3D Visualization System in Descemet Membrane Endothelial Keratoplasty (DMEK): A Six-Month Comparison with Conventional Microscope
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Materials
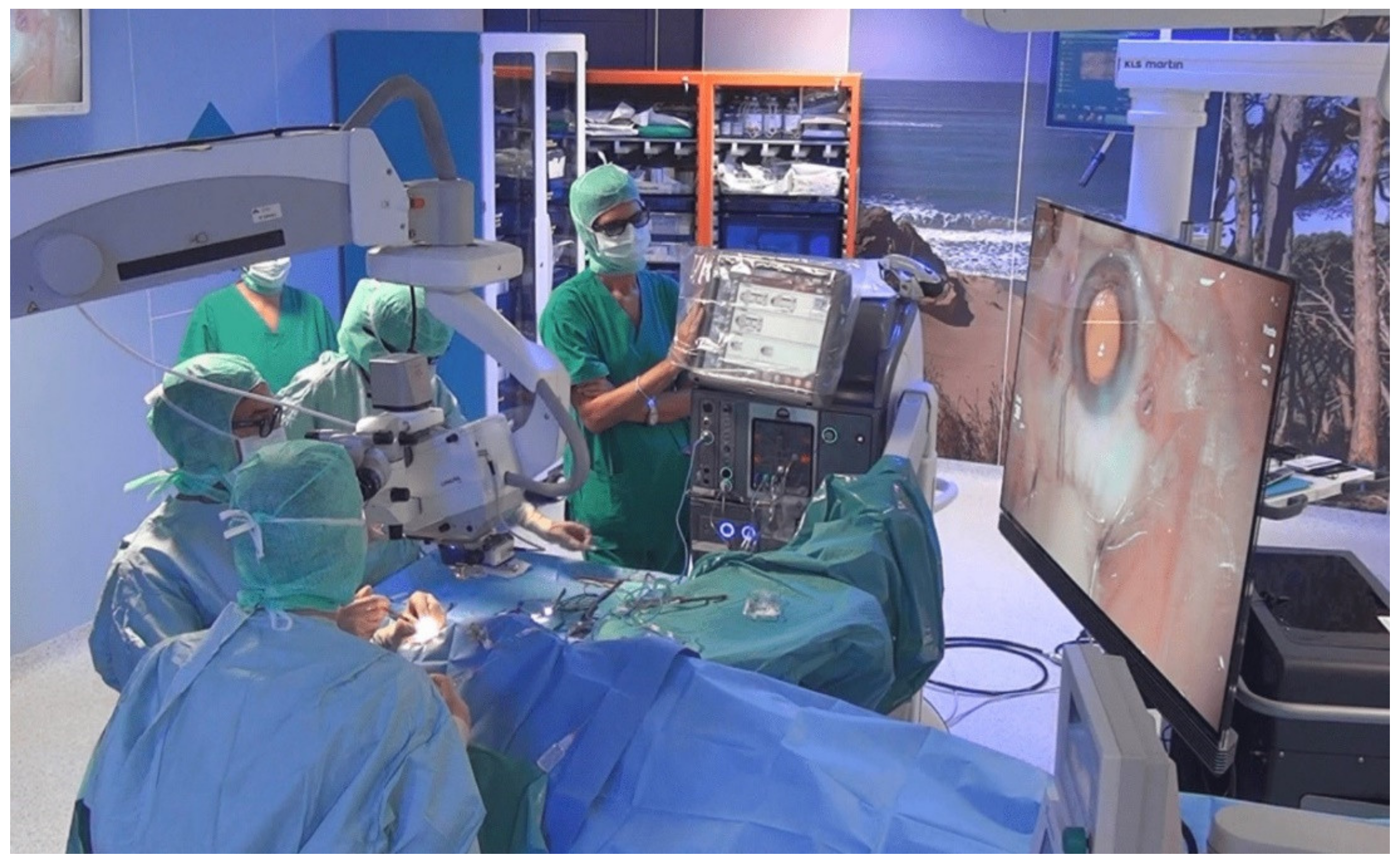
2.3. Surgery
2.4. Statistics
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nanavaty, M.A.; Wang, X.; Shortt, A.J. Endothelial keratoplasty versus penetrating keratoplasty for Fuchs endothelial dystrophy. Cochrane Database Syst. Rev. 2014, 2, CD008420. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Wilkins, M.R.; Mehta, J.S.; Tan, D. Descemet membrane endothelial keratoplasty. Br. J. Ophthalmol. 2016, 100, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, W.H.; Gillings, M.J.; Robertson, D.M.; Petroll, W.M.; Mootha, V.V. Lower Corneal Haze and Aberrations in Descemet Membrane Endothelial Keratoplasty Versus Descemet Stripping Automated Endothelial Keratoplasty in Fellow Eyes for Fuchs Endothelial Corneal Dystrophy. Cornea 2020, 39, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Pahuja, N.; Shetty, R.; Subbiah, P.; Nagaraja, H.; Nuijts, R.M.; Jayadev, C. Corneal Densitometry: Repeatability in Eyes with Keratoconus and Postcollagen Cross-Linking. Cornea 2016, 35, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Alnawaiseh, M.; Zumhagen, L.; Wirths, G.; Eveslage, M.; Eter, N.; Rosentreter, A. Corneal Densitometry, Central Corneal Thickness, and Corneal Central-to Peripheral Thickness Ratio in Patients with Fuchs Endothelial Dystrophy. Cornea 2016, 35, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Schaub, F.; Enders, P.; Bluhm, C.; Bachmann, B.O.; Cursiefen, C.; Heindl, L.M. Two-Year Course of Corneal Densitometry after Descemet Membrane Endothelial Keratoplasty. Am. J. Ophthalmol. 2017, 175, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, C.; Paulo, E.B. Heads-up surgery for vitreoretinal procedures: An experimental and clinical study. Retina 2016, 36, 137–147. [Google Scholar] [CrossRef]
- Kunikata, H.; Abe, T.; Nakazawa, T. Heads-up macular surgery with a 27-gauge microincision vitrectomy system and minimal illumination. Case Rep. Ophthalmol. 2016, 7, 265–269. [Google Scholar] [CrossRef]
- Skinner, C.C.; Riemann, C.D. ‘Heads up’ digitally assisted surgical viewing for retinal detachment repair in a patient with severe kyphosis. Retin. Cases Brief Rep. 2018, 12, 257–259. [Google Scholar] [CrossRef]
- Coppola, M.; La Spina, C.; Rabiolo, A.; Querques, G.; Bandello, F. Heads-up 3D vision system for retinal detachment surgery. Int. J. Retina. Vitr. 2017, 3, 46. [Google Scholar] [CrossRef]
- Mohamed, Y.H.; Uematsu, M.; Inoue, D.; Kitaoka, T. First experience of nDASEK with heads-up surgery. Medicine 2017, 96, e12287. [Google Scholar] [CrossRef]
