Signs and Symptoms of Temporomandibular Dysfunction and Radiographic Condylar Morphology in Patients with Idiopathic Condylar Resorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Signs and Symptoms
2.3. CBCT Images
2.4. Image Measurements
2.5. Statistical Analysis
3. Results
3.1. Bilateral ICR Group
3.2. Unilateral ICR Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gunson, M.J.; Arnett, G.W.; Milam, S.B. Pathophysiology and pharmacologic control of osseous mandibular condylar resorption. J. Oral Maxillofac. Surg. 2012, 70, 1918–1934. [Google Scholar] [CrossRef]
- Gunson, M.J.; Arnett, G.W.; Formby, B.; Falzone, C.; Mathur, R.; Alexander, C. Oral contraceptive pill use and abnormal menstrual cycles in women with severe condylar resorption: A case for low serum 17beta-estradiol as a major factor in progressive condylar resorption. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 772–779. [Google Scholar] [CrossRef]
- Wolford, L.M. Idiopathic condylar resorption of the temporomandibular joint in teenage girls (cheerleaders syndrome). Proc. Bayl. Univ. Med. Cent. 2001, 14, 246–252. [Google Scholar] [CrossRef]
- Posnick, J.C.; Fantuzzo, J.J. Idiopathic condylar resorption: Current clinical perspectives. J. Oral Maxillofac. Surg. 2007, 65, 1617–1623. [Google Scholar] [CrossRef]
- Arnett, G.W.; Milam, S.B.; Gottesman, L. Progressive mandibular retrusion—Idiopathic condylar resorption. Part II. Am. J. Orthod. Dentofac. Orthop. 1996, 110, 117–127. [Google Scholar] [CrossRef]
- Wolford, L.M.; Cardenas, L. Idiopathic condylar resorption. Diagnosis, treatment protocol, and outcomes. Am. J. Orthod. Dentofac. Orthop. 1999, 116, 667–677. [Google Scholar] [CrossRef]
- Arnett, G.W.; Milam, S.B.; Gottesman, L. Progressive mandibular retrusion—Idiopathic condylar resorption. Part I. Am. J. Orthod. Dentofac. Orthop. 1996, 110, 8–15. [Google Scholar] [CrossRef]
- Mehra, P.; Nadershah, M.; Chigurupati, R. Is alloplastic temporomandibular joint reconstruction a viable option in the surgical management of adult patients with idiopathic condylar resorption? J. Oral Maxillofac. Surg. 2016, 74, 2044–2054. [Google Scholar] [CrossRef]
