Retinal Microvascular Signs in Pre- and Early-Stage Diabetic Retinopathy Detected Using Wide-Field Swept-Source Optical Coherence Tomographic Angiography
Abstract
:1. Introduction
2. Method
2.1. Inclusion and Exclusion Criteria
2.2. Ophthalmic Examinations and Blood Test
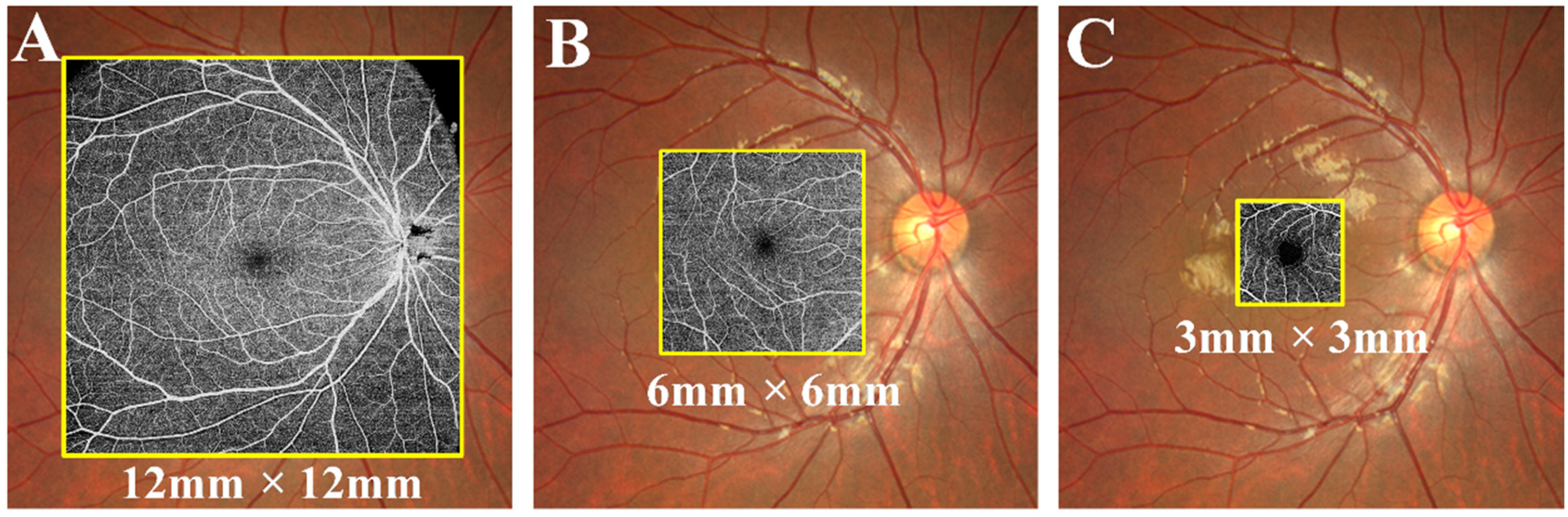
3. OCTA Image Analysis
Outcome Measures
4. Statistical Analysis
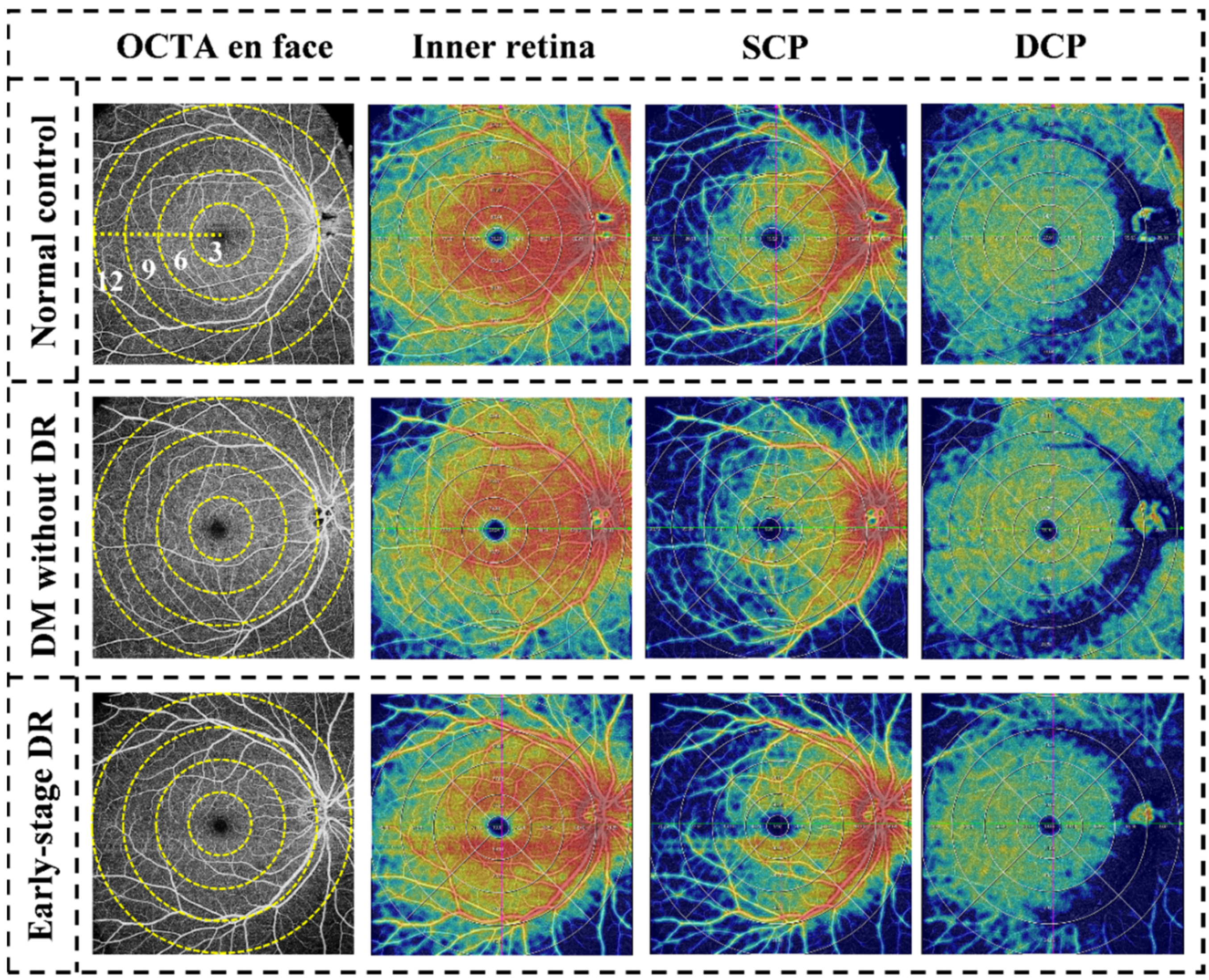
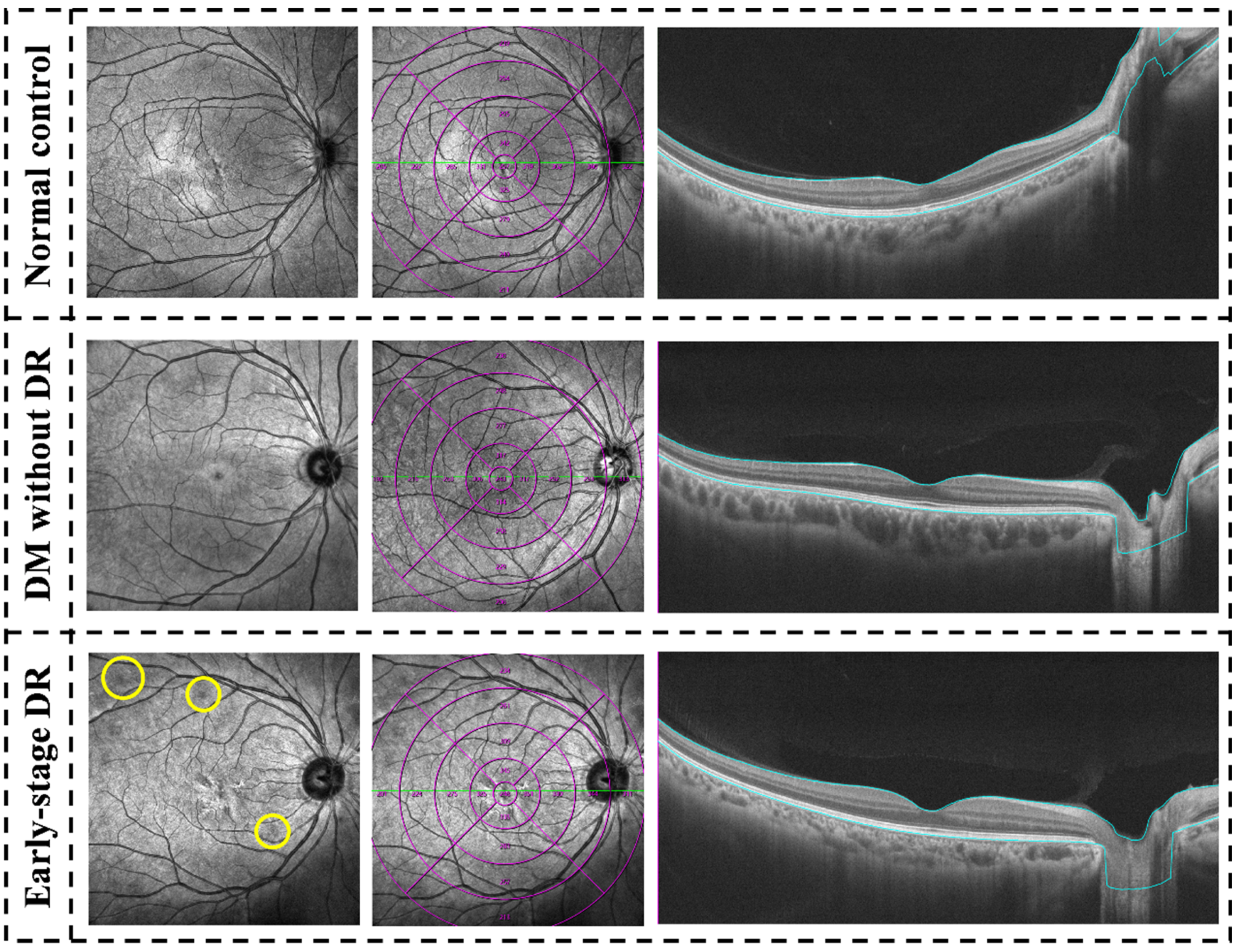
5. Results
5.1. Baseline Demographic Data
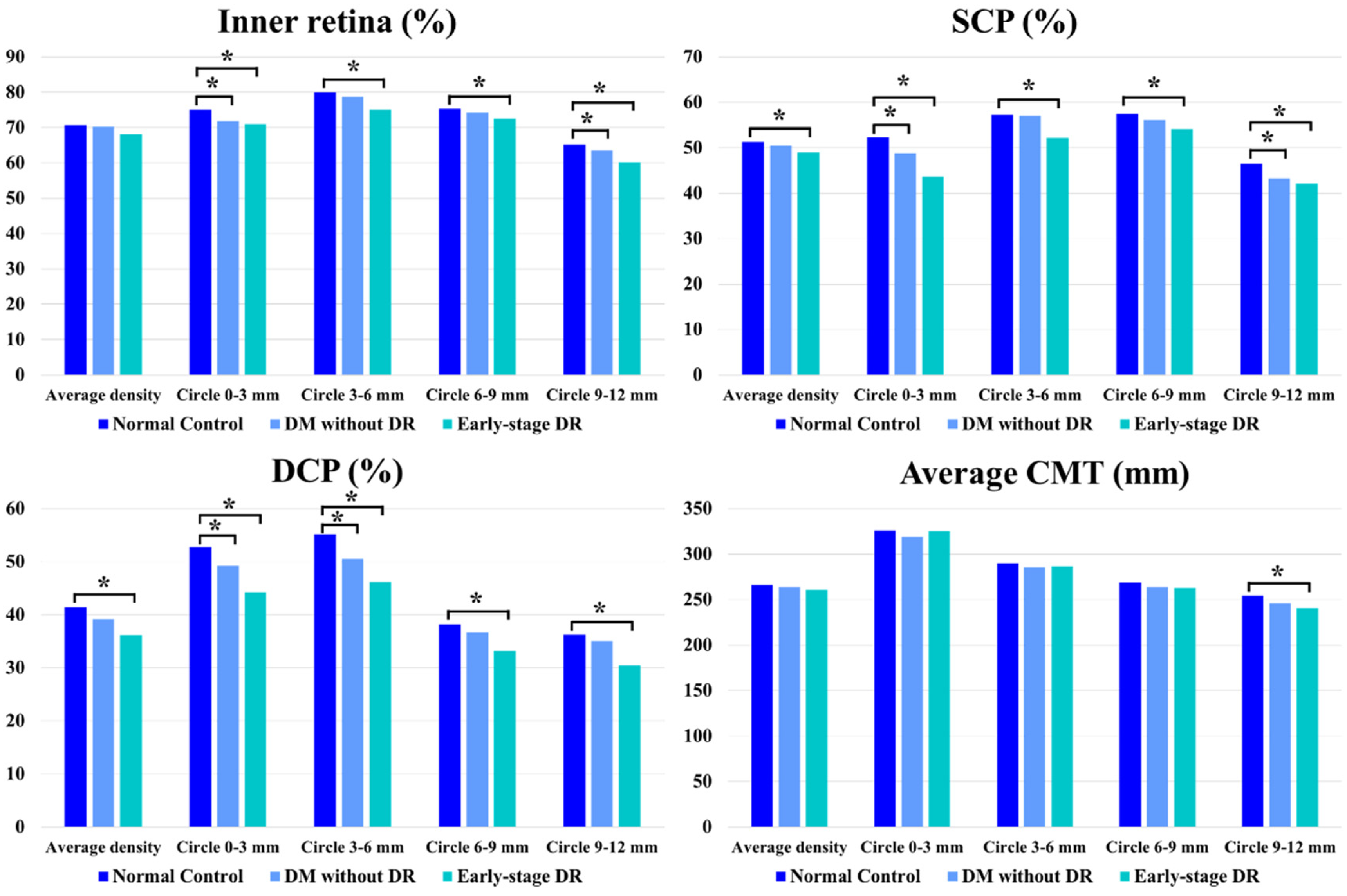
5.2. Quantitative Evaluation of the Inner Retina
5.3. Quantitative Evaluation of SCP
5.4. Quantitative Evaluation of DCP
5.5. Quantitative Evaluation of CMT
5.6. Qualitative Evaluation of the Concentric Ring Area
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bek, T. Transretinal histopathological changes in capillary-free areas of diabetic retinopathy. Acta Ophthalmol. 1994, 72, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, T.; Sato, E.; Takahashi, A.; Yokota, H.; Sogawa, K.; Yoshida, A. Impaired retinal circulation in patients with type 2 diabetes mellitus: Retinal laser Doppler velocimetry study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6729–6734. [Google Scholar] [CrossRef]
- Stefánsson, E.; Bek, T.; Porta, M.; Larsen, N.; Kristinsson, J.K.; Agardh, E. Screening and prevention of diabetic blindness. Acta Ophthalmol. Scand. 2000, 78, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.H.; Stevens, G.A.; et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef] [Green Version]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Sabanayagam, C.; Banu, R.; Chee, M.L.; Lee, R.; Wang, Y.X.; Tan, G.; Jonas, J.B.; Lamoureux, E.L.; Cheng, C.Y.; Klein, B.E.K.; et al. Incidence and progression of diabetic retinopathy: A systematic review. Lancet Diabetes Endocrinol. 2019, 7, 140–149. [Google Scholar] [CrossRef]