- Galvis, V.; Berrospi, R.D.; Arias, J.D.; Tello, A.; Bernal, J.C. Heads up Descemet membrane endothelial keratoplasty performed using a 3D visualization system. J. Surg. Case Rep. 2017, 2017, rjx231. [Google Scholar] [CrossRef]
- Panthier, C.; Courtin, R.; Moran, S.; Gatinel, D. Heads-up Descemet Membrane Endothelial Keratoplasty Surgery: Feasibility, Surgical Duration, Complication Rates, and Comparison with a Conventional Microscope. Cornea 2021, 40, 415–419. [Google Scholar] [CrossRef]
- Weinstock, R.J.; Diakonis, V.F.; Schwartz, A.J.; Weinstock, A.J. Heads-up cataract surgery: Complication rates, surgical duration, and comparison with traditional microscopes. J. Refract. Surg. 2019, 35, 318–322. [Google Scholar] [CrossRef]
- Moura-Coelho, N.; Henriques, J.; Nascimento, J.; Dutra-Medeiros, M. Three-dimensional Display Systems in Ophthalmic Surgery—A Review. Eur. Ophthalmic. Rev. 2019, 13, 31. [Google Scholar] [CrossRef]
- Berquet, F.; Henry, A.; Barbe, C.; Cheny, T.; Afriat, M.; Benyelles, A.K.; Bartolomeu, D.; Arndt, C. Comparing Heads-Up versus Binocular Microscope Visualization Systems in Anterior and Posterior Segment Surgeries: A Retrospective Study. Ophthalmologica 2020, 243, 347–354. [Google Scholar] [CrossRef]
- Dhubhghaill, S.N.; Rozema, J.J.; Jongenelen, S.; Hidalgo, I.R.; Zakaria, N.; Tassignon, M.J. Normative values for corneal densitometry analysis by Scheimpflug optical assessment. Investig. Ophthalmol. Vis. Sci. 2014, 55, 162–168. [Google Scholar] [CrossRef]
- Ong, H.S.; Ang, M.; Mehta, J. Evolution of therapies for the corneal endothelium: Past, present and future approaches. Br. J. Ophthalmol. 2021, 105, 454–467. [Google Scholar] [CrossRef]
- Weinstock, R.J.; Ainslie-Garcia, M.H.; Ferko, N.C.; Qadeer, R.A.; Morris, L.P.; Cheng, H.; Ehlers, J.P. Comparative Assessment of Ergonomic Experience with Heads-Up Display and Conventional Surgical Microscope in the Operating Room. Clin. Ophthalmol. 2021, 15, 347–356. [Google Scholar] [CrossRef]
- Borroni, D.; Rocha-de-Lossada, C.; Bonci, P.; Rechichi, M.; Rodríguez-Calvo-de-Mora, M.; Rachwani-Anil, R.; Sánchez González, J.M.; Urbinati, F.; Lorente, M.G.; Vigo, L.; et al. Glasses-Assisted 3D Display System-Guided Descemet Membrane Endothelial Keratoplasty Tissue Preparation. Cornea 2022. [Google Scholar] [CrossRef]
- Agha, B.; Dawson, D.G.; Kohnen, T.; Schmack, I. Corneal Densitometry after Secondary Descemet Membrane Endothelial Keratoplasty. Cornea 2019, 38, 1083–1092. [Google Scholar] [CrossRef]
- Ta Kim, D.; Chow, D. The effect of latency on surgical performance and usability in a three-dimensional heads-up display visualization system for vitreoretinal surgery. Graefes. Arch. Clin. Exp. Ophthalmol. 2022, 260, 471–476. [Google Scholar] [CrossRef]