- Sinha, V.P.; Pradhan, H.; Gupta, H.; Mohammad, S.; Singh, R.K.; Mehrotra, D.; Pant, M.C.; Pradhan, R. Efficacy of plain radiographs, CT scan, MRI and ultra sonography in temporomandibular joint disorders. Natl. J. Maxillofac. Surg. 2012, 3, 2–9. [Google Scholar] [CrossRef]
- Young, A. Idiopathic condylar resorption. The current understanding in diagnosis and treatment. J. Indian Prosthodont. Soc. 2017, 17, 128–135. [Google Scholar] [CrossRef]
- Ahmad, M.; Hollender, L.; Anderson, Q.; Kartha, K.; Ohrbach, R.; Truelove, E.L.; John, M.T.; Schiffman, E.L. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): Development of image analysis criteria and examiner reliability for image analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, 844–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, T.; Schreurs, R.; van Loon, B.; de Koning, M.; Bergé, S.; Hoppenreijs, T.; Maal, T. 3D analysis of condylar remodeling and skeletal relapse following bilateral sagittal split advancement osteotomies. J. Cranio-Maxillofac. Surg. 2015, 43, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Peck, C.C.; Goulet, J.P.; Lobbezoo, F.; Schiffman, E.L.; Alstergren, P.; Anderson, G.C.; Leeuw, R.; Jensen, R.; Michelotti, A.; Ohrbach, R.; et al. Expanding the taxonomy of the diagnostic criteria for temporomandibular disorders. J. Oral Rehabil. 2014, 41, 2–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6. [Google Scholar] [CrossRef] [PubMed]
- Soon-Jung, H.; Piet, E.H.; Burkhardt, S.; Hermann, F.S. Non-surgical risk factors for condylar resorption after orthognathic surgery. J. Cranio-Maxillofac. Surg. 2004, 32, 103–111. [Google Scholar]
- Silva, R.J.; Valadares Souza, C.V.; Souza, G.A.; Ambrosano, G.M.B.; Freitas, D.Q.; Sant’Ana, E.; Oliveira Santos, C. Changes in condylar volume and joint spaces after orthognathic surgery. Int. J. Oral Maxillofac. Surg. 2018, 47, 511–517. [Google Scholar] [CrossRef]
