Global and Regional Myocardial Work in Female Adolescents with Weight Disorders
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Anthropometric and Clinical Assessments
2.3. Echocardiographic Recordings
2.4. Cardiac Morphology
2.5. Left Ventricular (LV) Systolic and Diastolic Functions
2.6. Myocardial Work Quantification
2.7. Statistical Analyses
3. Results
3.1. Population Characteristics and Resting Echocardiography
3.2. Global Longitudinal Strain and Myocardial Work
3.3. Regional Longitudinal Strains and Myocardial Work
4. Discussion
4.1. Global Myocardial Work in Anorexia Nervosa (AN) and Obesity (OB) Patients
4.2. Regional Analysis of Myocardial Work in AN and OB Patients
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giovinazzo, S.; Sukkar, S.G.; Rosa, G.M.; Zappi, A.; Bezante, G.P.; Balbi, M.; Brunelli, C. Anorexia nervosa and heart disease: A systematic review. Eat. Weight. Disord.-Stud. Anorexia, Bulim. Obes. 2018, 24, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Koopman, L.P.; Mertens, L. Impact of Childhood Obesity on Cardiac Structure and Function. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 345. [Google Scholar] [CrossRef] [PubMed]
- Garrido, B.J.; Lobera, I.J. Cardiovascular complications in eating disorders. In Relevant topics in Eating Disorders; IntechOpen: London, UK, 2012. [Google Scholar]
- Hutchinson, J.; Emerick, J.; Saxena, H. The Future of Pediatric Obesity. Prim. Care Clin. Off. Pr. 2016, 43, 1–17. [Google Scholar] [CrossRef]
- Fayssoil, A.; Melchior, J.C.; Hanachi, M. Heart and anorexia nervosa. Heart Fail. Rev. 2019, 26, 65–70. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.; Larson, M.; Kannel, W.B.; Vasan, R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Sachs, K.V.; Harnke, B.; Mehler, P.S.; Krantz, M.J. Cardiovascular complications of anorexia nervosa: A systematic review. Int. J. Eat. Disord. 2015, 49, 238–248. [Google Scholar] [CrossRef]
- Schusterova, I.; Jurko, A.; Minarik, M. Left ventricular systolic and diastolic function in children with overweight and obesity. Bratisl. Lek. List. 2013, 114, 526–530. [Google Scholar] [CrossRef]
