Pancreas Preservation with a Neutrophil Elastase Inhibitor, Alvelestat, Contributes to Improvement of Porcine Islet Isolation and Transplantation
Abstract
1. Introduction
2. Materials and Methods
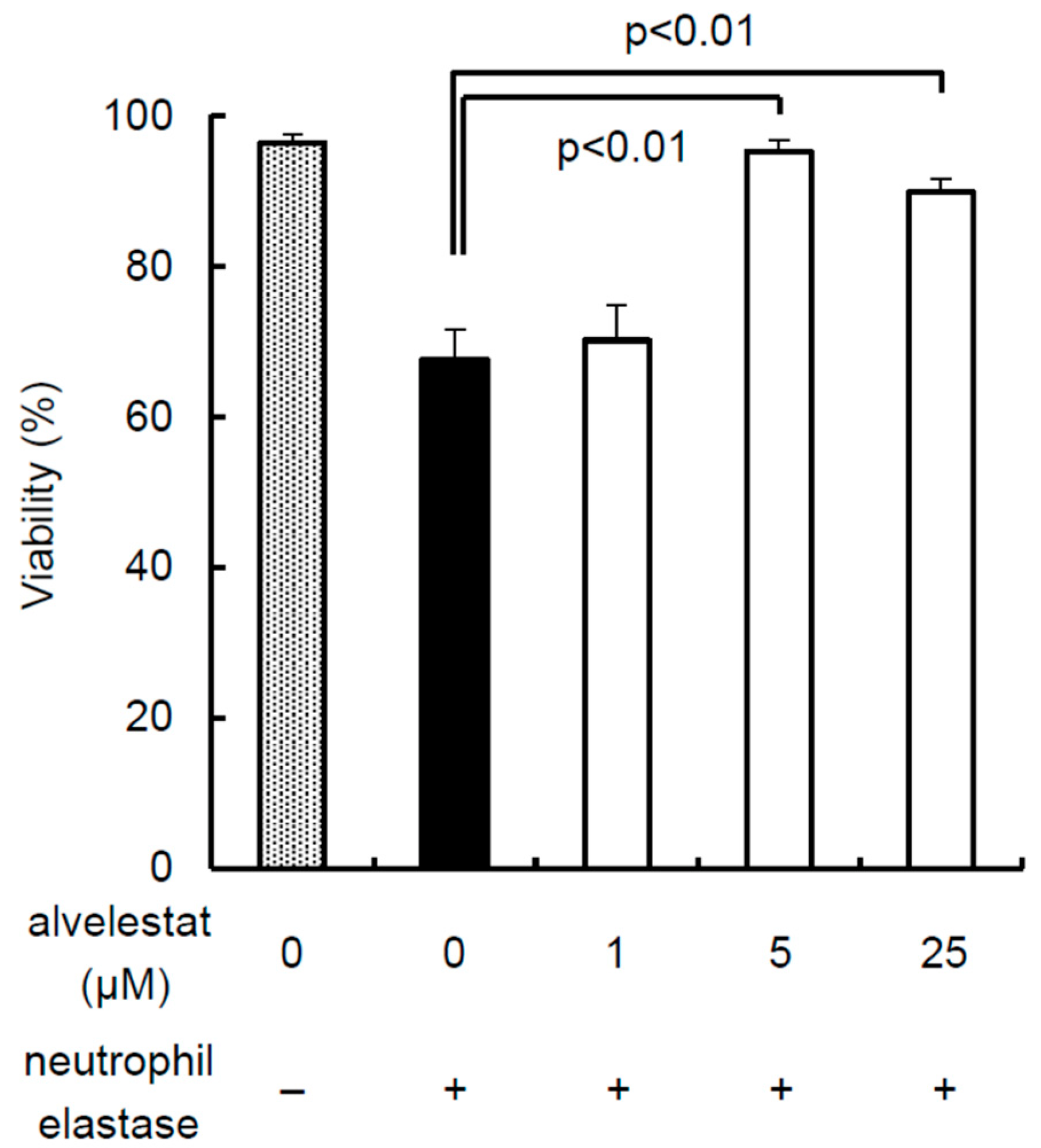
2.1. Inhibitory Effects of Alvelestat against the Cytotoxicity of NE
2.2. Pancreas Preservation Solution
2.3. Procurement, Preservation, and Islet Isolation of Porcine Pancreata
2.4. Assessment of Islet Function
2.5. Measurement of ATP Production
2.6. In Vivo Assessment
2.7. Statistical Analysis
3. Results
3.1. Inhibitory Effects of Alvelestat against the Cytotoxicity of NE
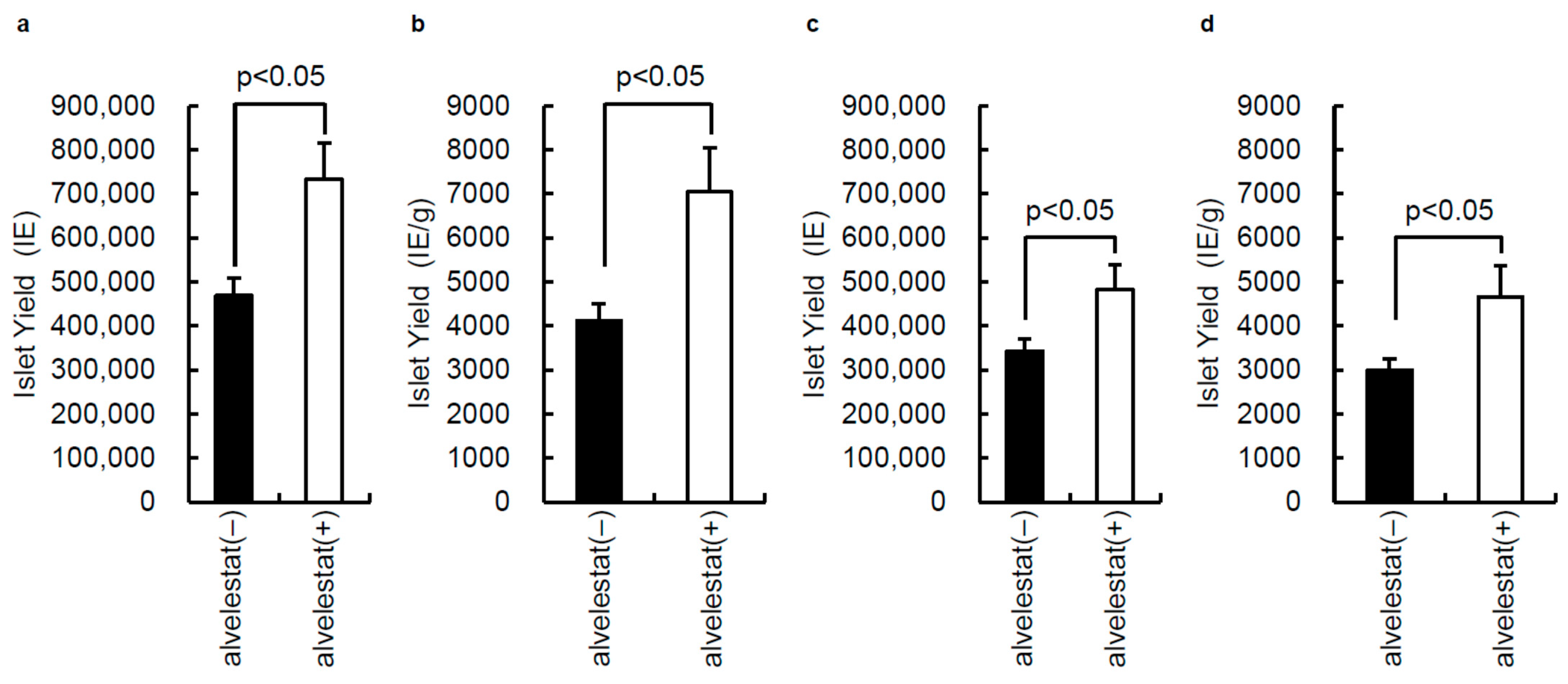
3.2. Effect of Alvelestat on Porcine Islet Isolation
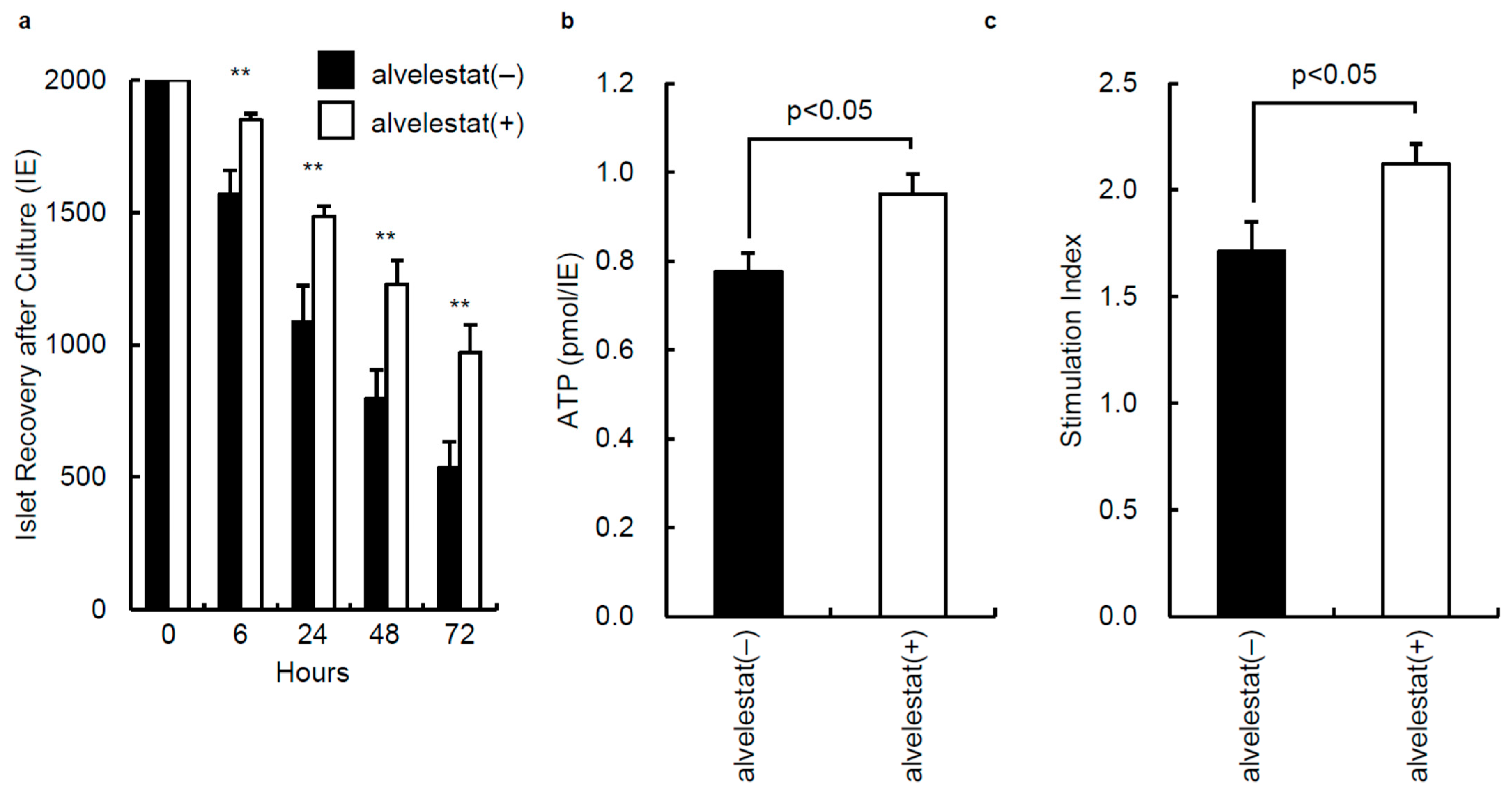
3.3. In Vitro Assessment of Isolated Islets from Porcine Pancreata Preserved with or without Alvelestat
3.4. In Vivo Assessment of Porcine Islets from Alvelestat (+) and Alvelestat (−) Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.; Ricordi, C.; Hering, B.J.; Auchincloss, H.; Lindblad, R.; Robertson, R.P.; Secchi, A.; Brendel, M.D.; Berney, T.; Brennan, D.C.; et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 2006, 355, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Hering, B.J.; Kandaswamy, R.; Harmon, J.V.; Ansite, J.D.; Clemmings, S.M.; Sakai, T.; Paraskevas, S.; Eckman, P.M.; Sageshima, J.; Nakano, M.; et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am. J. Transplant. 2004, 4, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Froud, T.; Ricordi, C.; Baidal, D.A.; Hafiz, M.M.; Ponte, G.; Cure, P.; Pileggi, A.; Poggioli, R.; Ichii, H.; Khan, A.; et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am. J. Transplant. 2005, 5, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Iwanaga, Y.; Okitsu, T.; Nagata, H.; Yonekawa, Y.; Matsumoto, S. Evaluation of islet transplantation from non-heart beating donors. Am. J. Transplant. 2006, 6, 2476–2482. [Google Scholar] [CrossRef]