- Bandello, F.; Corbelli, E.; Carnevali, A.; Pierro, L.; Querques, G. Optical Coherence Tomography Angiography of Diabetic Retinopathy. Dev. Ophthalmol. 2016, 56, 107–112. [Google Scholar] [CrossRef]
- Kim, A.Y.; Chu, Z.; Shahidzadeh, A.; Wang, R.K.; Puliafito, C.A.; Kashani, A.H. Quantifying Microvascular Density and Morphology in Diabetic Retinopathy Using Spectral-Domain Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT362-370. [Google Scholar] [CrossRef]
- Salz, D.A.; de Carlo, T.E.; Adhi, M.; Moult, E.; Choi, W.; Baumal, C.R.; Witkin, A.J.; Duker, J.S.; Fujimoto, J.G.; Waheed, N.K. Select Features of Diabetic Retinopathy on Swept-Source Optical Coherence Tomographic Angiography Compared with Fluorescein Angiography and Normal Eyes. JAMA Ophthalmol. 2016, 134, 644–650. [Google Scholar] [CrossRef]
- Simonett, J.M.; Scarinci, F.; Picconi, F.; Giorno, P.; De Geronimo, D.; Di Renzo, A.; Varano, M.; Frontoni, S.; Parravano, M. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 2017, 95, e751–e755. [Google Scholar] [CrossRef] [Green Version]
- Dimitrova, G.; Chihara, E.; Takahashi, H.; Amano, H.; Okazaki, K. Quantitative Retinal Optical Coherence Tomography Angiography in Patients with Diabetes without Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Neves, C.; Marques, I.P.; Pires, I.; Schwartz, C.; Costa, M.A.; Santos, T.; Durbin, M.; Cunha-Vaz, J. Comparison of diabetic retinopathy classification using fluorescein angiography and optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Ng, D.S.; Lam, A.; Luk, F.; Wong, R.; Chan, C.; Mohamed, S.; Fong, A.; Lok, J.; Tso, T.; et al. Determinants of Quantitative Optical Coherence Tomography Angiography Metrics in Patients with Diabetes. Sci. Rep. 2017, 7, 2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendis, K.R.; Balaratnasingam, C.; Yu, P.; Barry, C.J.; McAllister, I.L.; Cringle, S.J.; Yu, D.Y. Correlation of histologic and clinical images to determine the diagnostic value of fluorescein angiography for studying retinal capillary detail. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5864–5869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Ma, Q.; Wu, C.; Tan, F.; Chen, F.; Wu, Q.; Zhou, R.; Zhuang, X.; Lu, F.; Qu, J.; et al. Macular Vascular Fractal Dimension in the Deep Capillary Layer as an Early Indicator of Microvascular Loss for Retinopathy in Type 2 Diabetic Patients. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3785–3794. [Google Scholar] [CrossRef]
- Scarinci, F.; Picconi, F.; Giorno, P.; Boccassini, B.; De Geronimo, D.; Varano, M.; Frontoni, S.; Parravano, M. Deep capillary plexus impairment in patients with type 1 diabetes mellitus with no signs of diabetic retinopathy revealed using optical coherence tomography angiography. Acta Ophthalmol. 2018, 96, e264–e265. [Google Scholar] [CrossRef] [Green Version]
- Safi, H.; Safi, S.; Hafezi-Moghadam, A.; Ahmadieh, H. Early detection of diabetic retinopathy. Surv. Ophthalmol. 2018, 63, 601–608. [Google Scholar] [CrossRef]
- Rosen, R.B.; Andrade Romo, J.S.; Krawitz, B.D.; Mo, S.; Fawzi, A.A.; Linderman, R.E.; Carroll, J.; Pinhas, A.; Chui, T.Y.P. Earliest Evidence of Preclinical Diabetic Retinopathy Revealed Using Optical Coherence Tomography Angiography Perfused Capillary Density. Am. J. Ophthalmol. 2019, 203, 103–115. [Google Scholar] [CrossRef]
- Li, Z.; Wen, X.; Zeng, P.; Liao, Y.; Fan, S.; Zhang, Y.; Li, Y.; Xiao, J.; Lan, Y. Do microvascular changes occur preceding neural impairment in early-stage diabetic retinopathy? Evidence based on the optic nerve head using optical coherence tomography angiography. Acta Diabetol. 2019, 56, 531–539. [Google Scholar] [CrossRef]