- Potter, E.; Marwick, T.H. Assessment of left ventricular function by echocardiography. JACC Cardiovasc. Imaging 2018, 11, 260–274. [Google Scholar] [CrossRef]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Hear. J.-Cardiovasc. Imaging 2018, 20, 582–590. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure–strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef]
- Loncaric, F.; Marciniak, M.; Nunno, L.; Mimbrero, M.; Fernandes, J.F.; Fabijanovic, D.; Sanchis, L.; Doltra, A.; Montserrat, S.; Cikes, M.; et al. Distribution of myocardial work in arterial hypertension: Insights from non-invasive left ventricular pressure-strain relations. Int. J. Cardiovasc. Imaging 2020, 37, 145–154. [Google Scholar] [CrossRef]
- Hubert, A.; Le Rolle, V.; Leclercq, C.; Galli, E.; Samset, E.; Casset, C.; Mabo, P.; Hernandez, A.; Donal, E. Estimation of myocardial work from pressure–strain loops analysis: An experimental evaluation. Eur. Hear. J. Cardiovasc. Imaging 2018, 19, 1372–1379. [Google Scholar] [CrossRef]
- Larsen, C.; Aalen, J.M.; Stokke, C.; Fjeld, J.G.; Kongsgaard, E.; Duchenne, J.; Degtiarova, G.; Gheysens, O.; Voigt, J.-U.; A Smiseth, O.; et al. Regional myocardial work by cardiac magnetic resonance and non-invasive left ventricular pressure: A feasibility study in left bundle branch block. Eur. Heart J.-Cardiovasc. Imaging 2020, 21, 143–153. [Google Scholar] [CrossRef]
- Bogaert, J.; Rademakers, F.E. Regional nonuniformity of normal adult human left ventricle. Am. J. Physiol. Circ. Physiol. 2001, 280, H610–H620. [Google Scholar] [CrossRef]
- Bussadori, C.; Moreo, A.; Di Donato, M.; De Chiara, B.; Negura, D.; Dall’Aglio, E.; Lobiati, E.; Chessa, M.; Arcidiacono, C.; Dua, J.; et al. A new 2D-based method for myocardial velocity strain and strain rate quantification in a normal adult and paediatric population: Assessment of reference values. Cardiovasc. Ultrasound 2009, 7, 8. [Google Scholar] [CrossRef]
- Mangner, N.; Scheuermann, K.; Winzer, E.; Wagner, I.; Hoellriegel, R.; Sandri, M.; Zimmer, M.; Mende, M.; Linke, A.; Kiess, W.; et al. Childhood Obesity. JACC Cardiovasc. Imaging 2014, 7, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Binnetoglu, F.K.; Yildirim, S.; Topaloglu, N.; Tekin, M.; Kaymaz, N.; Aylanc, H.; Karakurt, H. Early detection of myocardial deformation by 2D speckle tracking echocardiography in normotensive obese children and adolescents. Anadolu Kardiyol. Dergisi/Anatol. J. Cardiol. 2015, 15, 151. [Google Scholar] [CrossRef]