- Kristensen, K.D.; Schmidt, B.; Stoustrup, P.; Pedersen, T.K. Idiopathic condylar resorptions: 3-dimensional condylar bony deformation, signs and symptoms. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 214–223. [Google Scholar] [CrossRef]
- Kerstens, H.C.; Tuinzing, D.B.; Golding, R.P.; van der Kwast, W.A. Condylar atrophy and osteoarthrosis after bimaxillary surgery. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 274–280. [Google Scholar] [CrossRef]
- Hoppenreijs, T.J.; Freihofer, H.P.; Stoelinga, P.J.; Tuinzing, D.B.; van’t Hof, M.A. Condylar remodelling and resorption after Le Fort I and bimaxillary osteotomies in patients with anterior open bite. A clinical and radiological study. Int. J. Oral Maxillofac. Surg. 1998, 27, 81–91. [Google Scholar] [CrossRef]
- Handelman, C.S.; Greene, C.S. Progressive/idiopathic condylar resorption: An orthodontic perspective. Semin. Orthod. 2013, 19, 55–70. [Google Scholar] [CrossRef]
- Hajati, A.K.; Nasstrom, K.; Alstergren, P.; Bratt, J.; Kopp, S. Temporomandibular joint bone tissue resorption in patients with early rheumatoid arthritis can be predicted by joint crepitus and plasma glutamate level. Mediat. Inflamm. 2010, 2010, 627803. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lin, H.; Lin, Q.; Lu, L.; Li, M.; Li, Q.; Xue, J.; Xu, Y. Morphologic changes in idiopathic condylar resorption with different degrees of bone loss. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Kajii, T.S.; Fujita, T.; Sakaguchi, Y.; Shimada, K. Osseous changes of the mandibular condyle affect backward-rotation of the mandibular ramus in Angle Class II orthodontic patients with idiopathic condylar resorption of the temporomandibular joint. Craniomandibular 2019, 37, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Yang, Y.M.; Kim, Y.I.; Cho, B.H.; Jung, Y.H.; Hwang, D.S. Effect of bimaxillary surgery on adaptive condylar head remodeling: Metric analysis and image interpretation using cone-beam computed tomography volume superimposition. J. Oral Maxillofac. Surg. 2012, 70, 1951–1959. [Google Scholar] [CrossRef]
