Abstract
Our aim is to assess the optimal levels of oxygen and carbon dioxide for the prognosis of favorable neurologic outcomes in survivors after extracorporeal cardiopulmonary resuscitation (ECPR). We obtained the mean levels of PaCO2 and PaO2 in arterial blood gas samples 72 h after ECPR. The primary outcome was the neurological status, according to the Cerebral Performance Categories (CPC) scale, upon discharge. Of 119 (48.6%) survivors, 95 (38.8%) had favorable neurologic outcomes (CPC 1 or 2). There was a U-shaped relationship between mean arterial blood gas tensions and poor neurological outcomes. The risk of poor neurological outcome was lowest in patients with the second tertile of mean PaCO2 (30–42 mm Hg) and PaO2 (120–160 mm Hg). In a multivariable analysis, third tertile of mean PaCO2, third tertile of mean PaO2, age, shockable rhythm, out of hospital cardiac arrest, duration of cardiopulmonary resuscitation, and ECPR at cardiac catheterization lab were found to be significantly associated with poor neurologic outcomes. Additionally, hypercapnia and extreme hyperoxia were found to be significantly associated with poor neurological outcomes after ECPR. Therefore, maintaining adequate arterial levels of oxygen and carbon dioxide may be important for favorable neurological prognoses in survivors after ECPR.
1. Introduction
Neurological prognoses in survivors after successful cardiopulmonary resuscitation (CPR) from cardiac arrest are of the utmost importance [1]. To improve these prognoses, there are several recommendations in the current guidelines for post-cardiac arrest care, including targeted temperature management, control of blood pressure and glucose level, seizure management, oxygenation, and ventilation [2,3]. The guidelines recommend titrating inspired oxygen and ventilation to achieve normal oxygen and carbon dioxide levels after the return of spontaneous circulation [2,3,4,5].
Aberrant oxygen levels may be associated with poor prognosis after cardiac arrest [6,7]. Hyperoxia can exacerbate oxygen free radical formation and cause subsequent reperfusion injury [8]. Hypoxia has also been reported to be associated with hospital mortality in survivors after cardiac arrest [6]. Hypercapnia can provoke cerebral vasodilatation and increased cerebral blood flow (CBF), while hypocapnia can lead to cerebral vasoconstriction and decreased CBF [9]. In survivors of cardiac arrest, neurological recovery is related to rapid improvement of CBF to meet the metabolic needs of the brain [10]. Therefore, it is hypothesized that oxygen and carbon dioxide levels may be closely connected with clinical outcomes in survivors after cardiac arrest.
It remains unknown whether the optimal target of arterial blood gas tension after extracorporeal cardiopulmonary resuscitation (ECPR) is similar to that of conventional CPR. In survivors after ECPR, brain recovery may be related to CBF autoregulation, native circulatory restoration, and amount of extracorporeal membrane oxygenation (ECMO) support [1,11]. However, it is not known in detail how the continuous flow of ECMO affects the autoregulation of CBF [11]. To date, no studies have determined the optimal levels of oxygen and carbon dioxide considering the changes in CBF autoregulation by ECMO. Therefore, in the present study, our aim is to assess the optimal levels of oxygen and carbon dioxide for favorable neurologic outcomes after ECPR.
2. Materials and Methods
2.1. Study Design and Variables
The present research comprised a retrospective, single-center, and observational study. In this study, ECPR was defined as successful implantation of veno-arterial ECMO and pump-on with cardiac compression during index procedure in patients with cardiac arrest [1,11]. ECPR was considered with confirmed witness arrest, reversible causes of cardiac arrest, and chest compression of 10 min or more [12]. ECPR was contraindicated with the patients with life expectancy less than 6 months, end-stage malignancies, an unwitnessed arrest, or chest compressions of 1 hour or more, but advanced age alone was not an absolute contraindication [11,12]. ECPR was performed when the indications for ECPR were appropriate, regardless of in-hospital or out-of-hospital cardiac arrest [11]. The CPR duration was defined as the total time from onset to halt of chest compression. Targeted temperature management was designated by an on-site intensivist according to the protocol [13].
