A Comparison of EQ-5D-3L, EQ-5D-5L, and SF-6D Utilities of Patients with Musculoskeletal Disorders of Different Severity: A Health-Related Quality of Life Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments
2.2. Sample and Data Collection
2.3. Ethical Issues
2.4. Analyses
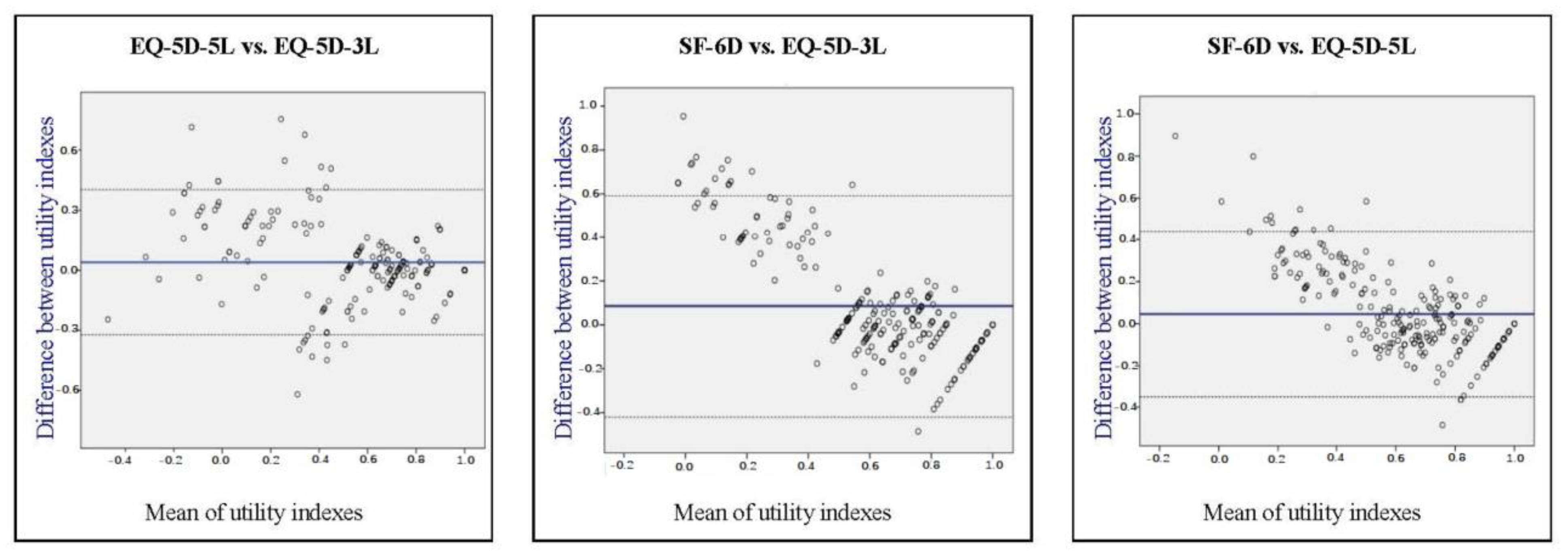
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef]
- Sebbag, E.; Felten, R.; Sagez, F.; Sibilia, J.; Devilliers, H.; Arnaud, L. The world-wide burden of musculoskeletal diseases: A systematic analysis of the World Health Organization Burden of Diseases Database. Ann. Rheum. Dis. 2019, 78, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Cortesi, P.A.; Scalone, L.; D’ Angiolella, L.; Belisari, A.; Fusco, F.; Olivieri, I.; Mantovani, L.G. Systematic literature review on economic implications and pharmacoeconomic issues of psoriatic arthritis. Clin. Exp. Rheumatol. 2012, 30, 126–131. [Google Scholar]
- Sieper, J.; Braun, J.; Rudwaleit, M.; Boonen, A.; Zink, A. Ankylosing spondylitis: An overview. Ann Rheum Dis. 2002, 61, iii8–iii18. [Google Scholar] [CrossRef] [Green Version]
- Oden, A.; McCloskey, E.V.; Kanis, J.A.; Harvey, N.C.; Johansson, H. Burden of high fracture probability worldwide: Secular increases 2010–2040. Osteoporosis Int. 2015, 26, 2243–2248. [Google Scholar] [CrossRef] [PubMed]
- Rasanen, P.; Roine, E.; Sintonen, H.; Semberg-Konttinen, V.; Ryynanen, O.P.; Roine, R. Use of quality-adjusted life years for the estimation of effectiveness of health care: A systematic literature review. Int. J. Tech. Assess. Health Care 2006, 22, 235–241. [Google Scholar] [CrossRef]
- Bryan, S.; Longworth, L. Measuring health-related utility: Why the disparity between EQ-5D and SF-6D? Eur. J. Health Econ. 2005, 6, 253–260. [Google Scholar] [CrossRef]
- Gerard, K.; Nicholson, T.; Mullee, M.; Mehta, R.; Roderick, P. EQ-5D versus SF-6D in an older, chronically ill patient group. Appl. Health Econ. Health Policy 2004, 3, 91–102. [Google Scholar] [CrossRef]
- Lamers, L.M.; Bouwmans, C.A.; van Straten, A.; Donker, M.C.; Hakkaart, L. Comparison of EQ-5D and SF-6D utilities in mental health patients. Health Econ. 2006, 15, 1229–1236. [Google Scholar] [CrossRef]
- Xie, F.; Li, S.C.; Luo, N.; Lo, N.N.; Yeo, S.J.; Yang, K.Y.; Fong, K.Y.; Thumboo, J. Comparison of the EuroQol and short form 6D in Singapore multiethnic Asian knee osteoarthritis patients scheduled for total knee replacement. Arthritis Rheum. 2007, 57, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Kontodimopoulos, N.; Argiriou, M.; Theakos, N.; Niakas, D. The impact of disease severity on EQ-5D and SF-6D utility discrepancies in chronic heart failure. Eur. J. Health Econ. 2011, 12, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wong, C.K.; McGhee, S.M.; Pang, P.K.; Yu, W.C. A comparison between the EQ-5D and the SF-6D in patients with chronic obstructive pulmonary disease (COPD). PLoS ONE 2014, 9, e112389. [Google Scholar] [CrossRef] [Green Version]