- Abdelli, S.; Ansite, J.; Roduit, R.; Borsello, T.; Matsumoto, I.; Sawada, T.; Allaman-Pillet, N.; Henry, H.; Beckmann, J.S.; Hering, B.J.; et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004, 53, 2815–2823. [Google Scholar] [CrossRef][Green Version]
- Paraskevas, S.; Maysinger, D.; Wang, R.; Duguid, T.P.; Rosenberg, L. Cell loss in isolated human islets occurs by apoptosis. Pancreas 2000, 20, 270–276. [Google Scholar] [CrossRef]
- Rosenberg, L.; Wang, R.; Paraskevas, S.; Maysinger, D. Structural and functional changes resulting from islet isolation lead to islet cell death. Surgery 1999, 126, 393–398. [Google Scholar] [CrossRef]
- Bottino, R.; Balamurugan, A.N.; Tse, H.; Thirunavukkarasu, C.; Ge, X.; Profozich, J.; Milton, M.; Ziegenfuss, A.; Trucco, M.; Piganelli, J.D. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes 2004, 53, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Morini, S.; Braun, M.; Onori, P.; Cicalese, L.; Elias, G.; Gaudio, E.; Rastellini, C. Morphological changes of isolated rat pancreatic islets: A structural, ultrastructural and morphometric study. J. Anat. 2006, 209, 381–392. [Google Scholar] [CrossRef]
- Machida, T.; Tanemura, M.; Ohmura, Y.; Tanida, T.; Wada, H.; Kobayashi, S.; Marubashi, S.; Eguchi, H.; Ito, T.; Nagano, H.; et al. Significant improvement in islet yield and survival with modified ET-Kyoto solution: ET-Kyoto/Neutrophil elastase inhibitor. Cell Transplant. 2013, 22, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Shinichiro, O.; Kuroki, T.; Adachi, T.; Kosaka, T.; Okamoto, T.; Tanaka, T.; Tajima, Y.; Kanematsu, T.; Eguchi, S. The effect of selective neutrophil elastase inhibitor on pancreatic islet yields and functions in rat with hypercytokinemia. Ann. Transplant. 2011, 16, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.C.; Shapiro, L.; Bowers, O.J.; Dinarello, C.A. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 12153–12158. [Google Scholar] [CrossRef]