2.2. Study Population
Patients were 18 years or older and underwent ECPR during hospitalization between January 2010 and December 2018 in Samsung Medical Center. All consecutive patients who underwent ECPR during the study period were included in the study. Of these patients, those aged below 18 years, those with Glasgow Coma Scale (GCS) > 12 on admission to the intensive care unit, those with an inappropriate indication for ECPR, those who had pre-existing severe neurologic disease or damage before arrest including traumatic brain injury, major stroke, malignant brain tumor or severe dementia, and those with insufficient medical records were excluded from the study (Figure 1). This study was approved by the Institutional Review Board of Samsung Medical Center (approved No. 2019-10-119). The requirement for informed consent was waived by the Institutional Review Board of Samsung Medical Center due to the retrospective nature of the study.
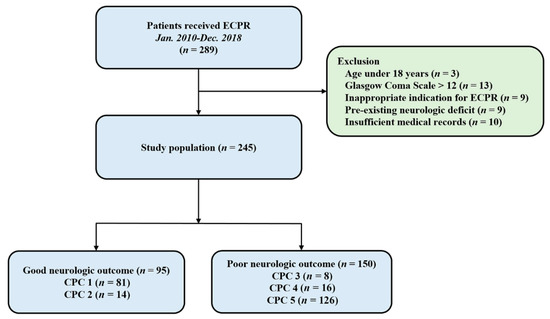
Figure 1.
Study flow chart. ECPR, extracorporeal cardiopulmonary resuscitation; CPC, Cerebral Performance Categories scale.
2.3. Data Collection Process
An expert nurse in the ECMO team was responsible for data collection and all data for all patients receiving ECPR were systematically recorded. We obtained the levels of partial pressure of carbon dioxide (PaCO2) and partial pressure of oxygen (PaO2) in arterial blood gas samples 72 h after ECPR. The sampling interval was determined by the treating physician. The mean PaCO2 and PaO2 were calculated using entire blood gas measurements 72 h after ECPR. The primary outcome was neurological status upon discharge, as assessed by the Glasgow-Pittsburgh Cerebral Performance Categories (CPC) scale (range: 1 to 5) [1].
Favorable neurological outcomes were defined as CPC scores 1 and 2, whereas poor neurologic outcomes were defined as CPC scores of 3 to 5. Two independent intensivists (SMH and JAR) assessed the CPC score by thoroughly reviewing the patient’s medical records. If the investigated scales did not match with each other, the intensivists discussed and corrected the scale.
2.4. Statistical Analyses
Continuous variables are presented as means ± standard deviations, and categorical variables are represented as numbers with subsequent percentages. Data comparisons were carried out using Student’s t-test for continuous variables, whereas the Chi-square test was used for categorical variables. We classified the subjects into three groups based on the distribution of the concentration of oxygen and carbon dioxide, using cut-off values between the groups. All possible ranges of oxygen and carbon dioxide were grid-searched to find the cutoffs of both variables. However, since the number of subjects with a low level of carbon dioxide was not sufficient, the cutoff value was fixed at 30 mm Hg, i.e., the generally accepted level [14,15,16,17]. Among all the logistic regression models that included the generated combination of oxygen and carbon dioxide as independent variables, the combination with the largest c-index was selected. For all analyses, multiple logistic regression was performed to correct clinically relevant variables. Eventually, the clinically relevant variables, including mean arterial blood gas tensions, age, first monitored rhythm, type of cardiac arrest, CPR duration, targeted temperature management, and location of ECMO insertion were obtained. All tests were two-sided and p values of less than 0.05 were considered statistically significant. Statistical analyses were performed with R Statistical Software (version 4.2.0; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Baseline Characteristics and Clinical Outcomes
In this study, 245 patients were analyzed (Figure 1). The mean age of the patients was 58.8 ± 15.7 years, and 176 patients (71.8%) were men. Hypertension (48.2%) and diabetes mellitus (35.1%) were identified as the most common comorbidities. Forty patients (16.3%) experienced out-of-hospital cardiac arrest. The mean CPR duration was 26.7 ± 20.6 min. The baseline characteristics of the ECPR patients are presented in Table 1. Compared with the favorable neurologic outcome group, the poor neurologic outcome group comprised elderly patients, higher incidence of out-of-hospital cardiac arrest, initial asystole rhythm and renal replacement therapy, lower incidence of ischemic heart disease as a cause of cardiac arrest, ECPR at cardiac catheterization lab, and longer CPR duration.