- Kontodimopoulos, N.; Stamatopoulou, E.; Brinia, A.; Talias, M.A.; Ferreira, L.N. Are condition-specific utilities more valid than generic preference-based ones in asthma? Evidence from a study comparing EQ-5D-3L and SF-6D with AQL-5D. Expert Rev. Pharmacoecon. Outcomes Res. 2018, 18, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Petrou, S.; Hockley, C. An investigation into the empirical validity of the EQ-5D and SF-6D based on hypothetical preferences in a general population. Health Econ. 2005, 14, 1169–1189. [Google Scholar] [CrossRef]
- Bharmal, M.; Thomas, J., 3rd. Comparing the EQ-5D and the SF-6D descriptive systems to assess their ceiling effects in the US general population. Value Health 2006, 9, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Barton, G.R.; Sach, T.H.; Avery, A.J.; Jenkinson, C.; Doherty, M.; Whynes, D.K.; Muir, K.R. A comparison of the performance of the EQ-5D and SF-6D for individuals aged >/= 45 years. Health Econ. 2008, 17, 815–832. [Google Scholar] [CrossRef]
- Kontodimopoulos, N.; Pappa, E.; Papadopoulos, A.A.; Tountas, Y.; Niakas, D. Comparing SF-6D and EQ-5D utilities across groups differing in health status. Qual. Life Res. 2009, 18, 87–97. [Google Scholar] [CrossRef]
- Janssen, M.F.; Pickard, S.A.; Golicki, D.; Gudex, C.; Niewada, M.; Scalone, L.; Swinburn, P.; Busschbach, J. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: A multi-country study. Qual. Life Res. 2013, 22, 1717–1727. [Google Scholar] [CrossRef] [Green Version]
- Greene, M.A.; Rader, K.A.; Garellick, G.; Malchau, H.; Freiberg, A.A.; Rolfson, O. The EQ-5D-5L improves on the EQ-5D-3L for health-related quality-of-life assessment in patients undergoing total hip arthroplasty. Clin. Orthop. Relat. Res. 2015, 473, 3383–3390. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.N.; Ferreira, P.L.; Ribeiro, F.P.; Pereira, L.N. Comparing the performance of the EQ-5D-3L and the EQ-5D-5L in young Portuguese adults. Health Qual. Life Outcomes 2016, 14, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poór, A.K.; Rencz, F.; Brodszky, V.; Gulácsi, L.; Beretzky, Z.; Hidvégi, B.; Holló, P.; Kárpáti, S.; Péntek, M. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L in psoriasis patients. Qual. Life Res. 2017, 26, 3409–3419. [Google Scholar] [CrossRef] [PubMed]
- Rencz, F.; Lakatos, P.L.; Gulácsi, L.; Brodszky, V.; Kürti, Z.; Lovas, S.; Banai, J.; Herszényi, L.; Cserni, T.; Molnár, T.; et al. Validity of the EQ-5D-5L and EQ-5D-3L in patients with Crohn’s disease. Qual. Life Res. 2019, 28, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Kangwanrattanakul, K.; Parmontree, P. Psychometric properties comparison between EQ-5D-5L and EQ-5D-3L in the general Thai population. Qual. Life Res. 2020, 29, 3407–3417. [Google Scholar] [CrossRef]