- Lewis, E.C.; Mizrahi, M.; Toledano, M.; Defelice, N.; Wright, J.L.; Churg, A.; Shapiro, L.; Dinarello, C.A. alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 16236–16241. [Google Scholar] [CrossRef]
- Kono, T.; Okada, S.; Saito, M. Neutrophil elastase inhibitor, sivelestat sodium hydrate prevents ischemia-reperfusion injury in the rat bladder. Mol. Cell Biochem. 2008, 311, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Iwasaki, Y.; Okada, T.; Sawada, T.; Kubota, K. Protective effect of Sivelestat in a porcine hepatectomy model prepared using an intermittent Pringle method. Eur. J. Pharmacol. 2008, 587, 248–252. [Google Scholar] [CrossRef]
- Uchida, Y.; Freitas, M.C.; Zhao, D.; Busuttil, R.W.; Kupiec-Weglinski, J.W. The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury. Transplantation 2010, 89, 1050–1056. [Google Scholar] [CrossRef]
- Kawabata, K.; Suzuki, M.; Sugitani, M.; Imaki, K.; Toda, M.; Miyamoto, T. ONO-5046, a novel inhibitor of human neutrophil elastase. Biochem. Biophys. Res. Commun. 1991, 177, 814–820. [Google Scholar] [CrossRef]
- Uchida, Y.; Freitas, M.C.; Zhao, D.; Busuttil, R.W.; Kupiec-Weglinski, J.W. The inhibition of neutrophil elastase ameliorates mouse liver damage due to ischemia and reperfusion. Liver Transpl. 2009, 15, 939–947. [Google Scholar] [CrossRef]
- Dokai, M.; Madoiwa, S.; Yasumoto, A.; Kashiwakura, Y.; Ishiwata, A.; Sakata, A.; Makino, N.; Ohmori, T.; Mimuro, J.; Sakata, Y. Local regulation of neutrophil elastase activity by endogenous α1-antitrypsin in lipopolysaccharide-primed hematological cells. Thromb. Res. 2011, 128, 283–292. [Google Scholar] [CrossRef]
- Salahuddin, P. Genetic variants of alpha1-antitrypsin. Curr. Protein Pept. Sci. 2010, 11, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Stoller, J.K.; Aboussouan, L.S. A review of α1-antitrypsin deficiency. Am. J. Respir. Crit. Care Med. 2012, 185, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Viglio, S.; Iadarola, P.; D’Amato, M.; Stolk, J. Methods of Purification and Application Procedures of Alpha1 Antitrypsin: A Long-Lasting History. Molecules 2020, 25, 4014. [Google Scholar] [CrossRef] [PubMed]