Table 1.
Baseline characteristics of patients.
Among 245 patients, 119 (48.6%) survived until discharge from the hospital. Of these, 95 (CPC 1 or 2) had favorable neurologic outcomes while 24 (CPC 3 or 4) had poor neurologic outcomes. The entire distribution of CPC scales is shown in Figure 1.
3.2. The Relationship between Mean Arterial Blood Gas Tensions and Neurologic Outcomes
Among the three groups of mean PaCO2 and PaO2, the second tertile groups had the highest number of patients with favorable outcomes (Table 2). There was a U-shaped relationship between the mean arterial blood gas tensions and poor neurological outcomes. The risk of poor neurological outcome was lowest in patients with 30–42 mm Hg of mean PaCO2 or 120–160 mm Hg of mean PaO2 (Figure 2). Considering mean PaCO2 and PaO2 simultaneously, the risk of poor neurological outcomes was lowest in patients with the second tertile of mean PaCO2 and PaO2 (Figure 3). In multivariable analysis, third tertile of mean PaCO2 (adjusted odds ratio [OR]: 12.02, 95% confidence interval [CI]: 1.703–84.760), third tertile of mean PaO2 (adjusted OR: 2.85, 95% CI: 1.043–7.804), age (adjusted OR: 1.05, 95% CI: 1.021–1.076), shockable rhythm (adjusted OR: 0.16, 95% CI: 0.047–0.555), out of hospital cardiac arrest (adjusted OR: 3.13, 95% CI: 1.074–9.133), CPR duration (adjusted OR: 3.57, 95% CI: 2.263–5.616), and ECPR at cardiac catheterization lab (adjusted OR: 0.17, 95% CI: 0.069–0.409) were found to be significantly associated with poor neurologic outcomes (Table 3).

Table 2.
Comparison of mean blood gas tension distribution between the favorable neurological outcome group and poor neurological outcome group.
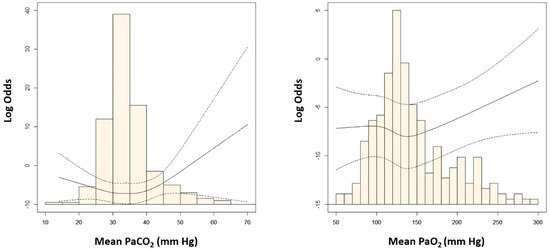
Figure 2.
Adjusted odds ratio (OR) of poor neurologic outcomes according to the mean levels of arterial carbon dioxide (PaCO2) and arterial oxygen (PaO2). OR was log−transformed to reduce skewness.
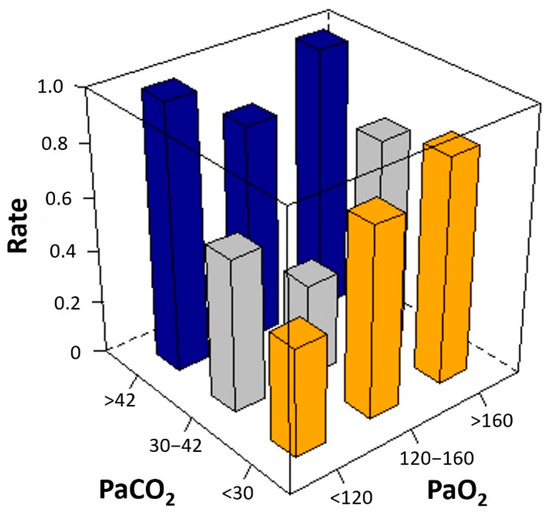
Figure 3.
Unadjusted rate of poor neurological outcomes according to the mean levels of arterial carbon dioxide (PaCO2) and arterial oxygen (PaO2). Considering mean PaCO2 and PaO2 simultaneously, the risk of poor neurological outcomes was lowest in patients with the second tertile of mean PaCO2 and PaO2 (the smallest gray square column in the middle).