- Morris, R.; Prasad, A.; Asaro, J.; Guzman, M.; Sanders, L.; Hauck, A.; Singh, G.K.; Levy, P.T. Markers of Cardiovascular dysfunction in adolescents with anorexia nervosa. Glob. Pediatr. Health 2017, 4, 2333794X17727423. [Google Scholar] [CrossRef]
- Duchenne, J.; Aalen, J.M.; Cvijic, M.; Larsen, C.K.; Galli, E.; Bézy, S.; Beela, A.S.; Ünlü, S.; Pagourelias, E.D.; Winter, S.; et al. Acute redistribution of regional left ventricular work by cardiac resynchronization therapy determines long-term remodelling. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Loas, G. The DSM-V: An overview. Rev. Med. Brux. 2016, 37, 231–234. [Google Scholar] [PubMed]
- Pérez, W.; Melgar, P.; Garcés, A.; De Marquez, A.D.; Merino, G.; Siu, C. Overweight and obesity of school-age children in El Salvador according to two international systems: A population-based multilevel and spatial analysis. BMC Public Health 2020, 20, 687. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Daniels, S.R.; Devereux, R.B.; Meyer, R.A.; Roman, M.J.; De Divitiis, O.; Alderman, M.H. Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. J. Am. Coll. Cardiol. 1992, 20, 1251–1260. [Google Scholar] [CrossRef]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef]
- Maufrais, C.; Schuster, I.; Doucende, G.; Vitiello, D.; Rupp, T.; Dauzat, M.; Obert, P.; Nottin, S. Endurance training minimizes age-related changes of left ventricular twist-untwist mechanics. J. Am. Soc. Echocardiogr. 2014, 27, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Van der Bijl, P.; Kostyukevich, M.; El Mahdiui, M.; Hansen, G.; Samset, E.; Marsan, N.; Bax, J.; Delgado, V. A Roadmap to Assess Myocardial Work: From Theory to Clinical Practice. JACC Cardiovasc. Imaging 2019, 12, 2549–2554. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Gjesdal, O.; Edvardsen, T.; Smiseth, O.A. Assessment of wasted myocardial work: A novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am. J. Physiol. Circ. Physiol. 2013, 305, H996–H1003. [Google Scholar] [CrossRef]