- Ochayon, D.E.; Mizrahi, M.; Shahaf, G.; Baranovski, B.M.; Lewis, E.C. Human α1-Antitrypsin Binds to Heat-Shock Protein gp96 and Protects from Endogenous gp96-Mediated Injury In vivo. Front. Immunol. 2013, 4, 320. [Google Scholar] [CrossRef]
- Stevens, T.; Ekholm, K.; Gränse, M.; Lindahl, M.; Kozma, V.; Jungar, C.; Ottosson, T.; Falk-Håkansson, H.; Churg, A.; Wright, J.L.; et al. AZD9668: Pharmacological characterization of a novel oral inhibitor of neutrophil elastase. J. Pharmacol. Exp. Ther. 2011, 339, 313–320. [Google Scholar] [CrossRef]
- Delbosc, S.; Rouer, M.; Alsac, J.M.; Louedec, L.; Philippe, M.; Meilhac, O.; Whatling, C.; Michel, J.B. Elastase inhibitor AZD9668 treatment prevented progression of experimental abdominal aortic aneurysms. J. Vasc. Surg. 2016, 63, 486–492.e1. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Tan, H.; Hu, Y.; Zhang, L.; Liu, S.; Dai, M.; Li, Y.; Li, Q.; Mao, Z.; et al. Neutrophil extracellular traps contribute to the pathogenesis of acid-aspiration-induced ALI/ARDS. Oncotarget 2018, 9, 1772–1784. [Google Scholar] [CrossRef]
- Elborn, J.S.; Perrett, J.; Forsman-Semb, K.; Marks-Konczalik, J.; Gunawardena, K.; Entwistle, N. Efficacy, safety and effect on biomarkers of AZD9668 in cystic fibrosis. Eur. Respir. J. 2012, 40, 969–976. [Google Scholar] [CrossRef]
- Stockley, R.; De Soyza, A.; Gunawardena, K.; Perrett, J.; Forsman-Semb, K.; Entwistle, N.; Snell, N. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respir. Med. 2013, 107, 524–533. [Google Scholar] [CrossRef]
- Ricordi, C.; Gray, D.W.; Hering, B.J.; Kaufman, D.B.; Warnock, G.L.; Kneteman, N.M.; Lake, S.P.; London, N.J.; Socci, C.; Alejandro, R. Islet isolation assessment in man and large animals. Acta Diabetol. Lat. 1990, 27, 185–195. [Google Scholar] [CrossRef]
