Frontal Fibrosing Alopecia: A Histopathological Comparison of the Frontal Hairline with Normal-Appearing Scalp
Abstract
1. Introduction
2. Materials and Methods
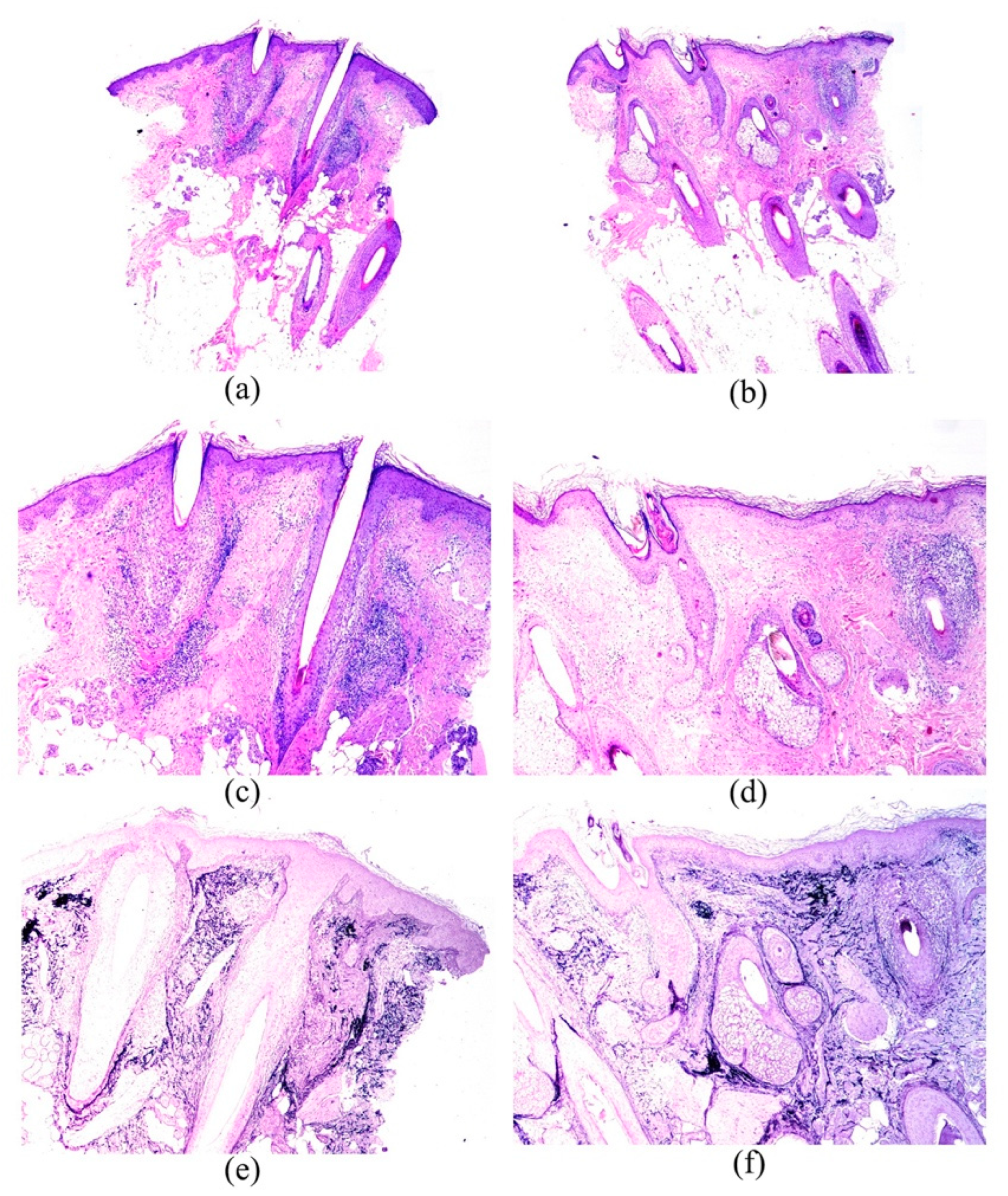
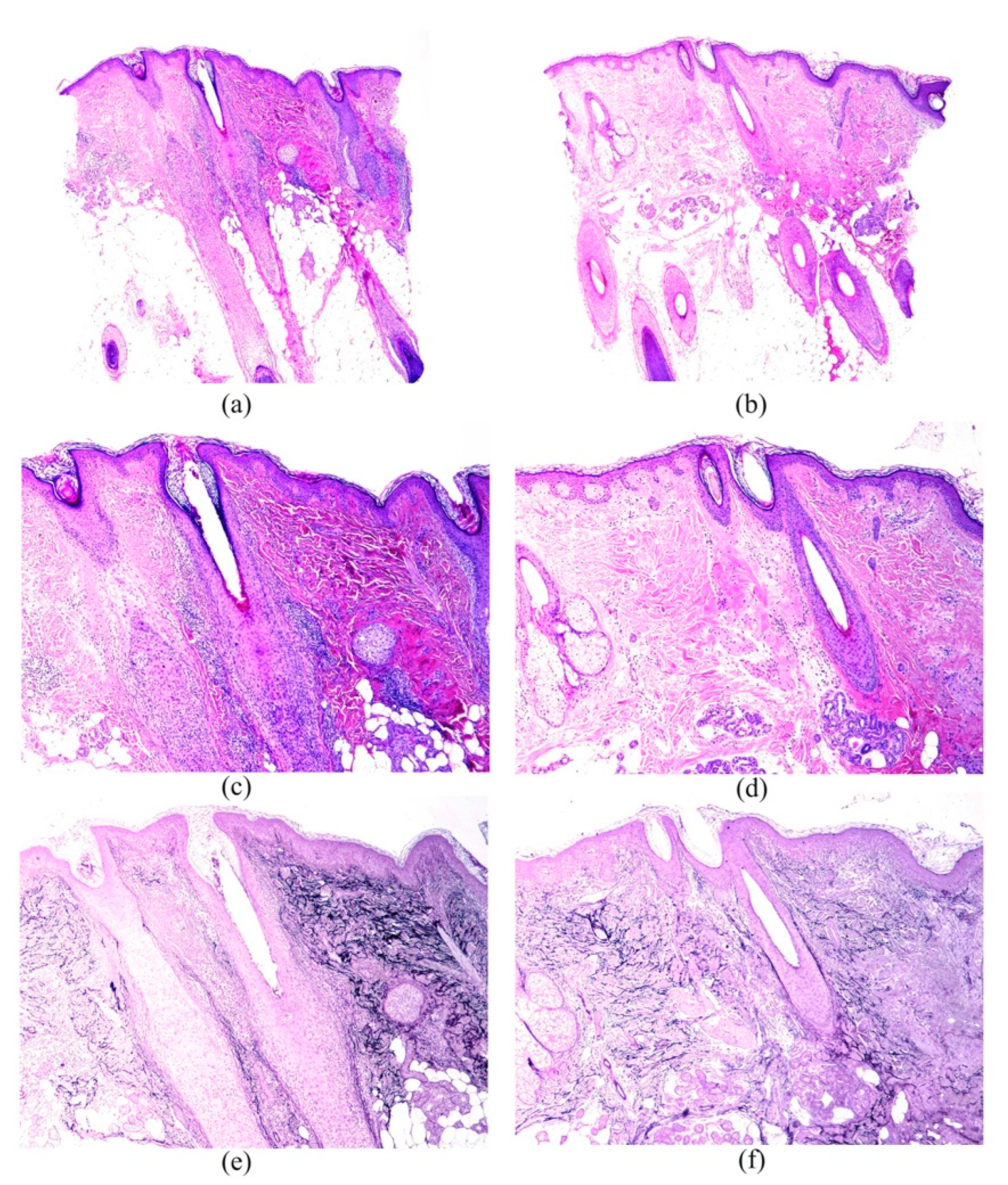
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vañó-Galván, S.; Saceda-Corralo, D.; Blume-Peytavi, U.; Cucchía, J.; Dlova, N.C.; Dias, M.F.R.G.; Grimalt, R.; Guzmán-Sánchez, D.; Harries, M.; Ho, A.; et al. Frequency of the Types of Alopecia at Twenty-Two Specialist Hair Clinics: A Multicenter Study. Ski. Appendage Disord. 2019, 5, 309–315. [Google Scholar] [CrossRef]
- Vañó-Galván, S.; Molina-Ruiz, A.M.; Serrano-Falcón, C.; Arias-Santiago, S.; Rodrigues-Barata, A.R.; Garnacho-Saucedo, G.; Martorell-Calatayud, A.; Fernández-Crehuet, P.; Grimalt, R.; Aranegui, B.; et al. Frontal fibrosing alopecia: A multicenter review of 355 patients. J. Am. Acad. Dermatol. 2014, 70, 670–678. [Google Scholar] [CrossRef]
- Porriño-Bustamante, M.L.; Fernández-Pugnaire, M.A.; Arias-Santiago, S. Frontal Fibrosing Alopecia: A Review. J. Clin. Med. 2021, 10, 1805. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.J.; Meyer, K.; Chaudhry, I.; Kloepper, J.E.; Poblet, E.; Griffiths, C.E.; Paus, R. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle’s epithelial stem cell niche. J. Pathol. 2013, 231, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.J.; Jimenez, F.; Izeta, A.; Hardman, J.; Panicker, S.P.; Poblet, E.; Paus, R. Lichen Planopilaris and Frontal Fibrosing Alopecia as Model Epithelial Stem Cell Diseases. Trends Mol. Med. 2018, 24, 435–448. [Google Scholar] [CrossRef]
- Kossard, S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. Arch. Dermatol. 1994, 130, 770–774. [Google Scholar] [CrossRef]