- Doucende, G.; Schuster, I.; Rupp, T.; Startun, A.; Dauzat, M.; Obert, P.; Nottin, S. Kinetics of Left Ventricular Strains and Torsion During Incremental Exercise in Healthy Subjects. Circ. Cardiovasc. Imaging 2010, 3, 586–594. [Google Scholar] [CrossRef]
- Chan, J.; Edwards, A.N.F.; Khandheria, B.K.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Scalia, G.M. A new approach to assess myocardial work by non-invasive left ventricular pressure–strain relations in hypertension and dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2018, 20, 31–39. [Google Scholar] [CrossRef]
- Labombarda, F.; Zangl, E.; Dugue, A.E.; Bougle, D.; Pellissier, A.; Ribault, V.; Maragnes, P.; Milliez, P.; Saloux, E. Alterations of left ventricular myocardial strain in obese children. Eur. Heart J. Cardiovasc. Imaging 2012, 14, 668–676. [Google Scholar] [CrossRef]
- Barbosa, J.A.A.; Mota, C.C.; Silva, A.C.S.; Nunes, M.D.C.P.; Barbosa, M.M. Assessing pre-clinical ventricular dysfunction in obese children and adolescents: The value of speckle tracking imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 882–889. [Google Scholar] [CrossRef]
- Kulkarni, A.; Gulesserian, T.; Lorenzo, J.M.M.D.; Haroonian, Y.; Ngyuyen, M.; Lo, Y.; Wang, D.; Hsu, D.; Kaskel, F.; Mahgerefteh, J. Left ventricular remodelling and vascular adaptive changes in adolescents with obesity. Pediatr. Obes. 2018, 13, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Haley, E.J.; Zhiqian, G.; Philip, K.R.; Nicolas, M.L.; Thomas, K.R.; Lawrence, D.M.; Elaine, U.M.; Gao, Z.; Khoury, P.R.; Madsen, N.L.; et al. Reduction in myocardial strain is evident in adolescents and young adults with obesity and type 2 diabetes. Pediatr. Diabetes 2019, 21, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.A.; Levy, P.T.; Sekarski, T.J.; Arbelaez, A.M.; Hildebolt, C.F.; Holland, M.R.; Singh, G.K. Markers of Cardiovascular Risk, Insulin Resistance, and Ventricular Dysfunction and Remodeling in Obese Adolescents. J. Pediatr. 2014, 166, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Büchi, M.; Hess, O.H.; Murakami, T.; Krayenbuehl, H.P. Left ventricular wall stress distribution in chronic pressure and volume overload: Effect of normal and depressed contractility on regional stress-velocity relations. Basic Res. Cardiol. 1990, 85, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.; Hoffman, J.I.; Mahajan, A.; Saleh, S.; Coghlan, C. Cardiac Mechanics Revisited. Circulation 2008, 118, 2571–2587. [Google Scholar] [CrossRef]