Table 3.
Multivariable analysis of factors associated with poor neurological outcomes.
4. Discussion
In the present study, we investigated the optimal levels of oxygen and carbon dioxide for the prognosis of neurologic outcomes after ECPR. The major findings were as follows: First, the risk of poor neurological outcomes was lowest in the second tertile groups: 30–42 mm Hg of mean PaCO2 and 120–160 mm Hg of mean PaO2. Second, in our multivariable analysis, the third tertile of mean PaCO2, the third tertile of mean PaO2, age, shockable rhythm, out-of-hospital cardiac arrest, CPR duration, and ECPR at cardiac catheterization lab were found to be significantly associated with poor neurologic outcomes. Finally, there was a U-shaped relationship between the mean arterial blood gas tensions and poor neurological outcomes, but low to normal levels of PaCO2 or PaO2 demonstrated no independent association with poor neurological outcomes. In contrast, hypercapnia and extreme hyperoxia were found to be independently associated with poor neurological outcomes after ECPR.
Aberrant arterial blood gas tensions have been reported to be associated with poor neurological outcomes after cardiac arrest, and the impact probably depends on the extent and duration of exposure [7]. Hypocapnia has been demonstrated to be independently associated with poor clinical outcomes [7,15]. Hypocapnia can lead to vasoconstriction, decreased CBF, and cerebral ischemia in neurocritically ill patients [18]. In addition, hypoxia has been reported to be independently associated with hospital mortality after cardiac arrest [6]. In the present study, we observed a U-shaped relationship between mean arterial blood gas tensions and poor neurological outcomes, but hypocapnia and low to normal levels of PaO2 demonstrated no independent association with poor neurological outcomes after ECPR. The ECPR survivors with simultaneous low levels of PaCO2 and PaO2 had favorable neurological outcomes. They had a low incidence of chronic renal disease and high incidences of in-hospital cardiac arrest and ECPR at the cardiac catheterization lab compared with other patients.
Extreme hyperoxia was found to be independently associated with poor neurological outcomes after ECPR in the present study. Survivors after ECPR may experience severe hyperoxia because oxygenated blood can be easily obtained through the oxygenator of ECMO [19]. Hyperoxia leads to the generation of noxious oxygen radicals and cerebral vasoconstriction, and this mechanism has been reported to provoke brain ischemia [18,20,21]. Early hyperoxia can lead to severe vascular failure, refractory circulatory shock, and exacerbating reperfusion injury after ECPR [22,23,24]. In addition, it is possible to obtain fully oxygenated blood through an oxygenator at the right radial arterial line if the survivor’s native cardiac function is severely depressed after ECPR. Therefore, hyperoxia may reflect markedly impaired cardiac function in such patients. It has been proposed that hyperoxia may be associated with poor neurological outcomes in survivors after ECPR [22,23,24].
A few studies have reported that hypercapnia may be associated with a greater likelihood of favorable outcomes [7,25]. However, hypercapnia may lead to cerebral vasodilatation and increased CBF, and might provoke cerebral edema in patients with impaired CBF autoregulation [9]. Another study reported that hypercapnia was common after cardiac arrest and demonstrated an independent association with poor neurological outcomes after resuscitation from cardiac arrest [15]. Therefore, further studies are needed to demonstrate whether hypercapnia during postcardiac arrest care improves the clinical outcome [14].
In our previous study, old age (>65 years) and prolonged CPR duration (>30 min) were related to poor neurologic outcomes after ECPR [1]. There was a significant interaction between age and CPR duration in predicting neurological outcomes after ECPR [1]. Despite initial hypoxic-ischemic injury, the considerable reserve and tolerance of young brains to hypoxic-ischemic injury may lead to favorable neurological outcomes [26]. In addition, age-induced altered cerebral hemodynamics may affect neurologic recovery after cardiac arrest [1]. In the present study, ECMO insertion in cardiac catheterization lab was associated with favorable neurologic outcomes. This may be associated with short ECMO pump-on time. A catheterization lab is the best place for ECPR in a short time; ECMO insertion can be safe and highly effective in this setting [27,28]. In our previous studies, we found that initial arrest rhythm and low-flow time may be associated with neurological outcome after ECPR [1,29]. Notably, shockable rhythm was associated with favorable neurological outcomes after ECPR [1,29]. ECPR patients after in-hospital cardiac arrest may have more favorable outcomes than those after out-of-hospital cardiac arrest [30]. Patients may have more prolonged arrest to ECMO pump-on time after out-of-hospital cardiac arrest compared with after in-hospital cardiac arrest.
