 (18.8) | 17 (21.2) | 0.69 |
| Hypertension, n (%) | 49 (61.3) | 53 (66.3) | 0.50 |
| Diabetes mellitus, n (%) | 8 (10.0) | 8 (10.0) | 1.00 |
| Dyslipidemia, n (%) | 43 (53.8) | 43 (53.8) | 1.00 |
| CTA characteristics | |||
| Diastolic triggering, n (%) | 73 (91.3) | 73 (91.3) | 1.00 |
| DLP (mGy*cm) | 245.4 (243.2–343.1) | 362.3 (356.7–375.9) | <0.001 |
| Effective dose (mSv) | 3.4 (3.4–4.8) | 5.1 (5.0–5.3) | <0.001 |
| Average heart rate (1/min) | 65.0 (60.0–70.5) | 65.0 (60.0–70.0) | 0.40 |
| DCCT | MDCT | p | |
|---|---|---|---|
| Overall interpretability | |||
| Per-patient | 74/80 (92.5) | 52/80 (65.0) | <0.001 |
| Per-coronary | 232/240 (96.7) | 199/240 (82.9) | <0.001 |
| Per-segment | 978/989 (98.9) | 934/1019 (91.7) | <0.001 |
| Interpretability by coronary artery | |||
| LM-LAD | 79/80 (98.8) | 69/80 (86.3) | 0.003 |
| LCX | 78/80 (97.5) | 66/80 (82.5) | 0.002 |
| RCA | 75/80 (93.8) | 60/80 (75.0) | 0.002 |
| DCCT | MDCT | p | |
|---|---|---|---|
| Overall image quality | |||
| Per-patient | 3.7 ± 0.4 | 3.3 ± 0.7 | <0.001 |
| Per-coronary | |||
| LM-LAD | 3.8 ± 0.3 | 3.5 ± 0.6 | <0.001 |
| LCx | 3.8 ± 0.5 | 3.3 ± 0.9 | <0.001 |
| RCA | 3.5 ± 0.6 | 3.0 ± 0.9 | <0.001 |
| Per-segment | |||
| Proximal segments | 3.7 ± 0.3 | 3.4 ± 0.6 | <0.001 |
| Distal segments | 3.6 ± 0.5 | 3.2 ± 0.8 | <0.001 |
| HR < 60/min | |||
| Per-patient | 3.9 ± 0.1 | 3.7 ± 0.4 | 0.09 |
| Per-coronary | |||
| LM-LAD | 3.9 ± 0.3 | 3.9 ± 0.3 | 0.53 |
| LCx | 4.0 ± 0.1 | 3.8 ± 0.4 | 0.08 |
| RCA | 3.8 ± 0.3 | 3.5 ± 0.9 | 0.13 |
| Per-segment | |||
| Proximal segments | 3.9 ± 0.1 | 3.7 ± 0.4 | 0.14 |
| Distal segments | 3.9 ± 0.2 | 3.7 ± 0.5 | 0.16 |
| HR: 60–65/min | |||
| Per-patient | 3.9 ± 0.2 | 3.7 ± 0.2 | 0.008 |
| Per-coronary | |||
| LM-LAD | 3.9 ± 0.1 | 3.9 ± 0.2 | 0.42 |
| LCx | 3.9 ± 0.3 | 3.7 ± 0.5 | 0.15 |
| RCA | 3.8 ± 0.4 | 3.4 ± 0.4 | 0.003 |
| Per-segment | |||
| Proximal segments | 3.9 ± 0.2 | 3.7 ± 0.3 | 0.01 |
| Distal segments | 3.9 ± 0.2 | 3.7 ± 0.3 | 0.06 |
| HR: 66–70/min | |||
| Per-patient | 3.5 ± 0.5 | 3.2 ± 0.6 | 0.048 |
| Per-coronary | |||
| LM-LAD | 3.7 ± 0.4 | 3.4 ± 0.6 | 0.04 |
| LCx | 3.5 ± 0.7 | 3.2 ± 0.7 | 0.12 |
| RCA | 3.1 ± 0.6 | 2.9 ± 0.8 | 0.41 |
| Per-segment | |||
| Proximal segments | 3.6 ± 0.4 | 3.3 ± 0.5 | 0.06 |
| Distal segments | 3.3 ± 0.7 | 3.0 ± 0.8 | 0.20 |
| HR > 70/min | |||
| Per-patient | 3.5 ± 0.4 | 2.7 ± 0.7 | <0.001 |
| Per-coronary | |||
| LM-LAD | 3.7 ± 0.3 | 3.0 ± 0.6 | 0.002 |
| LCx | 3.6 ± 0.5 | 2.5 ± 1.0 | 0.001 |
| RCA | 3.3 ± 0.5 | 2.3 ± 0.9 | 0.003 |
| Per-segment | |||
| Proximal segments | 3.6 ± 0.3 | 2.8 ± 0.7 | <0.001 |
| Distal segments | 3.5 ± 0.5 | 2.5 ± 0.9 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecsey-Nagy, M.; Jermendy, Á.L.; Kolossváry, M.; Vattay, B.; Boussoussou, M.; Suhai, F.I.; Panajotu, A.; Csőre, J.; Borzsák, S.; Fontanini, D.M.; et al. Heart Rate-Dependent Degree of Motion Artifacts in Coronary CT Angiography Acquired by a Novel Purpose-Built Cardiac CT Scanner. J. Clin. Med. 2022, 11, 4336. https://doi.org/10.3390/jcm11154336
Vecsey-Nagy M, Jermendy ÁL, Kolossváry M, Vattay B, Boussoussou M, Suhai FI, Panajotu A, Csőre J, Borzsák S, Fontanini DM, et al. Heart Rate-Dependent Degree of Motion Artifacts in Coronary CT Angiography Acquired by a Novel Purpose-Built Cardiac CT Scanner. Journal of Clinical Medicine. 2022; 11(15):4336. https://doi.org/10.3390/jcm11154336
Chicago/Turabian StyleVecsey-Nagy, Milán, Ádám Levente Jermendy, Márton Kolossváry, Borbála Vattay, Melinda Boussoussou, Ferenc Imre Suhai, Alexisz Panajotu, Judit Csőre, Sarolta Borzsák, Daniele Mariastefano Fontanini, and et al. 2022. "Heart Rate-Dependent Degree of Motion Artifacts in Coronary CT Angiography Acquired by a Novel Purpose-Built Cardiac CT Scanner" Journal of Clinical Medicine 11, no. 15: 4336. https://doi.org/10.3390/jcm11154336
APA StyleVecsey-Nagy, M., Jermendy, Á. L., Kolossváry, M., Vattay, B., Boussoussou, M., Suhai, F. I., Panajotu, A., Csőre, J., Borzsák, S., Fontanini, D. M., Csobay-Novák, C., Merkely, B., Maurovich-Horvat, P., & Szilveszter, B. (2022). Heart Rate-Dependent Degree of Motion Artifacts in Coronary CT Angiography Acquired by a Novel Purpose-Built Cardiac CT Scanner. Journal of Clinical Medicine, 11(15), 4336. https://doi.org/10.3390/jcm11154336