- Zeng, Y.; Cao, D.; Yu, H.; Yang, D.; Zhuang, X.; Hu, Y.; Li, J.; Yang, J.; Wu, Q.; Liu, B.; et al. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br. J. Ophthalmol. 2019, 103, 1747–1752. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wan, C.; Zhao, L.; Liu, S.; Hong, J.; Xiang, Y.; You, Q.; Zhou, L.; Li, Z.; Gong, S.; et al. Predicting Post-Therapeutic Visual Acuity and OCT Images in Patients with Central Serous Chorioretinopathy by Artificial Intelligence. Front. Bioeng. Biotechnol. 2021, 9, 649221. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Liu, X.; Yu, X. Multi-path cascaded U-net for vessel segmentation from fundus fluorescein angiography sequential images. Comput. Methods Programs Biomed. 2021, 211, 106422. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Pereira, S.; Morais-Sarmento, T.; Marques, R.E. Optical coherence tomography features of neovascularization in proliferative diabetic retinopathy: A systematic review. Int. J. Retin. Vitr. 2020, 6, 26. [Google Scholar] [CrossRef]
- Hormel, T.T.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Huang, D.; Jia, Y. Artificial intelligence in OCT angiography. Prog. Retin. Eye Res. 2021, 85, 100965. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, J.; Liang, A.; Zhao, C.; Gao, F.; Zhang, M. Widefield Swept-Source Optical Coherence Tomography Angiography Assessment of Choroidal Changes in Vogt-Koyanagi-Harada Disease. Front. Med. 2021, 8, 698644. [Google Scholar] [CrossRef]
- Pang, Y.; Sparschu, L.; Nylin, E.; Wang, J. Validation of an Automated Early Treatment Diabetic Retinopathy Study Low-contrast Letter Acuity Test. Optom. Vis. Sci. 2020, 97, 370–376. [Google Scholar] [CrossRef]
- Aiello, L.P.; Odia, I.; Glassman, A.R.; Melia, M.; Jampol, L.M.; Bressler, N.M.; Kiss, S.; Silva, P.S.; Wykoff, C.C.; Sun, J.K.; et al. Comparison of Early Treatment Diabetic Retinopathy Study Standard 7-Field Imaging with Ultrawide-Field Imaging for Determining Severity of Diabetic Retinopathy. JAMA Ophthalmol. 2019, 137, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Laiginhas, R.; Rosenfeld, P.J. Widefield Swept-Source Optical Coherence Tomography Angiography of a “Polypoidal-Like” Retinal Arteriovenous Anastomosis in Proliferative Diabetic Retinopathy. JAMA Ophthalmol. 2021, 139, e213859. [Google Scholar] [CrossRef]