- Moore, K.E.; Gooris, P.J.; Stoelinga, P.J. The contributing role of condylar resorption to skeletal relapse following mandibular advancement surgery: Report of five cases. J. Oral Maxillofac. Surg. 1991, 49, 448–460. [Google Scholar] [CrossRef]
- Hwang, S.J.; Haers, P.E.; Sailer, H.F. The role of a posteriorly inclined condylar neck in condylar resorption after orthognathic surgery. J. Cranio-Maxillofac. Surg. 2000, 28, 85–90. [Google Scholar] [CrossRef]

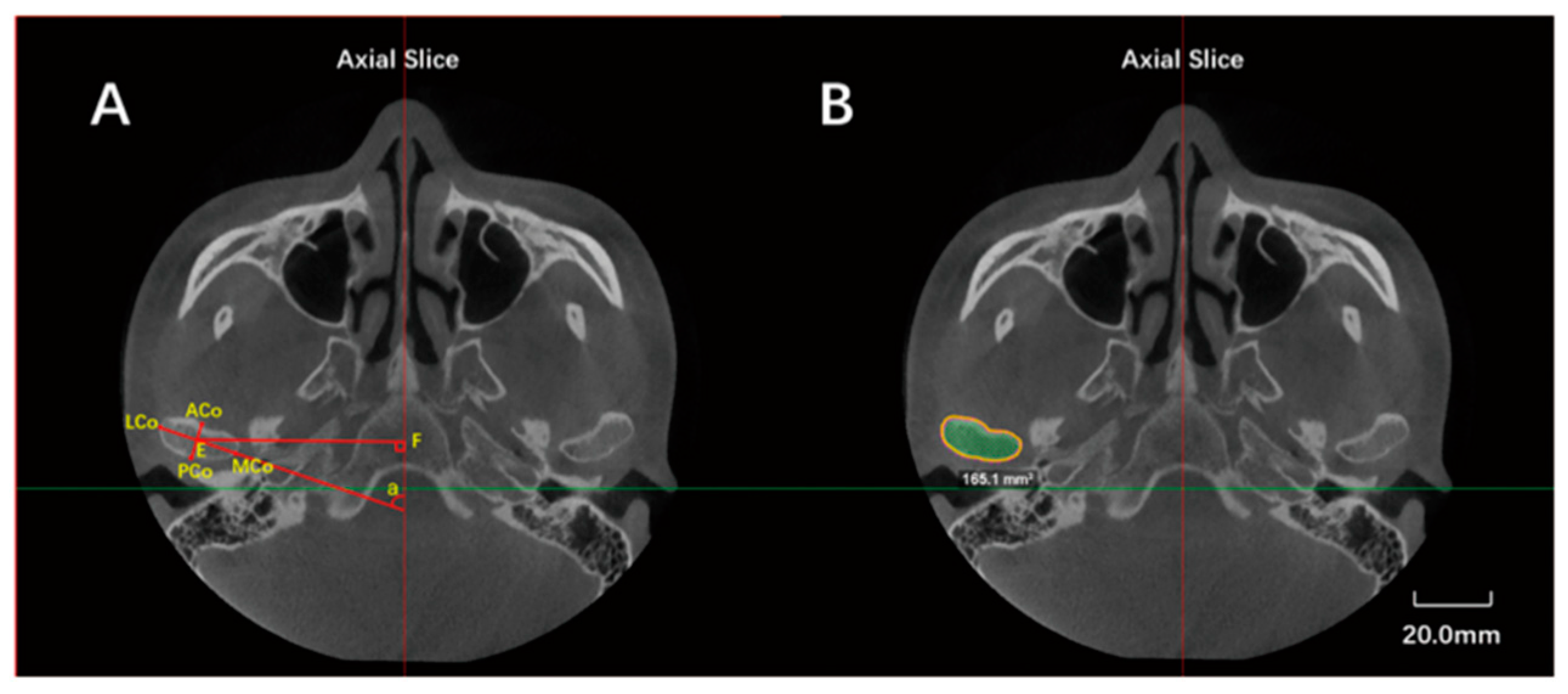

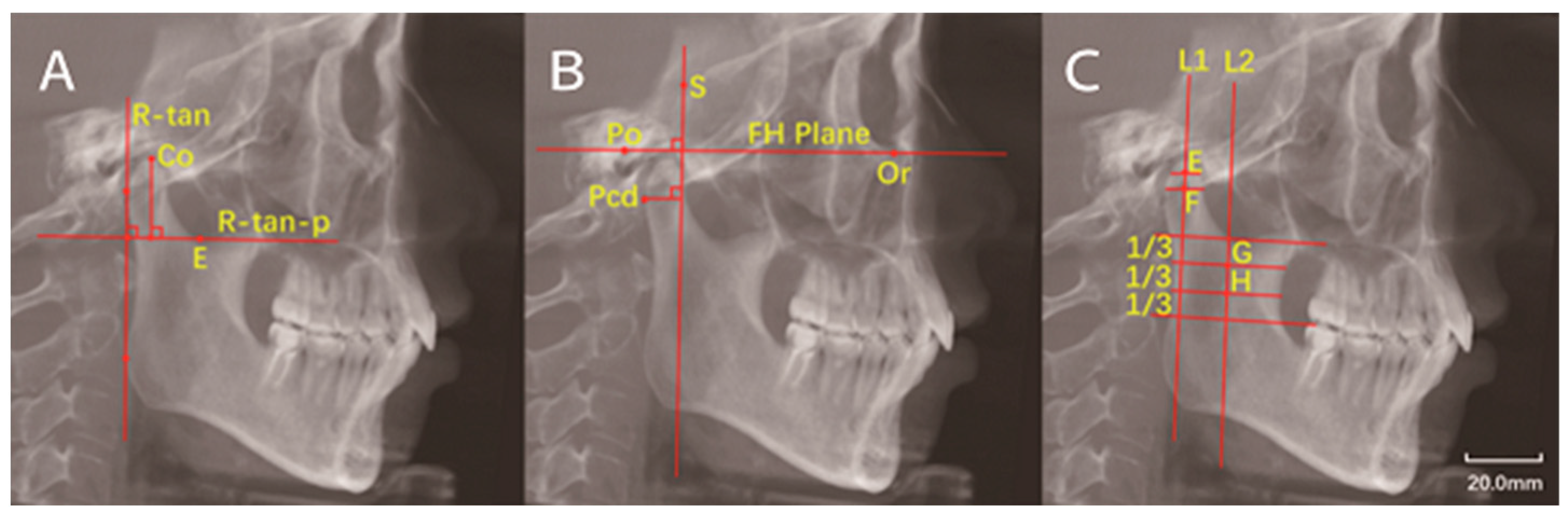
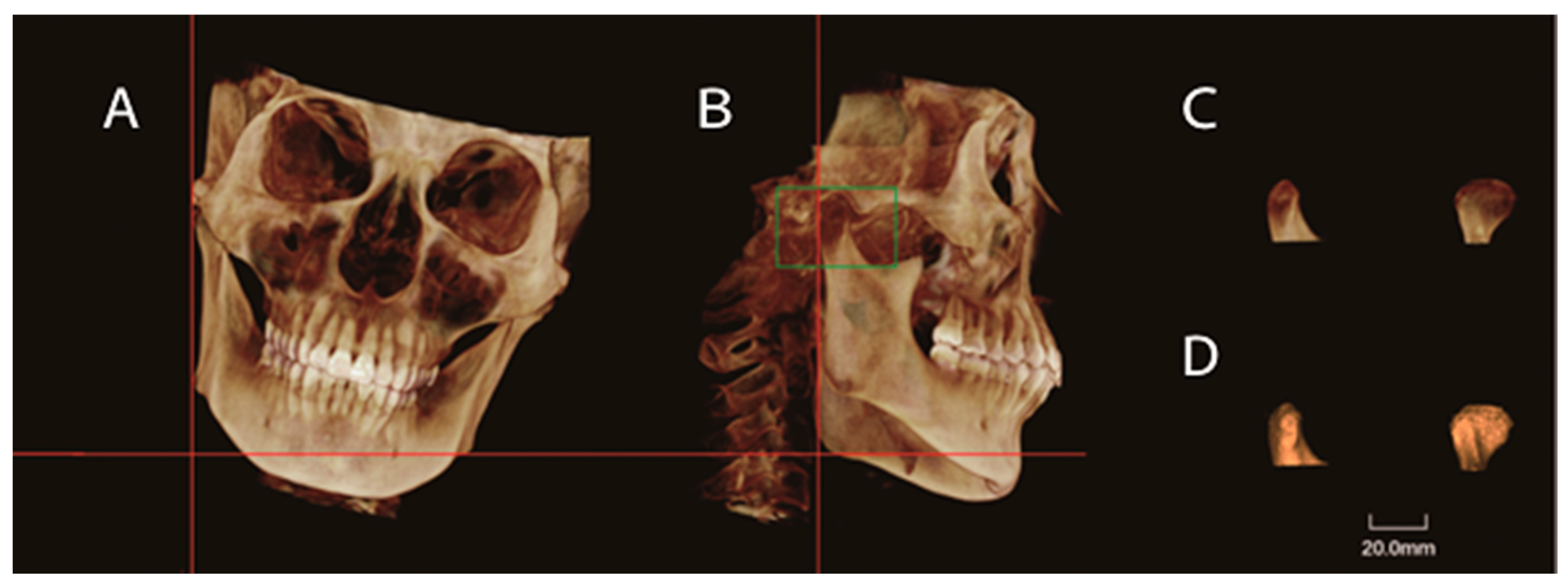
| Landmarks, Constructed Lines and Measurement Items | Abbreviation | Definition |
|---|---|---|
| Lateral condylar point | LCo | Most lateral point of the condyle |
| Medial condylar point | MCo | Most medial point of the condyle |
| Anterior condylar point | ACo | Most anterior point of the condyle |
| Superior condylar point | Co | Most superior point of the condyle |
| Posterior condylar point | PCo | Most posterior point of the condyle on axial plane |
| Posterior condylar border point | Pcd | Most posterior point of the condyle on sagittal plane |
| Sella | S | The center of sella on the median sagittal plane of skull |
| Porion | Po | The uppermost point of external auditory canal |
| Orbitale | Or | The lowest point of orbital margin |
| Ramus tangent line | R-tan | Tangent to the posterior border of the ramus |
| Ramus tangent line perpendicular | R-tan-p | Line perpendicular to R-tan and tanging the deepest point of the sigmoid notch |
| Frankfort horizontal plane | FH plane | Line from Po to Or |
| Anteroposterior diameter | ACo-PCo | Distance from ACo to PCo |
| Transverse diameter | LCo-MCo | Distance from LCO to MCo |
| Condylar height | - | Vertical distance from Co to R-tan-p |
| Maximal sectional area | Smax | Maximal sectional area of the condyle |
| Axial angle | - | Angle between LCo-MCo and midsagittal line |