- Poblet, E.; Jiménez, F.; Pascual, A.; Piqué, E. Frontal fibrosing alopecia versus lichen planopilaris: A clinicopathological study. Int. J. Dermatol. 2006, 45, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.A.; Imadojemu, S.; Beer, K.; Seykora, J.T. Inflammatory features of frontal fibrosing alopecia. J. Cutan. Pathol. 2017, 44, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Goldberg, L.J. The depth of inflammation in frontal fibrosing alopecia and lichen planopilaris: A potential distinguishing feature. J. Am. Acad. Dermatol. 2017, 76, 1183–1184. [Google Scholar] [CrossRef][Green Version]
- Porriño-Bustamante, M.L.; Fernández-Pugnaire, M.A.; Castellote-Caballero, L.; Arias-Santiago, S. Colour Doppler ultrasound study in patients with frontal fibrosing alopecia. Ski. Res. Technol. 2021, 27, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Mirmirani, P.; Willey, A.; Headington, J.T.; Stenn, K.; McCalmont, T.H.; Price, V.H. Primary cicatricial alopecia: Histopathologic findings do not distinguish clinical variants. J. Am. Acad. Dermatol. 2005, 52, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Saceda-Corralo, D.; Desai, K.; Pindado-Ortega, C.; Moreno-Arrones, O.M.; Vañó-Galván, S.; Miteva, M. Histological evidence for epidermal and dermal atrophy of the alopecic band in treatment-naïve patients with Frontal Fibrosing Alopecia. J. Eur. Acad. Dermatol. Venereol. 2022, 35, e47–e49. [Google Scholar] [CrossRef]
- Pindado-Ortega, C.; Perna, C.; Saceda-Corralo, D.; Fernández-Nieto, D.; Jaén-Olasolo, P.; Vañó-Galván, S. Frontal fibrosing alopecia: Histopathological, immunohistochemical and hormonal study of clinically unaffected scalp areas. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e84–e85. [Google Scholar] [CrossRef] [PubMed]
- Doche, I.; Romiti, R.; Hordinsky, M.K.; Valente, N.S. “Normal-appearing” scalp areas are also affected in lichen planopilaris and frontal fibrosing alopecia: An observational histopathologic study of 40 patients. Exp. Dermatol. 2020, 29, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Chew, A.L.; Bashir, S.J.; Wain, E.M.; Fenton, D.A.; Stefanato, C.M. Expanding the spectrum of frontal fibrosing alopecia: A unifying concept. J. Am. Acad. Dermatol. 2010, 63, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Sperling, L.C. Hair density in African Americans. Arch. Dermatol. 1999, 135, 656–658. [Google Scholar] [CrossRef]