- Bank, H.L. Rapid assessment of islet viability with acridine orange and propidium iodide. In Vitro Cell Dev. Biol. 1988, 24, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Faust, A.; Rothe, H.; Schade, U.; Lampeter, E.; Kolb, H. Primary nonfunction of islet grafts in autoimmune diabetic nonobese diabetic mice is prevented by treatment with interleukin-4 and interleukin-10. Transplantation 1996, 62, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y.; Saitoh, I.; Watanabe, M. Novel cell-permeable p38-MAPK inhibitor efficiently prevents porcine islet apoptosis and improves islet graft function. Am. J. Transplant. 2020, 20, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Ueda, M.; Hayashi, S.; Kobayashi, N.; Okitsu, T.; Iwanaga, Y.; Nagata, H.; Nakai, Y.; Matsumoto, S. Ductal injection of preservation solution increases islet yields in islet isolation and improves islet graft function. Cell Transplant. 2008, 17, 69–81. [Google Scholar] [CrossRef]
- Kuwae, K.; Miyagi-Shiohira, C.; Hamada, E.; Tamaki, Y.; Nishime, K.; Sakai, M.; Yonaha, T.; Makishi, E.; Saitoh, I.; Watanabe, M.; et al. Excellent Islet Yields after 18-h Porcine Pancreas Preservation by Ductal Injection, Pancreas Preservation with MK Solution, Bottle Purification, and Islet Purification Using Iodixanol with UW Solution and Iodixanol with MK Solution. J. Clin. Med. 2019, 8, 1561. [Google Scholar] [CrossRef]
- Ricordi, C.; Lacy, P.E.; Finke, E.H.; Olack, B.J.; Scharp, D.W. Automated method for isolation of human pancreatic islets. Diabetes 1988, 37, 413–420. [Google Scholar] [CrossRef]
- Hering, B.J.; Clarke, W.R.; Bridges, N.D.; Eggerman, T.L.; Alejandro, R.; Bellin, M.D.; Chaloner, K.; Czarniecki, C.W.; Goldstein, J.S.; Hunsicker, L.G.; et al. Clinical Islet Transplantation Consortium. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care 2016, 39, 1230–1240. [Google Scholar] [CrossRef]