This study has several limitations. First, it was conducted over a long period. In the meantime, post-cardiac arrest management may have evolved, which may have affected the clinical outcomes during the study period. Second, the effects of continuous ECMO flow on cerebral autoregulation were unclear in this study. Lastly, it is hypothesized that extreme hyperoxia may be obtained in ECPR patients with a markedly impaired heart. Extremely high oxygen levels in the right radial artery may mainly be caused by the flow of ECMO. It is unclear whether the poor clinical outcomes were related to hyperoxia itself or impaired heart function. Although the present study provides valuable insights, prospective large-scale studies are needed to evaluate the optimal levels of oxygen and carbon dioxide for favorable neurologic outcomes after ECPR.
5. Conclusions
In this study, we observed a U-shaped relationship between poor neurological outcomes and mean arterial blood gas tensions in the first 72 h after ECPR. However, hypocapnia, normocapnia, hypoxia, and normoxia demonstrated no relationship with poor neurological outcomes, whereas hypercapnia and extreme hyperoxia were found to be significantly associated with poor neurological outcomes. Therefore, it is proposed that maintaining adequate arterial levels of oxygen and carbon dioxide may be important for favorable prognoses of neurological outcomes in survivors who underwent ECPR.
Author Contributions
Conceptualization, S.H. and J.-A.R.; Data curation, J.H.J.; Formal analysis, J.H.Y., J.A. and J.-A.R.; Investigation, S.H., Y.H.C., J.A. and J.-A.R.; Methodology, S.H. and J.H.Y.; Project administration, J.H.Y.; Resources, J.H.J. and Y.H.C.; Software, Y.H.C.; Supervision, J.-A.R.; Validation, J.H.J., J.A. and J.-A.R.; Visualization, J.A.; Writing—original draft, S.H., J.A. and J.-A.R.; Writing—review & editing, J.-A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Samsung Medical Center (approval number: SMC 2019-10-119). Patients’ records were reviewed and published according to the Declaration of Helsinki.
Informed Consent Statement
Informed consent was waived because of the retrospective nature of this study.
Data Availability Statement
Regarding data availability, our data are available on the Harvard Dataverse Network (http://dx.doi.org/10.7910/DVN/P7FF28) as recommended repositories (The dataset was published at 20 June 2022).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
CBF, cerebral blood flow; CPC, cerebral performance categories; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; GCS, Glasgow Coma Scale; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen.
References
- Ryu, J.A.; Chung, C.R.; Cho, Y.H.; Sung, K.; Jeon, K.; Suh, G.Y.; Park, T.K.; Lee, J.M.; Song, Y.B.; Hahn, J.Y.; et al. Neurologic Outcomes in Patients Who Undergo Extracorporeal Cardiopulmonary Resuscitation. Ann. Thorac. Surg. 2019, 108, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 2021, 47, 369–421. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Soar, J.; Zideman, D.A.; Biarent, D.; Bossaert, L.L.; Deakin, C.; Koster, R.W.; Wyllie, J.; Böttiger, B. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation 2010, 81, 1219–1276. [Google Scholar] [CrossRef]
- Hazinski, M.F.; Nolan, J.P.; Billi, J.E.; Böttiger, B.W.; Bossaert, L.; de Caen, A.R.; Deakin, C.D.; Drajer, S.; Eigel, B.; Hickey, R.W.; et al. Part 1: Executive summary: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2010, 122, S250–S275. [Google Scholar] [CrossRef] [Green Version]