- Lu, E.S.; Cui, Y.; Le, R.; Zhu, Y.; Wang, J.C.; Lains, I.; Katz, R.; Lu, Y.; Zeng, R.; Garg, I.; et al. Detection of neovascularisation in the vitreoretinal interface slab using widefield swept-source optical coherence tomography angiography in diabetic retinopathy. Br. J. Ophthalmol. 2022, 106, 534–539. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, Y.; Wang, J.C.; Lu, Y.; Zeng, R.; Katz, R.; Vingopoulos, F.; Le, R.; Lains, I.; Wu, D.M.; et al. Comparison of widefield swept-source optical coherence tomography angiography with ultra-widefield colour fundus photography and fluorescein angiography for detection of lesions in diabetic retinopathy. Br. J. Ophthalmol. 2021, 105, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Schaal, K.B.; Munk, M.R.; Wyssmueller, I.; Berger, L.E.; Zinkernagel, M.S.; Wolf, S. Vascular Abnormalities in Diabetic Retinopathy Assessed with Swept-Source Optical Coherence Tomography Angiography Widefield Imaging. Retina 2019, 39, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Durbin, M.K.; An, L.; Shemonski, N.D.; Soares, M.; Santos, T.; Lopes, M.; Neves, C.; Cunha-Vaz, J. Quantification of Retinal Microvascular Density in Optical Coherence Tomographic Angiography Images in Diabetic Retinopathy. JAMA Ophthalmol. 2017, 135, 370–376. [Google Scholar] [CrossRef] [PubMed]

| Normal Control | Pre-DR | p Values | Early-Stage DR | p Values | |
|---|---|---|---|---|---|
| Patients (Female) | 21 (12) | 20 (11) | 0.953 | 21 (11) | 1.000 |
| Ages | 54.21 ± 9.18 | 56.56 ± 10.87 | 0.809 | 57.20 ± 12.07 | 0.743 |
| Eyes | 42 | 38 | 0.825 | 39 | 0.864 |