- Foster, E.D.; Bridges, N.D.; Feurer, I.D.; Eggerman, T.L.; Hunsicker, L.G.; Alejandro, R.; Clinical Islet Transplantation Consortium. Improved Health-Related Quality of Life in a Phase 3 Islet Transplantation Trial in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care 2018, 41, 1001–1008. [Google Scholar] [CrossRef]
- Noguchi, H.; Ueda, M.; Nakai, Y.; Iwanaga, Y.; Okitsu, T.; Nagata, H.; Yonekawa, Y.; Kobayashi, N.; Nakamura, T.; Wada, H.; et al. Modified two-layer preservation method (M-Kyoto/PFC) improves islet yields in islet isolation. Am. J. Transplant. 2006, 6, 496–504. [Google Scholar] [CrossRef]
- Noguchi, H. Pancreatic Islet Purification from Large Mammals and Humans Using a COBE 2991 Cell Processor versus Large Plastic Bottles. J. Clin. Med. 2020, 10, 10. [Google Scholar] [CrossRef]
- Nishime, K.; Miyagi-Shiohira, C.; Kuwae, K.; Tamaki, Y.; Yonaha, T.; Sakai-Yonaha, M.; Saitoh, I.; Watanabe, M.; Noguchi, H. Preservation of pancreas in the University of Wisconsin solution supplemented with AP39 reduces reactive oxygen species production and improves islet graft function. Am. J. Transplant. 2021, 21, 2698–2708. [Google Scholar] [CrossRef] [PubMed]
- Canelo, R.; Hakim, N.S.; Ringe, B. Experience with hystidine tryptophan ketoglutarate versus University Wisconsin preservation solutions in transplantation. Int. Surg. 2003, 88, 145–151. [Google Scholar] [PubMed]
- Brandhorst, H.; Theisinger, B.; Guenther, B.; Johnson, P.R.; Brandhorst, D. Pancreatic L-Glutamine Administration Protects Pig Islets from Cold Ischemic Injury and Increases Resistance Toward Inflammatory Mediators. Cell Transplant. 2016, 25, 531–538. [Google Scholar] [CrossRef]
- Ito, T.; Omori, K.; Rawson, J.; Todorov, I.; Asari, S.; Kuroda, A.; Shintaku, J.; Itakura, S.; Ferreri, K.; Kandeel, F.; et al. Improvement of canine islet yield by donor pancreas infusion with a p38MAPK inhibitor. Transplantation 2008, 86, 321–329. [Google Scholar] [CrossRef]
- Noguchi, H. Regulation of c-Jun NH(2)-Terminal Kinase for Islet Transplantation. J. Clin. Med. 2019, 8, 1763. [Google Scholar] [CrossRef]