- Cvijic, M.; Duchenne, J.; Ünlü, S.; Michalski, B.; Aarones, M.; Winter, S.; Aakhus, S.; Fehske, W.; Stankovic, I.; Voigt, J.-U. Timing of myocardial shortening determines left ventricular regional myocardial work and regional remodelling in hearts with conduction delays. Eur. Heart J. Cardiovasc. Imaging 2017, 19, 941–949. [Google Scholar] [CrossRef]
- Gaudron, P.D.; Liu, D.; Scholz, F.; Hu, K.; Florescu, C.; Herrmann, S.; Bijnens, B.; Ertl, G.; Störk, S.; Weidemann, F. The septal bulge—An early echocardiographic sign in hypertensive heart disease. J. Am. Soc. Hypertens. 2016, 10, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Goh, V.J.; Le, T.-T.; Bryant, J.; Wong, J.I.; Su, B.; Lee, C.-H.; Pua, C.J.; Sim, C.P.; Ang, B.; Aw, T.C.; et al. Novel index of maladaptive myocardial remodeling in hypertension. Circ. Cardiovasc. Imaging 2017, 10, e006840. [Google Scholar] [CrossRef] [PubMed]
| AN Patients (n = 26) | Controls (n = 33) | OB Patients (n = 28) | |
|---|---|---|---|
| Age (years) | 14.6 ± 1.9 | 14.0 ± 2.0 | 13.2 ± 1.4 # |
| Illness duration (months) | 19 ± 9 | - | 130 ± 27 ### |
| Anthropometry | |||
| Height (cm) | 159.8 ± 9.1 | 162.6 ± 10.0 | 162.9 ± 5.7 |
| Body mass (kg) | 40.7 ± 8.2 *** | 51.2 ± 9.8 | 90.4 ± 13.0 ***### |
| BMI (kg.m−2) | 15.8 ± 2.1 *** | 20.0 ± 3,2 | 34.0 ± 4.1 ***### |
| BMI z score | −1.8 ± 1.1 *** | 0.4 ± 1.3 | 4.1 ± 0.6 ***### |
| Ultrasound variables | |||
| LV septum thickness (cm) | 0.72 ± 0.15 | 0.76 ± 0.11 | 0.83 ± 0.18 # |
| LV posterior wall thickness (cm) | 0.67 ± 0.12 *** | 0.74 ± 0.11 | 0.88 ± 0.13 ***### |
| LV mass (g) | 79 ± 26 *** | 96 ± 24 | 128 ± 29 ***### |
| LV mass2.7 (g.m2.7) | 22 ± 6 *** | 26 ± 5 | 34 ± 7 ***### |
| RWT | 0.33 ± 0.06 | 0.36 ± 0.06 | 0.39 ± 0.06 ***### |
| LV end-diastolic volume (mL) | 79 ± 19 | 90 ± 23 | 119 ± 27 ***### |
| LV end-systolic volume (mL) | 29 ± 8 | 33 ± 9 | 42 ± 11 ***### |
| Ejection fraction (%) | 64 ± 5 | 64 ± 6 | 64 ± 6 |
| E wave (cm.s−1) | 84 ± 17 | 82 ± 14 | 90 ± 11 * |