| Duration of DM (months) | N/A | 8.14 ± 5.65 | N/A | 26.02 ± 12.34 | N/A |
| BCVA (ETDRS) | 82.24 ± 2.31 | 81.34 ± 1.98 | 0.962 | 82.41 ± 1.93 | 0.984 |
| GLU | N/A | 8.80 ± 7.63 | N/A | 9.01 ± 8.32 | N/A |
| HbA1c | N/A | 6.59 ± 4.06 | N/A | 8.78 ± 3.21 | N/A |
| CMT (μm) | 198.34 ± 11.36 | 202.46 ± 12.17 | 0.904 | 192.37 ± 10.95 | 0.837 |
| ChT (μm) | 267.36 ± 29.55 | 261.30 ± 30.11 | 0.914 | 256.07 ± 33.47 | 0.722 |
| Image Quality Index | 8.39 ± 1.56 | 8.58 ± 1.49 | 0.964 | 8.57 ± 1.63 | 0.959 |
| Layers | Location | Normal Control | Pre-DR | p Values | Early-Stage DR | p Values |
|---|---|---|---|---|---|---|
| Inner retina | Average density | 70.75 ± 2.28 | 70.26 ± 3.37 | p = 0.765 | 68.09 ± 3.00 | p = 0.210 |
| Ring 0–3 | 75.06 ± 3.01 | 71.81 ± 6.33 | p = 0.016 * | 70.96 ± 4.65 | p = 0.002 * | |
| Ring 3–6 | 79.99 ± 2.48 | 78.71 ± 4.57 | p = 0.542 | 75.02 ± 4.75 | p = 0.007 * | |
| Ring 6–9 | 75.32 ± 2.85 | 74.34 ± 3.37 | p = 0.461 | 72.50 ± 3.52 | p = 0.015 * | |
| Ring 9–12 | 65.25 ± 3.24 | 63.44 ± 3.81 | p = 0.008 * | 60.22 ± 3.63 | p < 0.001 * | |
| SCP | Average density | 51.27 ± 3.67 | 50.48 ± 4.55 | p = 0.410 | 48.94 ± 7.87 | p = 0.041 * |
| Ring 0–3 | 52.28 ± 4.31 | 48.77 ± 7.57 | p = 0.021 * | 43.67 ± 6.96 | p < 0.001 * | |
| Ring 3–6 | 57.28 ± 4.10 | 57.08 ± 5.40 | p = 0.823 | 52.15 ± 5.81 | p < 0.001 * | |
| Ring 6–9 | 57.38 ± 4.62 | 56.12 ± 4.40 | p = 0.548 | 54.13 ± 9.62 | p = 0.037 * | |
| Ring 9–12 | 46.42 ± 7.49 | 43.25 ± 6.64 | p = 0.002 * | 42.16 ± 6.94 | p < 0.001 * | |
| DCP | Average density | 41.36 ± 2.64 | 39.20 ± 4.45 | p = 0.143 | 36.23 ± 8.34 | p < 0.001 * |
| Ring 0–3 | 52.76 ± 4.03 | 49.22 ± 6.09 | p = 0.033 * | 44.21 ± 8.02 | p < 0.001 * | |
| Ring 3–6 | 55.14 ± 6.52 | 50.58 ± 6.72 | p < 0.001 * | 46.12 ± 7.63 | p < 0.001 * | |
| Ring 6–9 | 38.19 ± 3.81 | 36.69 ± 6.27 | p = 0.260 | 33.09 ± 6.24 | p < 0.001 * | |
| Ring 9–12 | 36.28 ± 2.78 | 35.08 ± 5.92 | p = 0.614 | 30.43 ± 6.92 | p < 0.001 * | |