- Land, W.G. The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation 2005, 79, 505–514. [Google Scholar] [CrossRef]
- Pratschke, J.; Wilhelm, M.J.; Kusaka, M.; Basker, M.; Cooper, D.K.; Hancock, W.W.; Tilney, N.L. Brain death and its influence on donor organ quality and outcome after transplantation. Transplantation 1999, 67, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Lakey, J.R.; Rajotte, R.V.; Warnock, G.L.; Kneteman, N.M. Human pancreas preservation prior to islet isolation. Cold ischemic tolerance. Transplantation 1995, 59, 689–694. [Google Scholar] [CrossRef]
- Ricordi, C. Quantitative and qualitative standards for islet isolation assessment in humans and large mammals. Pancreas 1991, 6, 242–244. [Google Scholar] [CrossRef]
- Barshes, N.R.; Wyllie, S.; Goss, J.A. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: Implications for intrahepatic grafts. J. Leukoc. Biol. 2005, 77, 587–597. [Google Scholar] [CrossRef]
- Hamada, E.; Ebi, N.; Miyagi-Shiohira, C.; Tamaki, Y.; Nakashima, Y.; Kobayashi, N.; Saitoh, I.; Watanabe, M.; Kinjo, T.; Noguchi, H. Comparison Between Modified Extracellular-Type Trehalose-Containing Kyoto Solution and University of Wisconsin Solution in 18-Hour Pancreas Preservation for Islet Transplantation. Pancreas 2018, 47, e46–e47. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Ueda, M.; Hayashi, S.; Kobayashi, N.; Nagata, H.; Iwanaga, Y.; Okitsu, T.; Matsumoto, S. Comparison of M-Kyoto solution and histidine-tryptophan-ketoglutarate solution with a trypsin inhibitor for pancreas preservation in islet transplantation. Transplantation 2007, 84, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H. Clinical Islet Transplantation Covered by Health Insurance in Japan. J. Clin. Med. 2022, 11, 3977. [Google Scholar] [CrossRef]
- Alvelestat (AZD9668). Available online: https://www.selleck.co.jp/products/avelestat-azd9668.html (accessed on 13 July 2022).