- Del Turco, C.; D’Amico Ricci, G.; Dal Vecchio, M.; Bogetto, C.; Panico, E.; Giobbio, D.C.; Romano, M.R.; Panico, C.; La Spina, C. Heads-up 3D eye surgery: Safety outcomes and technological review after 2 years of day-to-day use. Eur. J. Ophthalmol. 2022, 32, 1129–1135. [Google Scholar] [CrossRef]
- Mendez, B.M.; Chiodo, M.V.; Vandevender, D.; Patel, P.A. Heads-up 3D Microscopy: An Ergonomic and Educational Approach to Microsurgery. Plast. Reconstr. Surg. Glob. Open. 2016, 25, e717. [Google Scholar] [CrossRef]
- Bin Helayel, H.; Al-Mazidi, S.; AlAkeely, A. Can the Three-Dimensional Heads-Up Display Improve Ergonomics, Surgical Performance, and Ophthalmology Training Compared to Conventional Microscopy? Clin. Ophthalmol. 2021, 15, 679–686. [Google Scholar] [CrossRef]
| 3D Group | TM Group | ||||
|---|---|---|---|---|---|
| Mean ± SD (Range) | Absolute Number (%) | Mean ± SD (Range) | Absolute Number (%) | p Value | |
| Age (years) | 72.6 ± 6.9 (58–87) | 68.6 ± 7.4 (54–85) | 0.09 | ||
| Male | 7 (35%) | 9 (45%) | |||
| Female | 13 (65%) | 11 (55%) | 0.42 | ||
| Right Eye | 9 (45%) | 8 (40%) | |||
| Left Eye | 11 (55%) | 12 (60%) | 0.85 | ||
| BSCVA (logMAR) | 0.43 ± 0.21 (0.20–0.90) | 0.54 ± 0.42 (0.20–2.00) | 0.31 | ||
| CCT (µm) | 641.25 ± 50.2 (585–750) | 643.05 ± 46.62 (598–766) | 0.90 | ||
| IOP (mmHg) | 14.4 ± 1.4 (12–17) | 13.9 ± 1.4 (10–16) | |||
| 3D Group | TM Group | ||||
|---|---|---|---|---|---|
| Mean ± SD (Range) | Absolute Number (%) | Mean ± SD (Range) | Absolute Number (%) | p Value | |
| Age (years) | 68.7 ± 7.2 (54–76) | 64.9 ± 6.8 (45–74) | 0.10 | ||
| Male | 7 (35%) | 9 (45%) | |||
| Female | 13 (65%) | 11 (55%) | |||
| ECD (cells/mm2) | 2667.5 ± 192.1 (2300–3000) | 2742.8 ± 136.9 (2500–3000) | 0.16 | ||
| Preservation time (days) | 27.2 ± 3.3 (22–36) | 26.2 ± 2.4 (21–30) | 0.30 | ||
| 3D Group | TM Group | ||
|---|---|---|---|
| Mean ± SD (Range) | Mean ± SD (Range) | p Value | |
| Total surgical time (min) | 24.33 ± 3.56 (17.80–29.60) | 22.01 ± 3.58 (15.40–30.40) | 0.04 |
| Time to perform descemetorhexis (min) | 5.25 ± 2.03 (1.20–8.20) | 3.86 ± 1.59 (0.90–7.20) | 0.02 |
| Graft unfolding time (min) | 4.88 ± 1.38 (2.80–7.20) | 4.31 ± 1.31 (2.50–6.90) | 0.19 |
| Time | 3D Group | TM Group | p Value | |
|---|---|---|---|---|
| Mean ± SD (Range) | Mean ± SD (Range) | |||
| BSCVA (logMAR) | 1° month | 0.33 ± 0.25 (0.1–1.0) | 0.29 ± 0.21 (0.1–1.0) | 0.68 |
| 3° month | 0.22 ± 0.17 (0–0.6) | 0.21 ± 0.19 (0–0.8) | 0.86 | |
| 6° month | 0.20 ± 0.14 (0–0.4) | 0.19 ± 0.16 (0–0.6) | 0.92 | |
| CCT (µm) | 1° month | 543.1 ± 35.69 (515–657) | 542.95 ± 34.99 (520–680) | 0.99 |