- Thompson, A.J.; Turner, A.J. A Comparison of the EQ-5D-3L and EQ-5D-5L. Pharmacoeconomics 2020, 38, 575–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Lau, T.; Lee, E.; Vathsala, A.; Chia, K.S.; Luo, N. Comparison of the preference-based EQ-5D-5L and SF-6D in patients with end stage renal disease (ESRD). Eur. J. Health Econ. 2015, 16, 1019–1026. [Google Scholar] [CrossRef]
- Shiroiwa, T.; Fukuda, T.; Ikeda, S.; Igarashi, A.; Noto, S.; Saito, S.; Shimozuma, K. Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D. Qual. Life Res. 2016, 25, 707–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakthong, P.; Munpan, W. A head-to-head comparison of UK SF-6D and Thai and UK EQ-5D-5L value sets in Thai patients with chronic diseases. Appl. Health Econ. Health Policy 2017, 15, 669–679. [Google Scholar] [CrossRef]
- Kangwanrattanakul, K. A comparison of measurement properties between UK SF-6D and English EQ-5D-5L and Thai EQ-5D-5L value sets in general Thai population. Expert Rev. Pharmacoecon. Outcomes Res. 2021, 21, 765–774. [Google Scholar] [CrossRef]
- Brooks, R. EuroQol: The current state of play. Health Policy 1996, 37, 53–72. [Google Scholar] [CrossRef]
- Dolan, P. Modelling valuations for EuroQol health states. Med. Care 1997, 35, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Kontodimopoulos, N.; Pappa, E.; Niakas, D.; Yfantopoulos, J.; Dimitrakaki, C.; Tountas, Y. Validity of the EQ-5D instrument in a Greek general population. Value Health 2008, 11, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Yfantopoulos, J.N.; Chantzaras, A.E. Validation and comparison of the psychometric properties of the EQ-5D-3L and EQ-5D-5L instruments in Greece. Eur. J. Health Econ. 2017, 18, 519–531. [Google Scholar] [CrossRef] [PubMed]
- van Hout, B.; Janssen, M.F.; Feng, Y.S.; Kohlmann, T.; Busschbach, J.; Golicki, D.; Lloyd, A.; Scalone, L.; Kind, P.; Pickard, A.S. Interim scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012, 15, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Brazier, J.; Roberts, J.; Deverill, M. The estimation of a preference-based measure of health from the SF-36. J. Health Econ. 2002, 21, 271–292. [Google Scholar] [CrossRef] [Green Version]
- Pappa, E.; Kontodimopoulos, N.; Niakas, D. Validating and norming of the Greek SF-36 Health Survey. Qual. Life Res. 2005, 14, 1433–1438. [Google Scholar] [CrossRef]
- Kontodimopoulos, N.; Pappa, E.; Chadjiapostolou, Z.; Arvanitaki, E.; Papadopoulos, A.A.; Niakas, D. Comparing the sensitivity of EQ-5D, SF-6D and 15D utilities to the specific effect of diabetic complications. Eur. J. Health Econ. 2012, 13, 111–120. [Google Scholar] [CrossRef]
- Prevoo, M.L.; van ‘t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Van Gestel, A.M.; Prevoo, M.L.; van ‘t Hof, M.A.; van Rijswijk, M.H.; van de Putte, L.B.; van Riel, P.L. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996, 39, 34–40. [Google Scholar] [CrossRef]