| Average CMT | Average thickness | 265.73 ± 8.36 | 263.74 ± 14.40 | p = 0.754 | 261.08 ± 10.12 | p = 0.716 |
| Ring 0–3 | 325.74 ± 14.98 | 318.99 ± 15.01 | p = 0.219 | 325.12 ± 13.68 | p = 0.890 | |
| Ring 3–6 | 289.70 ± 10.54 | 285.39 ± 16.67 | p = 0.754 | 286.83 ± 15.62 | p = 0.773 | |
| Ring 6–9 | 268.88 ± 10.69 | 263.70 ± 13.16 | p = 0.684 | 263.05 ± 19.98 | p = 0.681 | |
| Ring 9–12 | 254.45 ± 9.38 | 245.54 ± 16.28 | p = 0.072 | 240.60 ± 24.39 | p = 0.024 * |
| Layers | Location | Normal Control | Pre-DR | p Values | Early-Stage DR | p Values |
|---|---|---|---|---|---|---|
| Inner retina | Average density | 70.75 ± 2.28 | 70.26 ± 3.37 | N/A | 68.09 ± 3.00 | N/A |
| Circle 0–3 | 4.89 ± 3.54 | 1.95 ± 4.36 | p = 0.021 * | 2.62 ± 3.84 | p = 0.105 | |
| Circle 3–6 | 9.45 ± 4.32 | 7.69 ± 4.29 | p = 0.483 | 7.25 ± 3.51 | p = 0.427 | |
| Circle 6–9 | 5.61 ± 2.84 | 3.96 ± 2.85 | p = 0.491 | 3.87 ± 3.19 | p = 0.465 | |
| Circle 9–12 | −5.81 ± 3.67 | −7.41 ± 3.74 | p = 0.038 * | −9.02 ± 3.42 | p < 0.001 * | |
| SCP | Average density | 51.27 ± 3.67 | 50.48 ± 4.55 | N/A | 48.94 ± 7.87 | N/A |
| Circle 0–3 | 0.93 ± 3.47 | 1.75 ± 6.54 | p = 0.338 | 4.36 ± 6.81 | p = 0.013 * | |
| Circle 3–6 | 6.28 ± 4.14 | 6.81 ± 5.83 | p = 0.851 | 4.21 ± 6.23 | p = 0.230 | |
| Circle 6–9 | 6.85 ± 3.86 | 5.92 ± 6.02 | p = 0.705 | 6.03 ± 7.28 | p = 0.861 | |
| Circle 9–12 | −4.96 ± 6.43 | −7.06 ± 7.32 | p = 0.009 * | −7.09 ± 5.36 | p = 0.008 * | |
| DCP | Average density | 41.36 ± 2.64 | 39.20 ± 4.45 | N/A | 36.23 ± 8.34 | N/A |
| Circle 0–3 | 9.65 ± 7.02 | 9.45 ± 6.63 | p = 0.921 | 9.65 ± 7.10 | p = 0.987 | |
| Circle 3–6 | 13.28 ± 7.74 | 12.36 ± 7.80 | p = 0.893 | 11.96 ± 6.90 | p = 0.765 | |
| Circle 6–9 | −3.53 ± 3.56 | −3.62 ± 5.39 | p = 0.950 | −1.86 ± 7.52 | p = 0.460 | |
| Circle 9–12 | −4.36 ± 2.96 | −4.85 ± 4.31 | p = 0.864 | −7.01 ± 5.86 | p = 0.038 * | |