| - | FH-p(S) | Line perpendicular to FH plane and through the sellar point |
| - | Pcd-FH-p(S) | Vertical distance from Pcd to FH-p(S) |
| Joints | Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| ICR Group | Bilateral ICR Group | Unilateral ICR Group | p | ICR Group | Bilateral ICR Group | Unilateral ICR Group | p | |
| Clicking | 76/120 (63.3%) | 57/82 (69.5%) | 19/38 (50.0%) | 0.039 * | 46/60 (76.7%) | 32/41 (78.0%) | 14/19 (73.7%) | 0.965 |
| TMJ pain | 35/120 (29.2%) | 25/82 (30.5%) | 10/38 (26.3%) | 0.640 | 22/60 (36.7%) | 15/41 (36.6%) | 7/19 (36.8%) | 0.985 |
| Joint locking | 22/120 (18.3%) | 13/82 (15.9%) | 9/38 (23.7%) | 0.302 | 14/60 (23.3%) | 8/41 (19.5%) | 6/19 (31.6%) | 0.484 |
| Crepitation | 16/120 (13.3%) | 13/82 (15.9%) | 3/38 (7.9%) | 0.233 | 10/60 (16.7%) | 8/41 (19.5%) | 2/19 (10.5%) | 0.620 |
| Limited mouth-opening | - | - | 7/60 (11.7%) | 5/41 (12.2%) | 2/19 (10.5%) | 1.000 | ||
| Symptoms | - | - | 49/60 (81.7%) | 34/41 (82.9%) | 15/19 (78.9%) | 0.990 | ||
| Joints | Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| ICR Group | Bilateral ICR Group | Unilateral ICR Group | Sig | ICR Group | Bilateral ICR Group | Unilateral ICR Group | Sig | |
| Opening–closing deviation | - | - | - | 35/60 (58.3%) | 20/41 (48.8%) | 15/19 (78.9%) | 0.027 * | |
| Crepitation | 51/120 (42.5%) | 38/82 (46.3%) | 13/38 (34.2%) | 0.211 | 32/60 (53.3%) | 23/41 (56.1%) | 9/19 (47.4%) | 0.528 |
| Clicking | 13/120 (10.8%) | 11/82 (13.4%) | 2/38 (5.3%) | 0.307 | 9/60 (15.0%) | 7/41 (17.1%) | 2/19 (10.5%) | 0.786 |
| Maximum opening limitation | - | - | - | 3/60 (5.0%) | 2/41 (4.9%) | 1/19 (5.3%) | 1.000 | |
| TMJ and muscle tenderness | 1/120 (0.8%) | 0 (0.0%) | 1/38 (2.6%) | 0.317 | 1/60 (1.7%) | 0 (0.0%) | 1/19 (5.3%) | 0.317 |
| Signs | - | - | - | 47/60 (78.3%) | 30/41 (73.2%) | 17/19 (89.5%) | 0.276 | |
| Control Group (n = 41) | Bilateral ICR Group (n = 41) | Resorption Side in Unilateral ICR Group (n = 19) | Healthy Side in Unilateral ICR Group (n = 19) | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Anteroposterior diameter (mm) | 8.07 ± 0.87 | 6.43 ± 1.11 *** | 6.27 ± 1.06 *** | 7.51 ± 1.13 * |