- Schoels, M.M.; Aletaha, D.; Alasti, F.; Smolen, J.S. Disease activity in psoriatic arthritis: Defining remission and treatment success using the DAPSA-score. Ann. Rheum. Dis. 2016, 75, 811–818. [Google Scholar] [CrossRef]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar] [PubMed]
- van Stralen, K.J.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Measuring agreement, more complicated than it seems. Nephron. Clin. Pract. 2012, 120, c162–c167. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.J.; Brazier, J.E. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual. Life Res. 2005, 14, 1523–1532. [Google Scholar] [CrossRef]
- Luo, N.; Johnson, J.A.; Coons, S.J. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med. Care 2010, 48, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B. Fundamental of Biostatistics, 8th ed.; Cengage Learning: Boston, MA, USA, 2015. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef]
- Nahvijou, A.; Safari, H.; Ameri, H. Comparing the performance of the EQ-5D-5L with two versions of the SF-6Dv2 in patients with breast cancer. Health Serv. Outcomes Res. Methodol. 2020, 20, 183–194. [Google Scholar] [CrossRef]
- Mulhern, B.; Feng, Y.; Shah, K.; Janssen, M.F.; Herdman, M.; van Hout, B.; Devlin, N. Comparing the UK EQ-5D-3L and English EQ-5D-5L value sets. PharmacoEconomics 2018, 36, 699–713. [Google Scholar] [CrossRef] [Green Version]
- Bleichrodt, H. A new explanation for the difference between time trade-off utilities and standard gamble utilities. Health Econ. 2002, 11, 447–456. [Google Scholar] [CrossRef]
- Green, C.; Brazier, J.; Deverill, M. Valuing health-related quality of life. A review of health state valuation techniques. Pharmacoeconomics 2000, 17, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Dolan, P.; Gudex, C.; Kind, P.; Williams, A. Valuing health states: A comparison of methods. J. Health Econ. 1996, 15, 209–231. [Google Scholar] [CrossRef]
- Bordens, K.S.; Abbott, B.B. Research Design and Methods: A Process Approach, 8th ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Janssen, M.F.; Birnie, E.; Haagsma, J.A.; Bonsel, G.J. Comparing the standard EQ-5D three-level system with a five-level version. Value Health 2008, 11, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattanaphesaj, J.; Thavorncharoensap, M. Measurement properties of the EQ-5D-5L compared to EQ-5D-3L in the Thai diabetes patients. Health Qual. Life Outcomes 2015, 13, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameri, H.; Safari, H.; Poder, T. Exploring the consistency of the SF-6Dv2 in a breast cancer population. Expert Rev. Pharmacoecon. Outcomes Res. 2021, 21, 1017–1024. [Google Scholar] [CrossRef]


| Characteristics | Total Sample (N = 315) | Rheumatoid Arthritis (N = 114) | Psoriatic Arthritis (N = 59) | Ankylosing Spondylitis (N = 47) | Osteopenia/ Osteoporosis (N = 95) | p-sig.1 |