- Miteva, M.; Sabiq, S. A New Histologic Pattern in 6 Biopsies from Early Frontal Fibrosing Alopecia. Am. J. Dermatopathol. 2019, 41, 118–121. [Google Scholar] [CrossRef]
- Gálvez-Canseco, A.; Sperling, L. Lichen planopilaris and frontal fibrosing alopecia cannot be differentiated by histopathology. J. Cutan. Pathol. 2018, 45, 313–317. [Google Scholar] [CrossRef]
- López-Pestaña, A.; Tuneu, A.; Lobo, C.; Ormaechea, N.; Zubizarreta, J.; Vildosola, S.; Del Alcazar, E. Facial lesions in frontal fibrosing alopecia (FFA): Clinicopathological features in a series of 12 cases. J. Am. Acad. Dermatol. 2015, 73, 987.e1–987.e6. [Google Scholar] [CrossRef]
- Saceda-Corralo, D.; Moreno-Arrones, O.M.; Rubio-Lambraña, M.; Gil-Redondo, R.; Bernárdez, C.; Hermosa-Gelbard, A.; Jaén-Olasolo, P.; Vañó-Galván, S. Trichoscopic features of mild frontal fibrosing alopecia. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e205–e207. [Google Scholar] [CrossRef]
- Dubin, C.; Glickman, J.W.; Del Duca, E.; Chennareddy, S.; Han, J.; Dahabreh, D.; Estrada, Y.D.; Zhang, N.; Kimmel, G.W.; Singer, G.; et al. Scalp and serum profiling of frontal fibrosing alopecia reveals scalp immune and fibrosis dysregulation with no systemic involvement. J. Am. Acad. Dermatol. 2022, 86, 551–562. [Google Scholar] [CrossRef] [PubMed]
| n: 52 | Mean |
|---|---|
| Age (years) | 63.04 (SD 9.69) |
| Age of menopause (years) | 50.5 (SD 3.61) |
| Age of onset of the alopecia (years) | 58.42 (SD 10.17) |
| Duration of the alopecia (months) | 55.58 (SD 37.63) |
| Frequencies | |
| Menopause | 88.5% (46/52) |
| Pruritus | 71.2% (37/52) |
| Trichodynia | 23.1% (12/52) |
| Occipital involvement | 9.6% (5/52) |
| Eyebrow alopecia | 86.5% (45/52) |
| Facial papules | 21.2% (11/52) |
| FFA severity grade: | |