| Average CMT | Average thickness | 265.73 ± 8.36 | 263.74 ± 14.40 | N/A | 261.08 ± 10.12 | N/A |
| Circle 0–3 | 56.34 ± 12.69 | 53.41 ± 14.03 | p = 0.854 | 60.21 ± 15.11 | p = 0.783 | |
| Circle 3–6 | 33.08 ± 11.24 | 27.02 ± 15.81 | p = 0.420 | 27.31 ± 14.63 | p = 0.601 | |
| Circle 6–9 | 3.61 ± 12.80 | 2.01 ± 11.08 | p = 0.813 | 3.01 ± 17.32 | p = 0.882 | |
| Circle 9–12 | −9.66 ± 10.20 | −12.05 ± 15.08 | p = 0.082 | −19.33 ± 20.52 | p = 0.002 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Li, Z.; Gao, Y.; Yang, X.; Huang, Z.; Li, Z.; Zhang, R.; Wang, S.; Guo, X.; Hou, X.; et al. Retinal Microvascular Signs in Pre- and Early-Stage Diabetic Retinopathy Detected Using Wide-Field Swept-Source Optical Coherence Tomographic Angiography. J. Clin. Med. 2022, 11, 4332. https://doi.org/10.3390/jcm11154332
Xu F, Li Z, Gao Y, Yang X, Huang Z, Li Z, Zhang R, Wang S, Guo X, Hou X, et al. Retinal Microvascular Signs in Pre- and Early-Stage Diabetic Retinopathy Detected Using Wide-Field Swept-Source Optical Coherence Tomographic Angiography. Journal of Clinical Medicine. 2022; 11(15):4332. https://doi.org/10.3390/jcm11154332
Chicago/Turabian StyleXu, Fabao, Zhiwen Li, Yang Gao, Xueying Yang, Ziyuan Huang, Zhiwei Li, Rui Zhang, Shaopeng Wang, Xinghong Guo, Xinguo Hou, and et al. 2022. "Retinal Microvascular Signs in Pre- and Early-Stage Diabetic Retinopathy Detected Using Wide-Field Swept-Source Optical Coherence Tomographic Angiography" Journal of Clinical Medicine 11, no. 15: 4332. https://doi.org/10.3390/jcm11154332
APA StyleXu, F., Li, Z., Gao, Y., Yang, X., Huang, Z., Li, Z., Zhang, R., Wang, S., Guo, X., Hou, X., Ning, X., & Li, J. (2022). Retinal Microvascular Signs in Pre- and Early-Stage Diabetic Retinopathy Detected Using Wide-Field Swept-Source Optical Coherence Tomographic Angiography. Journal of Clinical Medicine, 11(15), 4332. https://doi.org/10.3390/jcm11154332