| Transverse diameter (mm) | 18.70 ± 1.97 | 15.44 ± 2.99 *** | 14.81 ± 1.84 *** | 16.93 ± 1.90 ** |
| Condylar height (mm) | 21.22 ± 3.19 | 15.89 ± 2.86 *** | 15.34 ± 1.39 *** | 21.06 ± 3.47 |
| Smax (mm2) | 127.67 ± 18.27 | 88.72 ± 19.03 *** | 83.07 ± 13.66 *** | 107.77 ± 21.19 *** |
| Condylar volume (mm3) | 1626.74 ± 406.71 | 977.49 ± 302.50 *** | 893.74 ± 209.87 *** | 1442.79 ± 459.13 |
| Axial angle (°) | 71.85 ± 6.18 | 54.60 ± 10.53 *** | 55.58 ± 5.33 *** | 68.97 ± 6.83 |
| Condylar neck angle (°) | 0.99 ± 7.38 | 20.18 ± 7.13 *** | 18.34 ± 8.53 *** | 4.25 ± 7.29 |
| Axial distance (mm) | 51.30 ± 2.40 | 51.30 ± 2.40 | 52.51 ± 1.94 | 53.71 ± 2.19 |
| Pcd-FH-p(S) (mm) | 17.11 ± 2.41 | 13.87 ± 3.14 *** | 12.67 ± 2.54 *** | 15.84 ± 3.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Wang, S.; Wu, M.; Chen, X.; He, F. Signs and Symptoms of Temporomandibular Dysfunction and Radiographic Condylar Morphology in Patients with Idiopathic Condylar Resorption. J. Clin. Med. 2022, 11, 4289. https://doi.org/10.3390/jcm11154289
Yu Y, Wang S, Wu M, Chen X, He F. Signs and Symptoms of Temporomandibular Dysfunction and Radiographic Condylar Morphology in Patients with Idiopathic Condylar Resorption. Journal of Clinical Medicine. 2022; 11(15):4289. https://doi.org/10.3390/jcm11154289
Chicago/Turabian StyleYu, Yanfang, Sijie Wang, Mengjie Wu, Xiaoyan Chen, and Fuming He. 2022. "Signs and Symptoms of Temporomandibular Dysfunction and Radiographic Condylar Morphology in Patients with Idiopathic Condylar Resorption" Journal of Clinical Medicine 11, no. 15: 4289. https://doi.org/10.3390/jcm11154289
APA StyleYu, Y., Wang, S., Wu, M., Chen, X., & He, F. (2022). Signs and Symptoms of Temporomandibular Dysfunction and Radiographic Condylar Morphology in Patients with Idiopathic Condylar Resorption. Journal of Clinical Medicine, 11(15), 4289. https://doi.org/10.3390/jcm11154289