- Henriksen, P.A. The potential of neutrophil elastase inhibitors as anti-inflammatory therapies. Curr. Opin. Hematol. 2014, 21, 23–28. [Google Scholar] [CrossRef]
- Alvelestat (MPH966) for the Treatment of ALpha-1 ANTitrypsin Deficiency (ATALANTa). Available online: https://clinicaltrials.gov/ct2/show/NCT03679598 (accessed on 13 July 2022).
| Characteristic | Alvelestat (−) (n = 10) | Alvelestat (+) (n = 10) |
|---|---|---|
| Pancreas size (g) | 120.1 ± 8.9 | 107.9 ± 5.6 |
| Operation time (min) | 4.9 ± 0.4 | 5.2 ± 0.2 |
| Warm ischemic time (min) | 27.1 ± 1.2 | 27.2 ± 0.8 |
| Cold ischemic time (min) | 1116.3 ± 9.4 | 1087.0 ± 12.7 |
| Phase I period (min) | 11.1 ± 0.5 | 11.6 ± 0.5 |
| Phase II period (min) | 38.9 ± 0.8 | 40.5 ± 0.7 |
| Embedded islets (%) | 12.0 ± 1.9 | 12.0 ± 1.3 |
| Viability (%) | 96.7 ± 0.5 | 96.5 ± 0.6 |
| Purity (%) | 64.2 ± 2.4 | 59.8 ± 3.7 |
| Post-purification recovery (%) 1 | 76.3 ± 3.2 | 67.4 ± 3.1 |
| Score | 9.5 ± 0.2 | 9.7 ± 0.1 |
| Alvelestat (−) | Alvelestat (+) | |
|---|---|---|
| No. of Transplanted Mice | 20 | 20 |
| No. of Cured Mice | 1 | 11 |
| % | 5 | 55 |
| p-Value vs. alvelestat (−) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otsuka, R.; Miyagi-Shiohira, C.; Kuwae, K.; Nishime, K.; Tamaki, Y.; Yonaha, T.; Sakai-Yonaha, M.; Yamasaki, I.; Shinzato, M.; Saitoh, I.; et al. Pancreas Preservation with a Neutrophil Elastase Inhibitor, Alvelestat, Contributes to Improvement of Porcine Islet Isolation and Transplantation. J. Clin. Med. 2022, 11, 4290. https://doi.org/10.3390/jcm11154290
Otsuka R, Miyagi-Shiohira C, Kuwae K, Nishime K, Tamaki Y, Yonaha T, Sakai-Yonaha M, Yamasaki I, Shinzato M, Saitoh I, et al. Pancreas Preservation with a Neutrophil Elastase Inhibitor, Alvelestat, Contributes to Improvement of Porcine Islet Isolation and Transplantation. Journal of Clinical Medicine. 2022; 11(15):4290. https://doi.org/10.3390/jcm11154290
Chicago/Turabian StyleOtsuka, Ryusei, Chika Miyagi-Shiohira, Kazuho Kuwae, Kai Nishime, Yoshihito Tamaki, Tasuku Yonaha, Mayuko Sakai-Yonaha, Ikuo Yamasaki, Misaki Shinzato, Issei Saitoh, and et al. 2022. "Pancreas Preservation with a Neutrophil Elastase Inhibitor, Alvelestat, Contributes to Improvement of Porcine Islet Isolation and Transplantation" Journal of Clinical Medicine 11, no. 15: 4290. https://doi.org/10.3390/jcm11154290
APA StyleOtsuka, R., Miyagi-Shiohira, C., Kuwae, K., Nishime, K., Tamaki, Y., Yonaha, T., Sakai-Yonaha, M., Yamasaki, I., Shinzato, M., Saitoh, I., Watanabe, M., & Noguchi, H. (2022). Pancreas Preservation with a Neutrophil Elastase Inhibitor, Alvelestat, Contributes to Improvement of Porcine Islet Isolation and Transplantation. Journal of Clinical Medicine, 11(15), 4290. https://doi.org/10.3390/jcm11154290