| Grade I | 5.8% (3/52) |
| Grade II | 38.5% (20/52) |
| Grade III | 36.5% (19/52) |
| Grade IV | 11.5% (6/52) |
| Grade V | 7.7% (4/52) |
| Perifollicular erythema | 84.6% (44/52) |
| Follicular hyperkeratosis | 88.5% (46/52) |
| N52 | Mean (SD) | ||
|---|---|---|---|
| Follicular Count | Frontal Hairline (B1) | Healthy Scalp (B2) | p Value |
| Total | 5.10 (2.77) | 7.79 (3.87) | <0.001 |
| Terminal hairs | 4.25 (2.59) | 6.58 (3.48) | <0.001 |
| Intermediate hairs | 0.38 (0.91) | 0.50 (1.16) | 0.479 |
| Vellus hairs | 0.46 (0.85) | 0.71 (0.98) | 0.166 |
| Anagen hairs | 4.67 (2.90) | 7.35 (3.80) | <0.001 |
| Telogen hairs | 0.44 (0.67) | 0.44 (0.78) | 1.000 |
| N52 | Frontal Hairline (B1) | Healthy Scalp (B2) | p Value |
|---|---|---|---|
| Inflammatory infiltrate (yes) | 92.3% (48/52) | 86.5% (45/52) | 0.508 |
| Severity | |||
| Mild | 35.4% (17/48) | 71.1% (32/45) | 0.013 |
| Moderate | 60.4% (29/48) | 26.7% (12/45) | |
| Severe | 4.2% (2/48) | 2.2% (1/45) | |
| Specific location of the inflammatory infiltrate | |||
| Sebaceous gland (yes) | 13.5% (7/52) | 5.8% (3/52) | 0.368 |
| Dermis (general) (yes) | 88.5% (46/52) | 71.2% (37/52) | 0.035 |
| Interfollicular dermis (yes) | 86.5% (45/52) | 71.2% (37/52) | 0.057 |
| Superficial perivascular lymphohistiocytic infiltrate | 86.4% (44/52) | 69.2% (36/52) | 0.039 |
| Frontal Hairline (B1) | Healthy Scalp (B2) | p Value | |
|---|---|---|---|
| Epithelial changes | |||
| Corneum stratum changes (including hyperkeratosis, parakeratosis or both) (yes) | 11.5% (6/52) | 24.5% (12/49) | 0.180 |
| Epidermal changes (specified below) (yes) | 17.3% (9/52) | 6.1% (3/49) | 0.07 |
| Follicular epithelium changes (specified below) (yes) | 70.6% (36/51) | 48.1% (25/52) | 0.012 |
| Interface dermatitis lichenoid in the upper part of the hair follicle | 23.5% (12/51) | 15.4% (8/52) | 0.125 |
| Vacuolar degeneration basal layer outer root sheath | 64.7% (33/51) | 36.5% (19/52) | 0.001 |