- Helmerhorst, H.J.; Roos-Blom, M.J.; van Westerloo, D.J.; Abu-Hanna, A.; de Keizer, N.F.; de Jonge, E. Associations of arterial carbon dioxide and arterial oxygen concentrations with hospital mortality after resuscitation from cardiac arrest. Crit. Care 2015, 19, 348. [Google Scholar] [CrossRef] [Green Version]
- Schneider, A.G.; Eastwood, G.M.; Bellomo, R.; Bailey, M.; Lipcsey, M.; Pilcher, D.; Young, P.; Stow, P.; Santamaria, J.; Stachowski, E.; et al. Arterial carbon dioxide tension and outcome in patients admitted to the intensive care unit after cardiac arrest. Resuscitation 2013, 84, 927–934. [Google Scholar] [CrossRef]
- Balan, I.S.; Fiskum, G.; Hazelton, J.; Cotto-Cumba, C.; Rosenthal, R.E. Oximetry-guided reoxygenation improves neurological outcome after experimental cardiac arrest. Stroke 2006, 37, 3008–3013. [Google Scholar] [CrossRef] [Green Version]
- Kety, S.S.; Schmidt, C.F. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J. Clin. Investig. 1948, 27, 484–492. [Google Scholar] [CrossRef]
- Sundgreen, C.; Larsen, F.S.; Herzog, T.M.; Knudsen, G.M.; Boesgaard, S.; Aldershvile, J. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke 2001, 32, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.I.; Ko, R.E.; Yang, J.H.; Cho, Y.H.; Ahn, J.; Ryu, J.A. Optimal Mean Arterial Pressure for Favorable Neurological Outcomes in Survivors after Extracorporeal Cardiopulmonary Resuscitation. J. Clin. Med. 2022, 11, 290. [Google Scholar] [CrossRef]
- Ryu, J.A.; Chung, C.R.; Cho, Y.H.; Sung, K.; Suh, G.Y.; Park, T.K.; Song, Y.B.; Hahn, J.Y.; Choi, J.H.; Gwon, H.C.; et al. The association of findings on brain computed tomography with neurologic outcomes following extracorporeal cardiopulmonary resuscitation. Crit. Care 2017, 21, 15. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.A.; Park, T.K.; Chung, C.R.; Cho, Y.H.; Sung, K.; Suh, G.Y.; Lee, T.R.; Sim, M.S.; Yang, J.H. Association between Body Temperature Patterns and Neurological Outcomes after Extracorporeal Cardiopulmonary Resuscitation. PLoS ONE 2017, 12, e0170711. [Google Scholar] [CrossRef] [Green Version]
- Vaahersalo, J.; Bendel, S.; Reinikainen, M.; Kurola, J.; Tiainen, M.; Raj, R.; Pettila, V.; Varpula, T.; Skrifvars, M.B.; Group, F.S. Arterial blood gas tensions after resuscitation from out-of-hospital cardiac arrest: Associations with long-term neurologic outcome. Crit. Care Med. 2014, 42, 1463–1470. [Google Scholar] [CrossRef]
- Roberts, B.W.; Kilgannon, J.H.; Chansky, M.E.; Mittal, N.; Wooden, J.; Trzeciak, S. Association between postresuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with post-cardiac arrest syndrome. Circulation 2013, 127, 2107–2113. [Google Scholar] [CrossRef] [Green Version]
- Peluso, L.; Belloni, I.; Calabro, L.; Dell’Anna, A.M.; Nobile, L.; Creteur, J.; Vincent, J.L.; Taccone, F.S. Oxygen and carbon dioxide levels in patients after cardiac arrest. Resuscitation 2020, 150, 1–7. [Google Scholar] [CrossRef]
- Wang, H.E.; Prince, D.K.; Drennan, I.R.; Grunau, B.; Carlbom, D.J.; Johnson, N.; Hansen, M.; Elmer, J.; Christenson, J.; Kudenchuk, P.; et al. Post-resuscitation arterial oxygen and carbon dioxide and outcomes after out-of-hospital cardiac arrest. Resuscitation 2017, 120, 113–118. [Google Scholar] [CrossRef]