|---|---|---|---|---|---|---|
| Female, N (%) | 230 (73.0) | 94 (82.5) | 37 (62.7) | 10 (21.3) | 89 (93.7) | <0.001 |
| Age, (mean ± SD) | 60.1 ± 12.3 | 62.8 ± 12.5 | 56.4 ± 12.9 | 51.0 ± 12.4 | 63.7 ± 8.4 | <0.001 |
| Years disease duration, (mean ± SD) | 7.4 ± 8.1 | 9.2 ± 8.6 | 7.2 ± 8.2 | 9.3 ± 9.9 | 3.9 ± 4.4 | <0.001 |
| Educational level, N (% valid) | ||||||
| Primary | 98 (31.9) | 52 (46.4) | 16 (27.1) | 10 (21.3) | 20 (22.5) | 0.007 |
| Secondary | 141 (45.9) | 41 (36.6) | 29 (49.2) | 25 (53.2) | 46 (51.7) | |
| Tertiary | 58 (22.1) | 19 (17.0) | 14 (23.7) | 12 (25.5) | 23 (25.8) | |
| BMI Class, N (% valid) | ||||||
| Normal (18.5–24.9) | 126 (41.3) | 39 (35.5) | 11 (19.3) | 21 (45.7) | 55 (59.8) | <0.001 |
| Overweight (25.0–29.9) | 114 (37.4) | 42 (38.2) | 20 (35.1) | 19 (41.3) | 33 (35.9) | |
| Obese (≥30.0) | 65 (21.3) | 29 (26.4) | 26 (45.6) | 6 (13.0) | 4 (4.3) | |
| Comorbidities, N (%) | ||||||
| None | 166 (52.7) | 52 (45.6) | 30 (50.8) | 34 (72.3) | 50 (52.6) | 0.103 |
| One | 87 (27.6) | 34 (29.8) | 18 (30.5) | 9 (19.1) | 26 (27.4) | |
| Two or more | 62 (19.7) | 28 (24.5) | 11 (18.7) | 4 (8.5) | 19 (20.0) | |
| Disorder severity, N (% valid) | ||||||
| Remission/Controlled | 113 (54.3) | 49 (45.4) | 28 (50.0) | 36 (81.8) | - | |
| Low | 31 (14.9) | 16 (14.8) | 15 (26.8) | - | - | <0.001 |
| Moderate | 33 (15.9) | 23 (21.3) | 10 (17.9) | - | - | |
| High/Uncontrolled | 31 (14.9) | 20 (18.5) | 3 (5.4) | 8 (18.2) | - |
| Characteristics | Remission (N = 113) | Low Severity (N = 31) | Moderate Severity (N = 33) | High Severity (N = 31) | p-sig. 2 |
|---|---|---|---|---|---|
| Female, N (%) | 61 (54.0) | 28 (90.3) | 24 (72.7) | 20 (64.5) | 0.002 |
| Age, (mean ± SD) | 58.1 ± 14.1 | 58.2 ± 11.3 | 61.3 ± 12.1 | 58.6 ± 11.1 | 0.701 |
| Years disease duration, (mean ± SD) | 8.8 ± 8.5 | 9.9 ± 8.8 | 8.6 ± 8.6 | 7.0 ± 10.3 | 0.668 |
| Educational level, N (% valid) | |||||
| Primary | 34 (30.6) | 15 (48.4) | 15 (45.5) | 11 (35.5) | 0.437 |
| Secondary | 50 (45.0) | 10 (32.3) | 14 (42.4) | 14 (45.2) | |
| Tertiary | 27 (24.3) | 6 (19.4) | 4 (12.1) | 6 (19.4) | |
| BMI Class, N (% valid) | |||||
| Normal (18.5–24.9) | 48 (44.4) | 5 (16.1) | 7 (22.6) | 9 (29.0) | 0.017 |
| Overweight (25.0–29.9) | 33 (30.6) | 17 (54.8) | 11 (35.5) | 15 (48.4) | |
| Obese (≥30.0) | 27 (25.0) | 9 (29.0) | 13 (41.9) | 7 (22.6) | |
| Comorbidities, N (%) | |||||
| None | 64 (56.6) | 12 (38.7) | 14 (42.4) | 21 (67.7) | 0.080 |
| One | 33 (29.2) | 9 (29.0) | 10 (30.3) | 4 (12.9) | |
| Two or more | 16 (14.2) | 10 (32.3) | 9 (27.3) | 6 (19.4) |
| MSK Disorder | Between Groups Comparisons a | Within Groups Pairwise Comparisons/Mean Difference b (Effect Size) [ICC] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | EQ-5D-3L | N | EQ-5D-5L | N | SF-6D | N | EQ-5L/EQ-3L | N | SF-6D/EQ-3L | N | SF-6D/EQ-5L | |