| 3° month | 525.15 ± 20.56 (505–603) | 516.95 ± 25.21 (490–600) | 0.27 | |
| 6° month | 516.6 ± 23.66 (495–603) | 515.05 ± 32.88 (487–611) | 0.87 | |
| ECD (cells/mm2) | 1° month | 1787.6 ± 300.13 (1250–2302) | 1815.9 ± 220.66 (1470–2384) | 0.74 |
| 3° month | 1711.15 ± 282.87 (1200–2200) | 1712.05 ± 197.83 (1300–2200) | 0.99 | |
| 6° month | 1639.4 ± 268.32 (1200–2130) | 1654.9 ± 250.76 (1359–2280) | 0.85 |
| 3D Group | TM Group | |||
|---|---|---|---|---|
| Time | Mean ± SD (Range) | Mean ± SD (Range) | p Value | |
| CD 0–2 mm (GSU) | Baseline | 38.91 ± 9.13 (25.7–55.8) | 39.79 ± 10.64 (29.0–79.5) | 0.78 |
| 1° month | 24.53 ± 6.03 (16.0–39.0) | 23.03 ± 2.40 (19.0–27.0) | 0.40 | |
| 3° month | 22.78 ± 3.81 (16.0–30.0) | 22.38 ± 4.05 (16.0–29.0) | 0.75 | |
| 6° month | 20.98 ± 4.39 (14.0–29.0) | 19.93 ± 3.86 (14.0–27.0) | 0.43 | |
| CD 2–6 mm (GSU) | Baseline | 36.05 ± 77.97 (28.0–65.2) | 37.4 ±6.66 (30.2–62.3) | 0.56 |
| 1° month | 23.1 ± 3.7 (19.0–33.0) | 21.85 ±2.87 (17.0–19.0) | 0.24 | |
| 3° month | 21.88 ± 2.73 (18.0–28.0) | 20.9 ± 2.13 (16.0–25.0) | 0.27 | |
| 6° month | 20.70 ± 2.16 (17.0–25.0) | 19.85 ± 2.06 (16.0–24.0) | 0.22 | |
| CD 6–10 mm (GSU) | Baseline | 35.9 ± 5.11 (29.1–45.0) | 36.8 ± 4.27 (32.1–44.8) | 0.55 |
| 1° month | 25.8 ± 4.94 (18.0–37.0) | 25.4 ± 2.35 (21.0–29.0) | 0.75 | |
| 3° month | 24.55 ± 3.95 (17.0–33.0) | 25.6 ± 2.7 (21.0–31.0) | 0.33 | |
| 6° month | 23.9 ± 3.53 (16.0–30.0) | 25.3 ± 2.68 (21.0–30.0) | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelli, A.; Ferrandina, R.; Favuzza, E.; Cennamo, M.; Mencucci, R. 3D Visualization System in Descemet Membrane Endothelial Keratoplasty (DMEK): A Six-Month Comparison with Conventional Microscope. J. Clin. Med. 2022, 11, 4312. https://doi.org/10.3390/jcm11154312
Morelli A, Ferrandina R, Favuzza E, Cennamo M, Mencucci R. 3D Visualization System in Descemet Membrane Endothelial Keratoplasty (DMEK): A Six-Month Comparison with Conventional Microscope. Journal of Clinical Medicine. 2022; 11(15):4312. https://doi.org/10.3390/jcm11154312
Chicago/Turabian StyleMorelli, Alberto, Rosangela Ferrandina, Eleonora Favuzza, Michela Cennamo, and Rita Mencucci. 2022. "3D Visualization System in Descemet Membrane Endothelial Keratoplasty (DMEK): A Six-Month Comparison with Conventional Microscope" Journal of Clinical Medicine 11, no. 15: 4312. https://doi.org/10.3390/jcm11154312
APA StyleMorelli, A., Ferrandina, R., Favuzza, E., Cennamo, M., & Mencucci, R. (2022). 3D Visualization System in Descemet Membrane Endothelial Keratoplasty (DMEK): A Six-Month Comparison with Conventional Microscope. Journal of Clinical Medicine, 11(15), 4312. https://doi.org/10.3390/jcm11154312