- Muizelaar, J.P.; Marmarou, A.; Ward, J.D.; Kontos, H.A.; Choi, S.C.; Becker, D.P.; Gruemer, H.; Young, H.F. Adverse effects of prolonged hyperventilation in patients with severe head injury: A randomized clinical trial. J. Neurosurg. 1991, 75, 731–739. [Google Scholar] [CrossRef]
- Hutin, A.; Abu-Habsa, M.; Burns, B.; Bernard, S.; Bellezzo, J.; Shinar, Z.; Torres, E.C.; Gueugniaud, P.Y.; Carli, P.; Lamhaut, L. Early ECPR for out-of-hospital cardiac arrest: Best practice in 2018. Resuscitation 2018, 130, 44–48. [Google Scholar] [CrossRef]
- Douzinas, E.E.; Patsouris, E.; Kypriades, E.M.; Makris, D.J.; Andrianakis, I.; Korkolopoulou, P.; Boursinos, V.; Papalois, A.; Sotiropoulou, C.; Davaris, P.; et al. Hypoxaemic reperfusion ameliorates the histopathological changes in the pig brain after a severe global cerebral ischaemic insult. Intensive Care Med. 2001, 27, 905–910. [Google Scholar] [CrossRef]
- Becker, L.B. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc. Res. 2004, 61, 461–470. [Google Scholar] [CrossRef]
- Bonnemain, J.; Rusca, M.; Ltaief, Z.; Roumy, A.; Tozzi, P.; Oddo, M.; Kirsch, M.; Liaudet, L. Hyperoxia during extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest is associated with severe circulatory failure and increased mortality. BMC Cardiovasc. Disord. 2021, 21, 542. [Google Scholar] [CrossRef]
- Chang, W.T.; Wang, C.H.; Lai, C.H.; Yu, H.Y.; Chou, N.K.; Wang, C.H.; Huang, S.C.; Tsai, P.R.; Chou, F.J.; Tsai, M.S.; et al. Optimal Arterial Blood Oxygen Tension in the Early Postresuscitation Phase of Extracorporeal Cardiopulmonary Resuscitation: A 15-Year Retrospective Observational Study. Crit. Care Med. 2019, 47, 1549–1556. [Google Scholar] [CrossRef]
- Halter, M.; Jouffroy, R.; Saade, A.; Philippe, P.; Carli, P.; Vivien, B. Association between hyperoxemia and mortality in patients treated by eCPR after out-of-hospital cardiac arrest. Am. J. Emerg. Med. 2020, 38, 900–905. [Google Scholar] [CrossRef]
- Eastwood, G.M.; Schneider, A.G.; Suzuki, S.; Peck, L.; Young, H.; Tanaka, A.; Martensson, J.; Warrillow, S.; McGuinness, S.; Parke, R.; et al. Targeted therapeutic mild hypercapnia after cardiac arrest: A phase II multi-centre randomised controlled trial (the CCC trial). Resuscitation 2016, 104, 83–90. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Crit. Care 2017, 21, 90. [Google Scholar] [CrossRef] [Green Version]
- Arlt, M.; Philipp, A.; Voelkel, S.; Schopka, S.; Husser, O.; Hengstenberg, C.; Schmid, C.; Hilker, M. Early experiences with miniaturized extracorporeal life-support in the catheterization laboratory. Eur. J. Cardiothorac. Surg. 2012, 42, 858–863. [Google Scholar] [CrossRef]
- Goyal, V. ECPR in the cath lab. Qatar. Med. J. 2017, 1, 28. [Google Scholar] [CrossRef]
- Ko, R.E.; Ryu, J.A.; Cho, Y.H.; Sung, K.; Jeon, K.; Suh, G.Y.; Park, T.K.; Lee, J.M.; Song, Y.B.; Hahn, J.Y.; et al. The differential neurologic prognosis of low-flow time according to the initial rhythm in patients who undergo extracorporeal cardiopulmonary resuscitation. Resuscitation 2020, 148, 121–127. [Google Scholar] [CrossRef]
- Kagawa, E.; Inoue, I.; Kawagoe, T.; Ishihara, M.; Shimatani, Y.; Kurisu, S.; Nakama, Y.; Dai, K.; Takayuki, O.; Ikenaga, H.; et al. Assessment of outcomes and differences between in- and out-of-hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal life support. Resuscitation 2010, 81, 968–973. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).