| A wave (cm.s−1) | 30 ± 6 *** | 40 ± 8 | 39 ±7 ### |
| E/A | 2.9 ± 0.9 *** | 2.1 ± 0.5 | 2.4 ± 0.5 ***### |
| AN Patients (n = 26) | Controls (n = 37) | OB Patients (n = 28) | |
|---|---|---|---|
| Hemodynamic constants | |||
| Heart rate (bpm) | 56 ± 12 *** | 74 ± 11 | 76 ± 11 ### |
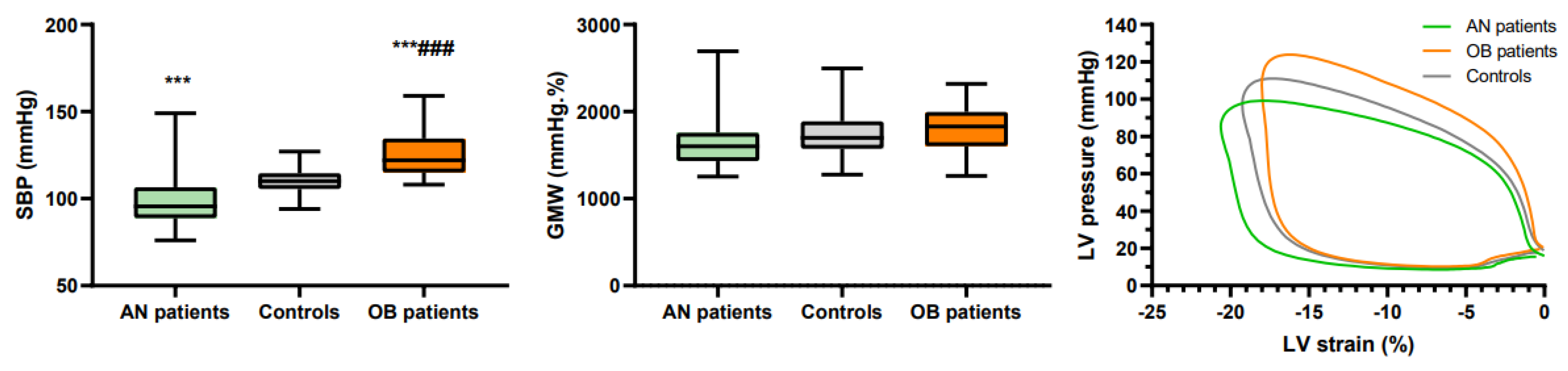
| SBP (mmHg) | 99 ± 14 *** | 110 ± 8 | 124 ± 12 ***### |
| DBP (mmHg) | 63 ± 11 | 67 ± 7 | 69 ± 13 |
| MBP (mmHg) | 75 ± 12 *** | 81 ± 7 | 87 ± 12 ***### |
| Longitudinal strains | |||
| GLS | −18.8 ± 2.0 ** | −16.9 ± 2.8 | −16.8 ± 1.9 ## |
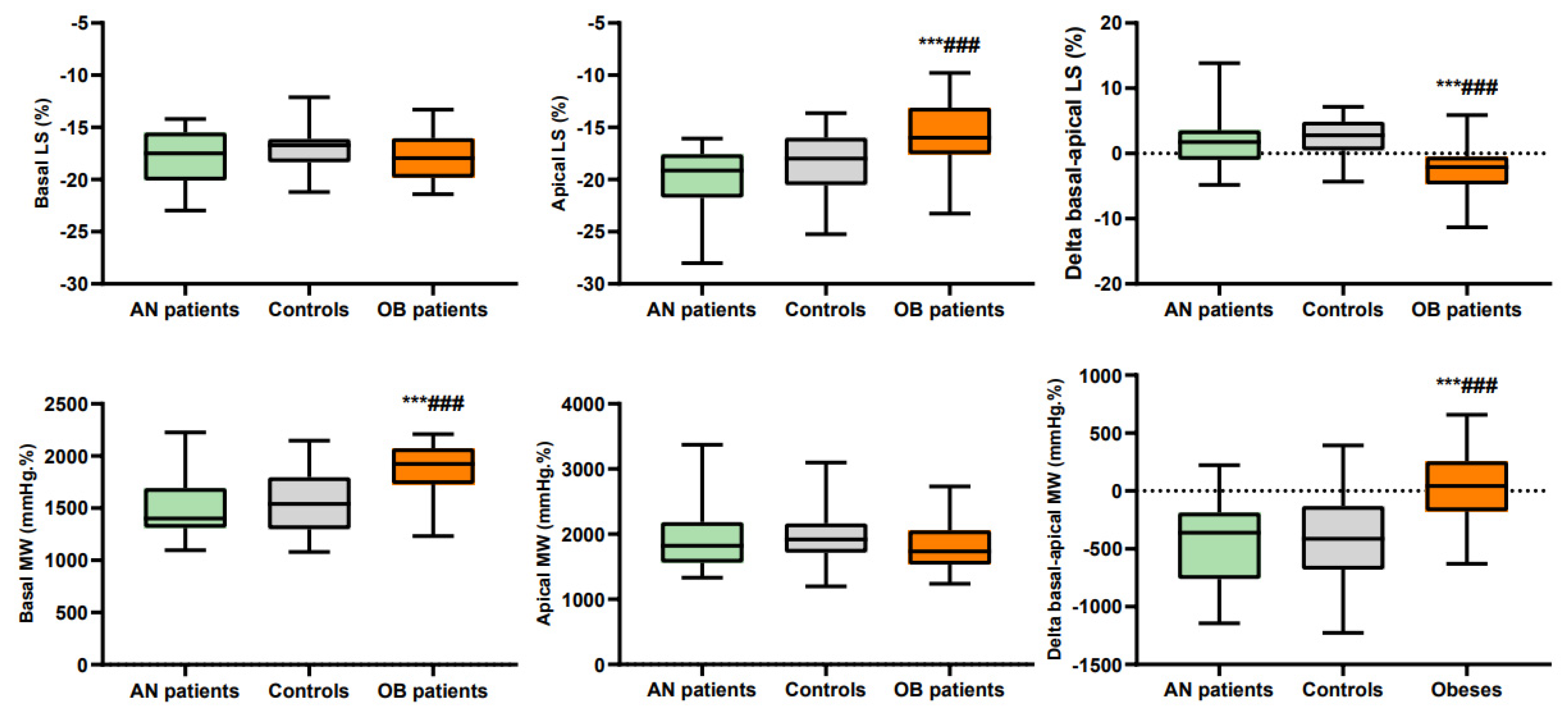
| Basal LS | −17.9 ± 2.6 | −16.9 ± 2.2 | −17.8 ± 2.1 |
| Median LS | −21.3 ± 2.0 *** | −20.1 ± 2.0 | −18.2 ± 2.1 ***### |
| Apical LS | −20.1 ± 3.4 | −18.4 ± 3.2 | −15.8 ± 3.5 ***### |
| Delta basal-apical LS | 2.1 ± 4.6 | 2.1 ± 3.7 | −2.5 ± 3.6 ***### |
| Myocardial work | |||
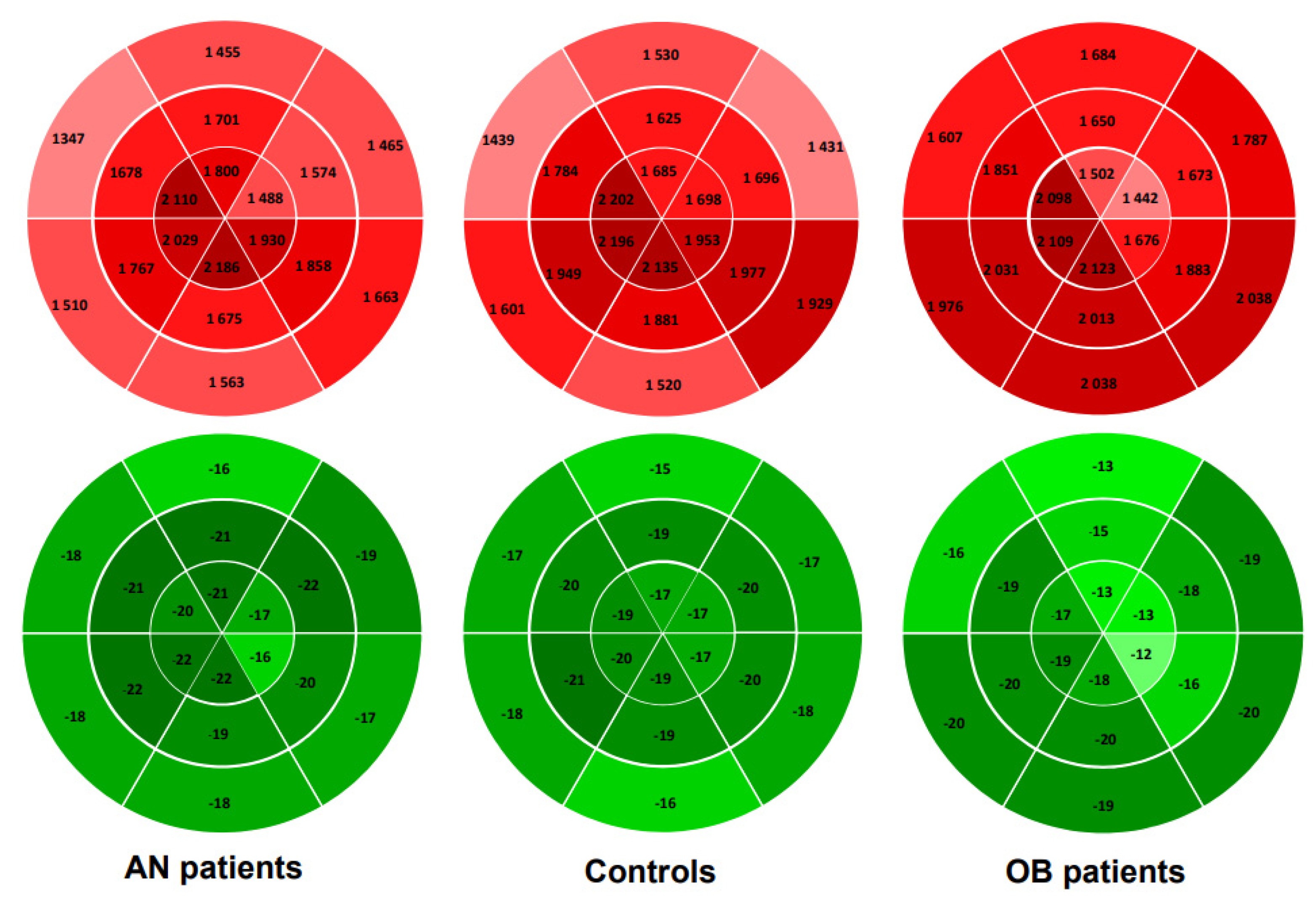
| GMW | 1 658 ± 335 | 1734 ± 287 | 1780 ± 292 |
| GWE | 92.8 ± 3.8 | 91.7 ± 5.2 | 92.7 ± 3.7 |
| Basal MW | 1501 ± 280 | 1575 ± 295 | 1855 ± 272 ***### |
| Median MW | 1709 ± 318 | 1819 ± 297 | 1850 ± 363 |
| Apical MW | 1924 ± 480 | 1978 ± 418 | 1825 ± 375 |
| Delta basal-apical MW | −423 ± 351 | −403 ± 404 | 30 ± 331 ***### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paysal, J.; Merlin, E.; Rochette, E.; Terral, D.; Nottin, S. Global and Regional Myocardial Work in Female Adolescents with Weight Disorders. J. Clin. Med. 2021, 10, 4671. https://doi.org/10.3390/jcm10204671
Paysal J, Merlin E, Rochette E, Terral D, Nottin S. Global and Regional Myocardial Work in Female Adolescents with Weight Disorders. Journal of Clinical Medicine. 2021; 10(20):4671. https://doi.org/10.3390/jcm10204671
Chicago/Turabian StylePaysal, Justine, Etienne Merlin, Emmanuelle Rochette, Daniel Terral, and Stéphane Nottin. 2021. "Global and Regional Myocardial Work in Female Adolescents with Weight Disorders" Journal of Clinical Medicine 10, no. 20: 4671. https://doi.org/10.3390/jcm10204671
APA StylePaysal, J., Merlin, E., Rochette, E., Terral, D., & Nottin, S. (2021). Global and Regional Myocardial Work in Female Adolescents with Weight Disorders. Journal of Clinical Medicine, 10(20), 4671. https://doi.org/10.3390/jcm10204671