| Necrosis of keratinocytes outer root sheath | 43.1% (22/51) | 15.4% (8/52) | 0.001 |
| Increased apoptotic activity in outer root sheath (more intense than the physiological grade commonly seen in any biopsy) | 45.1% (23/51) | 19.2% (10/52) | 0.001 |
| Infundibular dilatation and infundibular hypergranulosis | 13.7% (7/51) | 7.7% (4/52) | 0.508 |
| Fibrous tissular changes | 80.8% (42/52) | 53.8% (28/52) | 0.003 |
| Perifollicular fibrosis | 71.2% (37/52) | 30.8% (16/52) | <0.001 |
| Interfollicular dermal fibrosis | 53.8% (28/52) | 36.5% (19/52) | 0.122 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porriño-Bustamante, M.L.; Pinedo-Moraleda, F.J.; Fernández-Flores, Á.; Montero-Vílchez, T.; Fernández-Pugnaire, M.A.; Arias-Santiago, S. Frontal Fibrosing Alopecia: A Histopathological Comparison of the Frontal Hairline with Normal-Appearing Scalp. J. Clin. Med. 2022, 11, 4121. https://doi.org/10.3390/jcm11144121
Porriño-Bustamante ML, Pinedo-Moraleda FJ, Fernández-Flores Á, Montero-Vílchez T, Fernández-Pugnaire MA, Arias-Santiago S. Frontal Fibrosing Alopecia: A Histopathological Comparison of the Frontal Hairline with Normal-Appearing Scalp. Journal of Clinical Medicine. 2022; 11(14):4121. https://doi.org/10.3390/jcm11144121
Chicago/Turabian StylePorriño-Bustamante, María Librada, Fernando Javier Pinedo-Moraleda, Ángel Fernández-Flores, Trinidad Montero-Vílchez, María Antonia Fernández-Pugnaire, and Salvador Arias-Santiago. 2022. "Frontal Fibrosing Alopecia: A Histopathological Comparison of the Frontal Hairline with Normal-Appearing Scalp" Journal of Clinical Medicine 11, no. 14: 4121. https://doi.org/10.3390/jcm11144121
APA StylePorriño-Bustamante, M. L., Pinedo-Moraleda, F. J., Fernández-Flores, Á., Montero-Vílchez, T., Fernández-Pugnaire, M. A., & Arias-Santiago, S. (2022). Frontal Fibrosing Alopecia: A Histopathological Comparison of the Frontal Hairline with Normal-Appearing Scalp. Journal of Clinical Medicine, 11(14), 4121. https://doi.org/10.3390/jcm11144121