| Rheumatoid arthritis | 105 | 0.513 | 112 | 0.565 | 101 | 0.635 | 103 | 0.057 ** (0.161) [0.926 ***] | 96 | 0.125 *** (0.400) [0.738 ***] | 101 | 0.073 *** (0.284) [0.834 ***] |
| Psoriatic arthritis | 57 | 0.550 | 55 | 0.591 | 53 | 0.668 | 54 | 0.027 (0.069) [0.954 ***] | 52 | 0.109 ** (0.348) [0.773 ***] | 51 | 0.082 * (0.267) [0.832 ***] |
| Ankylosing spondylitis | 41 | 0.622 | 45 | 0.675 | 44 | 0.693 | 41 | 0.064 * (0.199) [0.923 ***] | 41 | 0.078 * (0.266) [0.804 ***] | 43 | 0.021 (0.087) [0.868 ***] |
| Osteopenia/Osteoporosis | 82 | 0.655 | 87 | 0.665 | 75 | 0.666 | 78 | 0.011 (0.039) [0.861 ***] | 70 | 0.021 (0.092) [0.635 ***] | 70 | −0.003 (0.014) [0.674 ***] |
| p = 0.061 | p = 0.071 | p = 0.327 | ||||||||||
| Severity group c | ||||||||||||
| Remission/Inactive | 105 | 0.731 | 107 | 0.753 | 100 | 0.750 | 102 | 0.020 (0.078) [0.951 ***] | 96 | 0.010 (0.038) [0.823 ***] | 98 | −0.005 (0.029) [0.856 ***] |
| Low | 28 | 0.504 | 30 | 0.628 | 27 | 0.683 | 27 | 0.003 (0.012) [0.857 ***] | 25 | 0.062 (0.308) [0.811 ***] | 26 | 0.058 (0.285) [0.858 ***] |
| Moderate | 30 | 0.349 | 32 | 0.409 | 29 | 0.544 | 29 | 0.053 (0.161) [0.912 ***] | 28 | 0.192 * (0.695) [0.511 *] | 29 | 0.131 * (0.590) [0.591 **] |
| High/Active | 28 | −0.006 | 31 | 0.186 | 30 | 0.423 | 28 | 0.197 *** (0.657) [0.704 ***] | 28 | 0.429 *** (1.820) [0.203] | 30 | 0.249 *** (1.214) [0.384 *] |
| p < 0.001 | p < 0.001 | p < 0.001 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontodimopoulos, N.; Stamatopoulou, E.; Gazi, S.; Moschou, D.; Krikelis, M.; Talias, M.A. A Comparison of EQ-5D-3L, EQ-5D-5L, and SF-6D Utilities of Patients with Musculoskeletal Disorders of Different Severity: A Health-Related Quality of Life Approach. J. Clin. Med. 2022, 11, 4097. https://doi.org/10.3390/jcm11144097
Kontodimopoulos N, Stamatopoulou E, Gazi S, Moschou D, Krikelis M, Talias MA. A Comparison of EQ-5D-3L, EQ-5D-5L, and SF-6D Utilities of Patients with Musculoskeletal Disorders of Different Severity: A Health-Related Quality of Life Approach. Journal of Clinical Medicine. 2022; 11(14):4097. https://doi.org/10.3390/jcm11144097
Chicago/Turabian StyleKontodimopoulos, Nikolaos, Eleni Stamatopoulou, Sousana Gazi, Dimitra Moschou, Michail Krikelis, and Michael A. Talias. 2022. "A Comparison of EQ-5D-3L, EQ-5D-5L, and SF-6D Utilities of Patients with Musculoskeletal Disorders of Different Severity: A Health-Related Quality of Life Approach" Journal of Clinical Medicine 11, no. 14: 4097. https://doi.org/10.3390/jcm11144097
APA StyleKontodimopoulos, N., Stamatopoulou, E., Gazi, S., Moschou, D., Krikelis, M., & Talias, M. A. (2022). A Comparison of EQ-5D-3L, EQ-5D-5L, and SF-6D Utilities of Patients with Musculoskeletal Disorders of Different Severity: A Health-Related Quality of Life Approach. Journal of Clinical Medicine, 11(14), 4097. https://doi.org/10.3390/jcm11144097








